What is neural tube defects surveillance?
minus icon
FAQ Topic Areas
Related Pages
- Neural tube defects surveillance or tracking is the ongoing collection of data on who is affected by neural tube defects in a population and where they are. It involves the analysis and interpretation of these data to help come up with the best medical and public health interventions for the population. These interventions, such as fortification of staple foods with folic acid, can reduce disability and death related to neural tube defects. Neural tube defects surveillance usually is done as part of a larger birth defects surveillance effort.
- A population-based neural tube defects surveillance program records the number of pregnancies and babies with neural tube defects within the population of a defined geographical area.
- A hospital- or facility-based neural tube defects surveillance program records the number of pregnancies and babies with neural tube defects that occur in selected facilities.
-
Neural tube defects surveillance data can be collected through active case finding, passive case finding, or a combination of the two (hybrid) (see below).
- With active case finding, data collectors regularly visit or have electronic access to databases at participating hospitals or facilities, and they review data from the multiple sources to identify pregnancies or newborn babies with a neural tube defect.
- With passive case finding, hospital or facility personnel identify pregnancies or newborn babies with a neural tube defect and report this information directly to the surveillance program. Identification can also occur by linking hospital or facility databases with the surveillance program database. With passive case finding, the information reported to the surveillance program usually is not reviewed and checked by surveillance program personnel.
- Hybrid case finding refers to a combination of passive and active case finding. A program can use passive case finding as a first step; then, for those cases passively reported, data collectors can verify the neural tube defects by reviewing the medical records of the cases at the hospitals or facilities
- Biomarker-based surveillance measures a biomarker within people in a specified population in order to help track health outcomes related to the biomarker. An example of biomarker-based surveillance is measuring the concentration of folate in red blood cells among people in a population in order to determine the proportion of reproductive-age women in the population who have a high risk of having babies with neural tube defects
- Blood folate concentration is the amount of folate that can be measured in the blood (many forms of folate are included in the measure). Folate concentrations can be measured in serum or plasma (the liquid part of blood) or in red blood cells (RBCs), which are the blood cells that carry oxygen throughout the body. By measuring the RBC folate concentrations among women in a population, it is possible to predict the risk within the population of having a pregnancy with a neural tube defect 11, 48.
- In a 2015 guideline, the World Health Organization (WHO) defined the level of RBC folate concentrations in a population that is high enough to help prevent neural tube defects in the population. According to WHO’s definition, at the population level, RBC folate concentrations should be above 400 nanograms per milliliter (906 nanomoles per liter) in women of reproductive age, to achieve the greatest reduction of neural tube defects.
- Folic acid fortification is a public health activity known as an intervention. RBC folate concentrations can be used to find out how effective this intervention is by monitoring how much folic acid is reaching women of reproductive age through staple food fortification programs.
- Governments can use RBC folate concentrations measured in their populations to find out if their folic acid fortification programs are working and to find ways to improve efforts to prevent neural tube defects.
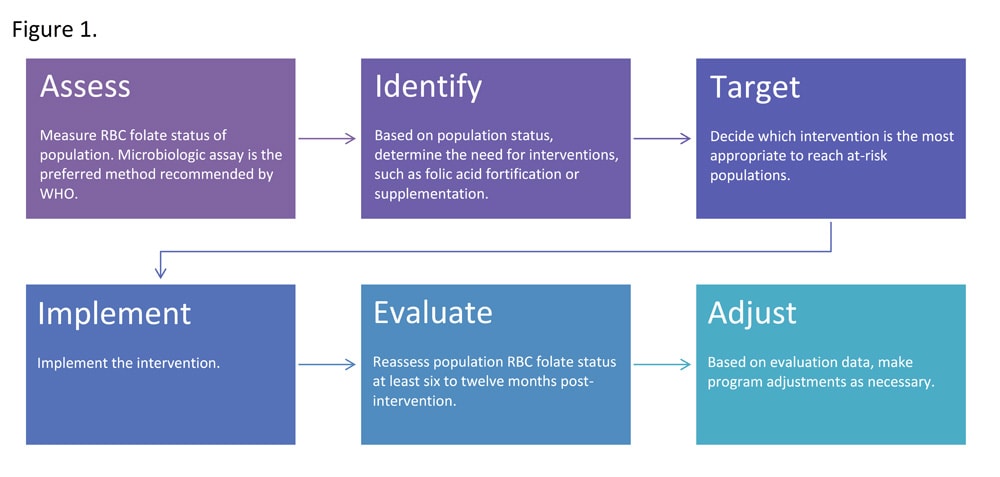

Download Image[JPG]
- There are many types of microbiologic assays, which are laboratory tests that can measure amounts of biomarkers in blood or cells. One particular microbiologic assay produces the most accurate results and is the recommended test for correctly identifying the amount of folate found in a person’s blood. It is the only laboratory test recommended for measuring red blood cell (RBC) folate concentrations. This methodology, detailed in the World Health Organization guidelines, should be used to determine RBC folate concentrations within a population.
Page last reviewed: June 17, 2022