Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Fatal Foodborne Clostridium perfringens Illness at a State Psychiatric Hospital — Louisiana, 2010
Clostridium perfringens, the third most common cause of foodborne illness in the United States (1), most often causes a self-limited, diarrheal disease lasting 12–24 hours. Fatalities are very rare, occurring in <0.03% of cases (1). Death usually is caused by dehydration and occurs among the very young, the very old, and persons debilitated by illness (2). On May 7, 2010, 42 residents and 12 staff members at a Louisiana state psychiatric hospital experienced vomiting, abdominal cramps, and diarrhea. Within 24 hours, three patients had died. The three fatalities occurred among patients aged 41–61 years who were receiving medications that had anti–intestinal motility side effects. For two of three decedents, the cause of death found on postmortem examination was necrotizing colitis. Investigation by the Louisiana Office of Public Health (OPH) and CDC found that eating chicken served at dinner on May 6 was associated with illness. The chicken was cooked approximately 24 hours before serving and not cooled in accordance with hospital guidelines. C. perfringens enterotoxin (CPE) was detected in 20 of 23 stool specimens from ill residents and staff members. Genetic testing of C. perfringens toxins isolated from chicken and stool specimens was carried out to determine which of the two strains responsible for C. perfringens foodborne illness was present. The specimens tested negative for the beta-toxin gene, excluding C. perfringens type C as the etiologic agent and implicating C. perfringens type A. This outbreak underscores the need for strict food preparation guidelines at psychiatric inpatient facilities and the potential risk for adverse outcomes among any patients with impaired intestinal motility caused by medications, disease, and extremes of age when exposed to C. perfringens enterotoxin.
On May 8, a state psychiatric hospital contacted OPH to report three resident deaths that occurred following an outbreak of gastrointestinal illness in patients and staff members that began late in the evening of May 6. The only common exposure was food from the hospital's kitchen. CDC joined the investigation on May 13 to help identify the outbreak cause. A case was defined as onset of any loose stools or vomiting from the evening of May 6 through the morning of May 8 in residents or staff members. Hospital infection control staff members identified 42 cases from among the 136 residents (attack rate = 31%). Illness onset ranged from 9:00 p.m. on May 6 through 3:00 p.m. on May 7 (Figure). Because of the tight clustering of symptom onset, food served at the evening meal on May 6 was considered to be the most likely cause of illness. The mean incubation time from eating the suspect meal was 13 hours (range: 5–21 hours). The most common symptoms were diarrhea (94%), abdominal cramps (51%), nausea (39%), and vomiting (27%).
Histories of food eaten were not obtained from patients because of their difficulties in recalling events, and food consumption was not recorded in nursing notes. However, 32 employees were interviewed, and 13 reported eating some portion of the kitchen-prepared suspect meal. Among these 13, nine had illness that met the case definition (attack rate = 69%). None of the staff members who did not eat the suspect dinner were ill (relative risk = infinity, 95% confidence interval = 3.7–infinity).
Interviews of the kitchen staff members revealed that the chicken served at the suspect meal was delivered frozen to the kitchen on May 4, and was cooked on May 5, the day before serving. Contrary to hospital guidelines, the chicken was placed in 6-inch deep pans after cooking and covered with aluminum foil, which slowed cooling, and the first temperature check was not until 16 hours later. During the 24 hours between cooking and serving, the chicken also was removed from cooling three times for preparation steps before being served as cold chicken sandwiches or chicken salad. Inspection of the hospital kitchen by OPH sanitarians found no critical violations of Louisiana sanitary code.
The state public health laboratory detected C. perfringens enterotoxin by reversed passive latex agglutination (RPLA) in 20 of 23 stool specimens from ill residents and staff members. CDC's Enteric Diseases Laboratory Branch detected C. perfringens enterotoxin by RPLA and polymerase chain reaction (PCR) assays for species-specific C. perfringens and CPE genes in 15 of 20 stool specimens available for testing. CDC's laboratory also isolated enterotoxin-producing C. perfringens and detected the CPE gene in all four of the samples of chicken served at the suspect meal. The stool specimens and bacterial isolates tested negative for the beta toxin gene, confirming that C. perfringens type C was not the etiologic agent and implicating C. perfringens type A.
In response to this outbreak, regional public health sanitarians conducted food safety presentations for all food service workers at the hospital. After reviewing its food preparation policies, the hospital additionally required all of its food service workers to attend a six-part food safety training course and temperature logs were developed to monitor cooling procedures.
Decedents
Patient 1. The first decedent was an ambulatory black woman aged 43 years with a history of bipolar-type schizoaffective disorder, seizures, and controlled hypothyroidism, but no known history of constipation. Her medications were citalopram, valproic acid, ziprasidone, quetiapine,* levothyroxine, and lithium. The morning after eating the suspect evening meal she experienced fecal incontinence, loose stools, clammy skin, and atypical behavior and was referred to a nearby medical center emergency department. In the emergency department, the patient was noted to have a progressive abdominal distention. Five hours after her arrival at the emergency department, the patient developed bradycardia, became apneic, and died. Her stool tested positive for C. perfringens enterotoxin by RPLA.
Autopsy revealed necrotizing colitis involving 95% of the colon and no evidence of perforation. Postmortem pathology using a C. perfringens–specific PCR assay targeting the alpha-toxin gene revealed no evidence of C. perfringens in stomach or colon. Immunohistochemical testing for C. perfringens using rabbit anti-Clostridium spp. antibody was also negative.
Patient 2. The second decedent was an ambulatory black man aged 41 years with a history of schizophrenia, hypertension, gastroesophageal reflux disorder, and frequent constipation. His medications were pantoprazole, atenolol, fluphenazine, asenapine, benztropine, hydrochlorothiazide, lithium, and lorazepam. Several hours after the suspect evening meal the patient complained of abdominal pain and was evaluated the next morning at an emergency department where radiography revealed a large amount of stool in the left colon. He was treated with magnesium citrate and discharged, but returned later in the day complaining of continued abdominal pain.
Shortly after his arrival in the emergency department, the patient passed a large loose stool and vomited once; he soon collapsed in cardiac arrest and died. Blood cultures and testing for Clostridium difficle toxin were negative. His stool tested positive for C. perfringens enterotoxin by RPLA.
Autopsy revealed necrotizing colitis involving 30% of the proximal half of the colon and 100% of the distal colon, but no evidence of perforation. Postmortem pathology using the PCR alpha-toxin assay and rabbit anti-Clostridium antibody revealed no evidence of C. perfringens in stomach and colon tissue.
Patient 3. The third decedent was an ambulatory black man aged 61 years with a history of schizophrenia, diabetes, and hypertension, and with no recorded history of constipation. His medications were clozapine, glipizide, omeprazole, ezetimibe, atenolol, losartan, and travoprost eye drops. The patient had been complaining of feeling unwell and having diarrhea throughout the day after eating the suspect evening meal, and was given loperamide (an anti–intestinal motility agent) that evening for his complaints of diarrhea.
At 5:00 a.m., the patient was found dead in his bed. His stool tested positive for C. perfringens enterotoxin by RPLA. An autopsy revealed distended, fluid-filled bowel, but with no colonic discoloration, hemorrhage, ulceration, or necrosis. Microscopic examination of the colon revealed only postmortem autolysis. Postmortem pathology using the alpha-toxin PCR assay revealed evidence of C. perfringens in stomach and colon tissue. The rabbit anti-Clostridium antibody test for C. perfringens was negative.
Reported by
Erin Delaune, MPH, Susanne Straif-Bourgeois, PhD, Raoult C. Ratard, MD, Infectious Disease Epidemiology Section, Louisiana Office of Public Health; Lee Tynes, MD, Tulane Univ School of Medicine, Louisiana Dept of Health and Hospitals. Paul C. Melstrom, PhD, Ellen Yard, PhD, Joshua G. Schier, MD, Div of Environmental Hazards and Health Effects, Sarah J. Reedy, MD, Officer of the Director, National Center for Environmental Health; Gerardo A. Gómez, Deborah F. Talkington, PhD, Samir V. Sodha, MD, Janet K. Dykes, MS, Carolina Luquez, PhD, Div of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases; Armand Sprecher, MD, EIS Officer, CDC. Corresponding contributor: Armand Sprecher, armand.sprecher@brussels.msf.org, +32 2 474 7543.
Editorial Note
The laboratory results, clinical course, and epidemiologic findings indicate that this outbreak was caused by C. perfringens type A. A related organism, C. perfringens type C, causes clostridial necrotizing enteritis or "pigbel," a type of foodborne illness characterized by necrotizing small bowel inflammation caused by the beta toxin. Necrotizing enteritis is caused when the normal trypsin-mediated degradation of beta toxin is impaired by a protein-poor diet or coingestion of foods such as sweet potatoes that contain trypsin inhibitors. Clostridial necrotizing enteritis has a mortality rate of 15%–25%, but it is rare in developed countries such as the United States (2) and was ruled out in this outbreak when samples tested negative for the beta-toxin gene.
This is the second reported outbreak of foodborne illness caused by C. perfringens type A with fatalities attributed to necrotizing colitis (3) that occurred in a U.S. psychiatric inpatient facility. In the first reported outbreak, which occurred in 2001, two of three patients who had necrotizing colitis died. Each had experienced a clinical course similar to that found in the 2010 outbreak. In one other report of a foodborne C. perfringens type A outbreak, two fatalities occurred in a psychogeriatric hospital in England (4). Evidence of chronic constipation and fecal impaction was found, but necrotizing colitis was not found on postmortem examination (4).
Psychiatric hospital residents exposed to C. perfringens might be at increased risk for developing necrotizing colitis because of impaired gastrointestinal motility from chronic use of anticholinergic medications. All three deceased patients in this outbreak were taking medications with anticholinergic side effects: quetiapine, fluphenazine, benztropine, and clozapine. Additionally, one patient was on a constipating diuretic medication, hydrochlorothiazide. Another was given an opiate antimotility agent (loperamide), but did not have necrotizing colitis. These medications delay the usual elimination of enterotoxin by C. perfringens–induced diarrhea, causing longer exposure to the toxin. Prolonged exposure to C. perfringens enterotoxin has been shown to cause severe intestinal damage and death in a rabbit model (5).
The findings in this report are subject to at least two limitations. First, patient information was obtained from secondary sources: hospital staff members, nursing notes, and emergency department records. These contained approximate measures of onset time, reducing the reliability of incubation time calculation. Second, no records were kept of the quantity of food each patient ate, which prevented a determination of correlation with disease severity.
Why these patients developed fatal necrotizing colitis when many other ill patients who also were taking psychiatric medications with anti–intestinal motility side effects experienced a self-limited illness with full recovery is unclear. The amount of inoculum ingested, the dose of antipsychotic medication administered to patients, and the variation in host susceptibility to anticholinergic side effects might affect disease outcome. The precise mechanism causing death remains in question; one patient who died in the outbreak had no evidence of necrotizing colitis or other abnormality on postmortem examination, raising the possibility that a systemic effect of clostridial toxin plays a role. Despite these unanswered questions, the results of this investigation suggest that psychiatric inpatients, especially those with constipation, are vulnerable to severe outcomes from C. perfringens intoxication. Institutions should ensure that precautions to prevent C. perfringens and other causes of foodborne illness are in place.† Providers of psychiatric care also should be aware that impaired intestinal motility places their patients at risk for adverse outcomes, including death, when exposed to enterotoxin-producing C. perfringens.
Acknowledgments
Shirley Burton, MPH, Michele Pogue, Randy Ducote, David Holcombe, MD, Louisiana Dept of Health and Hospitals; Kathy Nugent, Louisiana State Univ Health Sciences Center. Wun-Ju Shieh, Infectious Disease Pathology Br; Sue Reynolds, EIS Field Assignments Br, CDC.
References
- Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 2011;17:7–15.
- Brynestad S, Granum PE. Clostridium perfringens and foodborne infections. Int J Food Microbiol 2002;74:195–202.
- Bos J, Smithee L, McClane B, et al. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin Infect Dis 2005;40:e78–83.
- Pollock AM, Whitty PM. Outbreak of Clostridium perfringens food poisoning. J Hosp Infect 1991;17:179–86.
- Sarker MR, Carman RJ, McClane BA. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol Microbiol 1999;33:946–58.
* Drugs with constipation listed as a common side effect are italicized in the text and include benztropine, clozapine, fluphenazine, hydrochlorothiazide, loperamide, and quetiapine.
† Available resources include the Food and Drug Administration's Food Code, available at http://www.fda.gov/food/foodsafety/retailfoodprotection/foodcode/default.htm.
What is already known on this topic?
Clostridium perfringens is an underrecognized but common cause of foodborne illness that usually causes self-limited disease and rarely is fatal.
What is added by this report?
This is the second reported outbreak of C. perfringens foodborne illness with fatalities attributed to necrotizing colitis in the United States. That these outbreaks have occurred in psychiatric inpatient facilities increases concern that the impaired intestinal motility caused by antipsychotic medications renders these patients vulnerable to necrotizing colitis when exposed to C. perfringens enterotoxin.
What are the implications for public health practice?
Application of food preparation guidelines to prevent C. perfringens foodborne illness is warranted in any setting where institutional food preparation creates a risk for C. perfringens illness outbreaks. Given the potential for fatal outcomes, such guidelines deserve reinforcement in psychiatric hospitals and other settings where patients might have impaired intestinal motility for any reason to reduce the risk for necrotizing colitis after C. perfringens infection.
FIGURE. Date and time of symptom onset during an outbreak of Clostridium perfringens food poisoning at a state psychiatric hospital — Louisiana, 2010
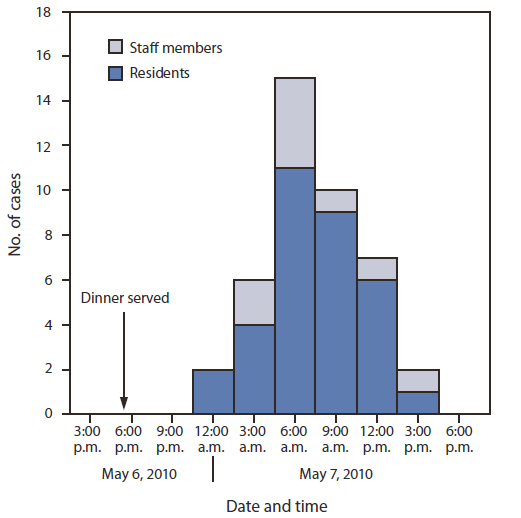
Alternate Text: The figure above shows the date and time of symptom onset during an outbreak of Clostridium perfringens food poisoning at a state psychiatric hospital in Louisiana, during 2010. On May 8, a state psychiatric hospital contacted OPH to report three resident deaths that occurred after an out¬break of gastrointestinal illness in patients and staff members that began late in the evening of May 6. The only common exposure was food from the hospital's kitchen. CDC joined the investigation on May 13 to help identify the outbreak cause. A case was defined as onset of any loose stools or vomiting from the evening of May 6 through the morning of May 8 in residents or staff members. Hospital infection control staff members identified 42 cases from among the 136 residents (attack rate = 31%). Illness onset ranged from 9:00 pm. on May 6 through 3:00 p.m. on May 7.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


