Clinical Inquiries Received by CDC Regarding Suspected Ebola Virus Disease in Children — United States, July 9, 2014–January 4, 2015
, MD1,2; , MD1,3; , PhD1,4; , MPH1,5; , PhD1,5; , MS1,2; , MD1,6; , MD1,3; , MD1,7,8; , MD1,6; , MD1,3; , ScD1,9; , MS, MPH1,10; , MD1,2; , MD1,10; , MD1,11
The 2014–2015 Ebola virus disease (Ebola) epidemic is the largest in history and represents the first time Ebola has been diagnosed in the United States (1,2). On July 9, 2014, CDC activated its Emergency Operations Center and established an Ebola clinical consultation service to assist U.S. state and local public health officials and health care providers with the evaluation of suspected cases. CDC reviewed all 89 inquiries received by the consultation service during July 9, 2014–January 4, 2015, about children (persons aged ≤18 years). Most (56 [63%]) children had no identifiable epidemiologic risk factors for Ebola; among the 33 (37%) who did have an epidemiologic risk factor, in every case this was travel from an Ebola-affected country. Thirty-two of these children met criteria for a person under investigation (PUI) because of clinical signs or symptoms (3,4). Fifteen PUIs had blood samples tested for Ebola virus RNA by reverse transcription–polymerase chain reaction; all tested negative. Febrile children who have recently traveled from an Ebola-affected country can be expected to have other common diagnoses, such as malaria and influenza, and in the absence of epidemiologic risk factors for Ebola, the likelihood of Ebola is extremely low. Delaying evaluation and treatment for these other more common illnesses might lead to poorer clinical outcomes. Additionally, many health care providers expressed concerns about whether and how parents should be allowed in the isolation room. While maintaining an appropriate level of vigilance for Ebola, public health officials and health care providers should ensure that pediatric PUIs receive timely triage, diagnosis, and treatment of other more common illnesses, and care reflecting best practices in supporting children's psychosocial needs (5).
CDC's Emergency Operations Center was activated to respond to the Ebola outbreak in West Africa. A clinical consultation service was established to assist state and local health departments and health care providers evaluate persons possibly at risk for Ebola. Children with any signs or symptoms consistent with Ebola (fever, nausea, vomiting, headache, diarrhea, abdominal pain, muscle pain, fatigue, or unexplained bleeding) and an epidemiologic risk factor (i.e., exposure to a person with Ebola or travel from an affected country during the 21 days before symptom onset) were considered PUIs (3,4). Ebola assessment can take ≥3 days, because the diagnosis cannot be ruled out until a blood specimen obtained 72 hours after symptom onset is negative for Ebola (6). Ebola testing might not be necessary if an alternative diagnosis is made or if symptoms resolve. All domestic Ebola diagnoses are confirmed at CDC or at local or state public health laboratories, most of which are part of the CDC Laboratory Response Network (7). All Ebola-related inquiries about children during July 9, 2014–January 4, 2015 were reviewed, including call logs and e-mails, as well as databases that contain the inquiry source, demographic information, epidemiologic risk factors, clinical presentation and course, isolation measures, Ebola virus test results, and discharge diagnoses, if available (3).
During July 9, 2014–January 4, 2015, CDC responded to clinical inquiries regarding 89 children in 27 states and the District of Columbia. CDC received an average of 1–2 Ebola-related inquiries about children per week (range = zero to eight) before September 30, 2014, when CDC confirmed the first imported Ebola case identified in the United States (Figure) (2). In the subsequent 4 weeks, calls increased, with CDC receiving an average of 12 pediatric Ebola-related inquiries per week (range = five to 25). However, only 20% (10 of 49) of these inquiries turned out to involve actual PUIs, significantly lower than the 55% (22 of 40 [p<0.01]) of pediatric Ebola-related inquires that involved PUIs during all other weeks combined.
Overall, 57 (64%) children about whom an Ebola-related inquiry was made did not meet PUI criteria. Among these, 56 had no identifiable epidemiologic risk factors for Ebola but nevertheless were perceived by the inquirer to be at risk. The most common misperception about risk involved confusion about which countries were affected by Ebola: 30 (54%) children had traveled from an unaffected country, seven (12%) had contact with a traveler from an unaffected country, and 15 (27%) were perceived erroneously to have been exposed to Ebola inside the United States. Most of these inquiries (49 of 56) were initiated by a health care provider; most (37) occurred during the 4 weeks after the first Ebola case was diagnosed in the United States.
Demographic characteristics were similar for children with and without epidemiologic risk factors (Table 1). Among 33 children with an epidemiologic risk factor for Ebola, all had traveled to an Ebola-affected country, and none had known contact with an Ebola patient; 32 (97%) also had one or more signs or symptoms consistent with Ebola and thus were considered PUIs. Fifteen (47%) of the 32 PUIs were tested for Ebola, and all tested negative. The most frequently reported sign or symptom among the 32 PUIs was subjective or objective fever (25 [78%]). Eight (25%) children had signs or symptoms suggestive of an alternative diagnosis, such as an upper respiratory tract infection (Table 2). Discharge diagnosis information was available for 19 (59%) PUIs; diagnoses included malaria (five), influenza (five), other viral illness (three), and other nonviral illness (six).
At health care facilities, at least 15 PUIs were placed in some form of isolation during evaluation, and at least 10 were transferred to another hospital for Ebola assessment. Intensive care was required for at least three children while they were being evaluated; diagnoses included malaria, dehydration, and nonspecific viral illness. Written records suggest that appropriate clinical care was delayed for at least five children, either because of difficulty finding a hospital to evaluate a pediatric PUI or hospital reluctance to perform testing in the laboratory for illnesses such as malaria and influenza, because of concern about potential laboratory contamination or exposure of staff members to Ebola. One PUI who received routine vaccinations within 21 days of travel from an Ebola-affected country developed a fever 2 days later and required a full clinical evaluation to determine whether the fever was related to the vaccines, Ebola, or another travel-related illness. During pediatric Ebola assessments, many health care providers expressed concerns about whether children's parents should be permitted in the isolation room and how to manage their presence. Health care facility challenges included a lack of established policies about parental presence with children in isolation, limited supplies of personal protective equipment (PPE), lack of available staff to supervise parental donning and doffing of PPE, and insufficient time to teach parents proper PPE use. In several instances, cell phone or laptop-based video connections were used to connect isolated school-aged children with their relatives in another room.
Discussion
Pediatric clinical care presents unique challenges during an Ebola assessment (5,8), and routine pediatric care for common pediatric illnesses (e.g., bacterial and viral infections, or malaria, a disease that is endemic in West Africa) was sometimes delayed because of concerns about Ebola. No child evaluated for Ebola had any known contact with an Ebola patient or their body fluids, or with a deceased patient, and to date, no pediatric Ebola case has been identified or managed in the United States. In light of this low risk, children can be expected to have other more common causes of febrile illness, and delaying evaluation and treatment of these other diagnoses might lead to poorer clinical outcomes. Malaria and influenza should be considered in the differential diagnosis of fever in travelers, and early testing and treatment can prevent severe illness (5). Furthermore, recent travelers and immigrant children, in particular, have specific health care needs, such as routine and catch-up vaccinations. Vaccine providers should be aware of the potential for vaccine-associated fever and can consult their local or state health department for assistance in weighing the benefits and risks of administering routine vaccinations to children who are under active monitoring for Ebola because they have traveled to the United States from an affected area during the preceding 21 days.
Children, especially those who are young or who have developmental delays, are dependent upon caregivers to provide physical, emotional, and social support. The presence of a parent or caregiver in the examination room during the clinical assessment for Ebola might be important for many pediatric patients, especially very young children. Risks and benefits of parental presence should be considered on a case-by-case basis. Parents or caregivers who provide bedside support to a child in isolation for an Ebola assessment should use PPE consistently, correctly, and in accordance with CDC guidance, state or local health department recommendations, and facility policies (9). Health care facilities that might perform pediatric Ebola assessments can anticipate and plan for issues specifically related to caring for a child, including establishing policies and plans for parental presence, PPE use, and communication. Transfers of pediatric patients between facilities for Ebola-related care involve challenges related to communication between clinical teams and health departments, infection control during transport, operations coordination, and other concerns (9,10).
Although the weekly number of pediatric PUIs varied little, the number of clinical inquiries related to children increased sharply during the month after the first domestic Ebola case (Figure). Most of these children did not meet PUI criteria, but all inquiries required rapid, accurate, and complete evaluation. By anticipating and planning for surges, public health officials can scale responses to meet changing community needs. Proactive steps, including clear and consistent risk communication, timely clinician education, and broad community outreach might reduce public misperception about Ebola risk and lessen strain on clinical and public health resources.
The findings in this report are subject to at least two limitations. First, clinical data and information regarding isolation and PPE were collected as part of a public health emergency response, and information on certain variables (e.g., symptoms, suspected exposure, and alternative diagnoses) might be incomplete. Second, although this report describes all domestic Ebola-related pediatric clinical inquiries to CDC, it might not include all inquiries to health departments.
During this epidemic, CDC, state and local public health departments, and health care providers developed strategies to monitor travelers and others who might be at risk for Ebola. The goals are to quickly isolate and evaluate PUIs, including children, to ensure that patients receive timely care while minimizing transmission risk. CDC provides up-to-date information about Ebola online at http://www.cdc.gov/vhf/ebola. While maintaining a high level of vigilance for Ebola among ill pediatric patients who have recently travelled from affected countries, U.S. public health officials and health care providers should provide child-focused care that includes timely diagnosis and appropriate treatment of common pediatric illnesses, as well as Ebola, and reflects overall best practices in supporting children's psychosocial needs.
Acknowledgments
Justin K. Arnold, Brenda Kinsella Balch, Michael Bartenfeld, Stephanie Bialek, Dianna Blau, Virginia B. Bowen, Timothy Campbell, Cristina Cardemil, Joseph Sean Cavanaugh, Steven W. Champaloux, Michelle S. Chevalier, Jennifer R. Cope, Whitni Davidson, Deborah Dowell, Lauren E. Finn, Anne Marie France, Scott Gardner, Shikha Garg, Jacqueline Gindler, Konrad Hayashi, Cindy Hinton, Susan Hocevar, Christopher Hsu, Brendan Jackson, Pallavi A. Kache, Barbara Knust, Shirley Lecher, Rebecca Levine, Benjamin A. Levy, Paul Mead, Ryan Maddox, Stephen Morris, Karen Neil, Bernadette Ng'eno, Minal K. Patel, Brett W. Petersen, John Pettay, David A. Price, Mark Rayfield, Don Sharp, Kanta Sircar, Charnetta L. Smith, Alaina Steck, Ute Ströher, Christopher A. Taylor, Chris Van Beneden, Laura Wilkinson, Jennifer Williams.
12014–2015 Ebola Response, CDC; 2National Center on Birth Defects and Developmental Disabilities, CDC; 3National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; 4National Center for Chronic Disease Prevention and Health Promotion, CDC; 5Center for Surveillance, Epidemiology, and Laboratory Services, CDC; 6Center for Global Health, CDC; 7Epidemic Intelligence Service, CDC; 8National Center for Environmental Health, CDC; 9Office of Infectious Diseases, CDC; 10National Center for Emerging and Zoonotic Infectious Diseases, CDC; 11Office of Public Health Preparedness and Response, CDC.
Corresponding author: Alyson B. Goodman, iym3@cdc.gov, 404-498-6269.
References
- CDC. 2014 Ebola outbreak in West Africa. Available at http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html.
- Karwowski MP, Meites E, Fullerton KE, et al. Clinical inquiries regarding Ebola virus disease received by CDC—United States, July 9–November 15, 2014. MMWR Morb Mortal Wkly Rep 2014;63:1175–9.
- CDC. Epidemiologic risk factors to consider when evaluating a person for exposure to Ebola virus. Available at http://www.cdc.gov/vhf/ebola/exposure/risk-factors-when-evaluating-person-for-exposure.html.
- CDC. Case definition for Ebola virus disease (EVD). Available at http://www.cdc.gov/vhf/ebola/hcp/case-definition.html.
- Peacock G, Uyeki TM, Rasmussen SA. Ebola virus disease and children: what pediatric health care professionals need to know. JAMA Pediatr 2014;168:1087–8.
- CDC. Interim guidance for preparing Ebola assessment hospitals. Available at http://www.cdc.gov/vhf/ebola/hcp/preparing-ebola-assessment-hospitals.html.
- CDC. Interim guidance for specimen collection, transport, testing, and submission for persons under investigation for Ebola virus disease in the United States. Available at http://www.cdc.gov/vhf/ebola/hcp/interim-guidance-specimen-collection-submission-patients-suspected-infection-ebola.html.
- CDC. Interim U.S. guidance for monitoring and movement of persons with potential Ebola virus exposure. Available at http://www.cdc.gov/vhf/ebola/exposure/monitoring-and-movement-of-persons-with-exposure.html.
- CDC. Guidance on personal protective equipment to be used by healthcare workers during management of patients with Ebola virus disease in U.S. hospitals, including procedures for putting on (donning) and removing (doffing). Available at http://www.cdc.gov/vhf/ebola/hcp/procedures-for-ppe.html.
- CDC. Q&A's about the transport of pediatric patients (<18 years of age) under investigation or with confirmed Ebola. Available at http://www.cdc.gov/vhf/ebola/healthcare-us/emergency-services/transporting-pediatric-patients.html.
Summary
What is already known on this topic?
The current epidemic of Ebola virus disease (Ebola) is the largest in history and represents the first time Ebola has been diagnosed in the United States. CDC's Emergency Operations Center offers consultation to state and local health departments and health care providers assessing adults and children for Ebola.
What is added by this report?
During July 9, 2014–January 4, 2015, CDC responded to clinical inquiries regarding 89 children in the United States. Only 33 had an epidemiologic risk factor; 15 were tested for Ebola. All were negative. Medical evaluation and treatment for other conditions were sometimes delayed while the child underwent Ebola assessment. Additionally, health care providers and hospitals expressed concerns about allowing parents in the isolation room.
What are the implications for public health practice?
Public health and health care providers in the United States, while maintaining a high level of vigilance for Ebola among ill pediatric patients, should be prepared to provide child-focused care that includes timely diagnosis and treatment of common pediatric illnesses, as well as Ebola, and reflects overall best practices in supporting children's psychosocial needs. Parents or caregivers who provide bedside support to a child in isolation for Ebola evaluation should use personal protective equipment in accordance with CDC guidance, health department recommendations, and facility policies.
FIGURE. Number of pediatric Ebola-related clinical inquiries to CDC, by week — United States, July 9, 2014–January 4, 2015
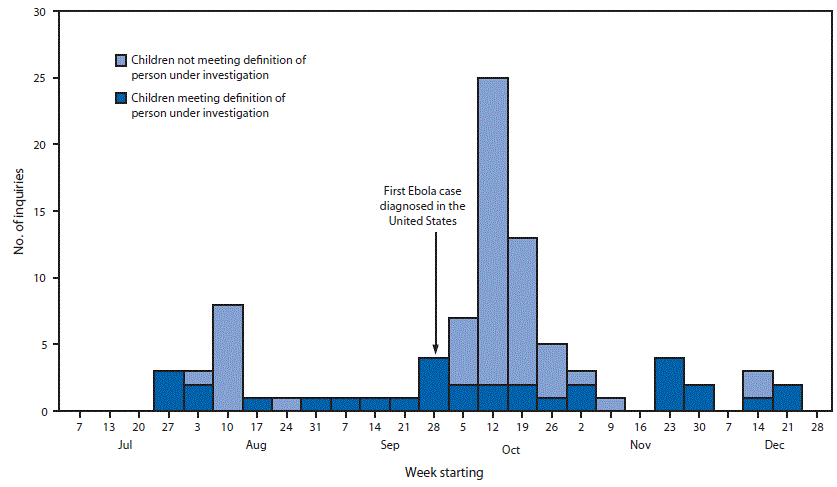
Alternate Text: The figure above is a bar chart showing the number of pediatric Ebola-related clinical inquiries to CDC, by week, in the United States during July 9, 2014–January 4, 2015.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


