Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
CDC Grand Rounds: Newborn Screening and Improved Outcomes
Newborn screening is the practice of testing every newborn for certain harmful or potentially fatal conditions, such as hearing loss and certain genetic, endocrine, and metabolic disorders that typically are not otherwise apparent at birth. Newborn screening in the United States began in the 1960s. Universal newborn screening has become a well-established, state-based, public health system involving education, screening, diagnostic follow-up, treatment and management, and system monitoring and evaluation (1). Each year, >98% of approximately 4 million newborns in the United States are screened (2,3). Through early identification, newborn screening provides an opportunity for treatment and significant reductions in morbidity and mortality (2,3).
Uniformity of Newborn Screening
In 2006, The American College of Medical Genetics (ACMG), under the aegis of the Health Resources and Services Administration (HRSA), convened a group of experts to address the substantial variation in the number of disorders screened for in each state. The experts evaluated scientific and medical information related to screened conditions and recommended a uniform screening panel of 29 core (or primary) conditions to be included in state newborn screening panels: 20 inborn errors of metabolism, three hemoglobinopathies, and six other conditions (4). This panel was endorsed by the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) and designated by the Secretary of the U.S. Department of Health and Human Services as a national standard for newborn screening programs (4). Its adoption has led to increased uniformity of screening in the United States and its territories (Figure 1) (2,3). Additional conditions for screening continue to be identified and nominated for inclusion in the panel.
Expansion of Newborn Screening
ACHDNC reviews nominations of conditions to be included in the uniform panel. The committee encourages nomination by persons and organizations with expertise on the condition being nominated. The nomination process is transparent, allows for public commentary, and follows a systematic protocol for evidence-based review (5). Since adoption of the core panel of 29 conditions, nine additional conditions have been submitted and reviewed. ACHDNC recommendations to include two of the conditions, severe combined immunodeficiency and critical congenital heart disease, into the uniform newborn screening panel were approved by the Secretary in 2010 and 2011, respectively. Six of the conditions submitted for inclusion have been forwarded for an external review, and four have been referred back to nominators for additional studies.
Public Health Burden
Of the 4 million infants who are screened each year, approximately 12,500 are diagnosed with one of the 29 core conditions of the uniform screening panel. The five most commonly diagnosed conditions in the United States are 1) hearing loss, 2) primary congenital hypothyroidism, 3) cystic fibrosis, 4) sickle cell disease, and 5) medium-chain acyl-CoA dehydrogenase deficiency (Table) (3.6). Newborn screening can help prevent death or disability, if treatment follows (1,3). Each year, for example, one in 2,000 newborns is diagnosed with congenital hypothyroidism. Screening followed by thyroid hormone treatment can prevent intellectual disability (intelligence quotient [IQ] score <70) (7,8). Congenital hearing loss occurs in one to three newborns per 1,000 live births. Each year, newborn hearing screening identifies hearing loss in >5,000 infants. Without screening, these children might have delayed language acquisition, low educational attainment, increased behavior problems, decreased psychosocial well-being, and poor adaptive skills (9). Untreated phenylketonuria can result in severe cognitive impairment. Prompt initiation of treatment following newborn screening is essential for optimal development and prevention of disability (10).
Assessing the health benefits and return on investment of newborn screening has its challenges, given the diversity of conditions and their varying outcomes. Overall, screening and treating disabling conditions can reduce health-care costs. The conditions on the uniform newborn screening panel, with the exception of hearing loss and critical congenital heart disease, all are detected by dried blood spot screening. The cost of the U.S. newborn blood spot screening system was assessed by the U.S. Government Accountability Office in 2003 at $120 million per year, or $30 per infant (11). For congenital hypothyroidism alone, the most recent estimate of the annual cost of testing is $5 per infant, or $20 million for the entire country (12). The potential health benefit of testing 4 million infants for congenital hypothyroidism is the prevention of 160 cases of intellectual disability and, among 1,010 infants in whom milder impairments (i.e., IQ scores lower than expected for the population) were prevented, a total gain of nearly 15,000 IQ points (8).
Laboratory expertise for newborn screening tests. Most newborn screening is conducted by state health laboratories, which follow prescribed procedures to ensure high-quality screening and communicate results and information with other segments of the newborn screening system, such as hospitals and health-care practitioners. They also play an important role in conducting translational research by identifying and designing new screening tests and focusing on quality improvement of current screening tests. Their challenges include an environment of restricted state budgets, an increase in the number of new conditions that need to be detected, and the need to stay current with evolving technologies and automate processes to reduce cost.
CDC works with state and regional newborn screening laboratories to develop and improve the quality of screening tests. CDC administers the Newborn Screening Quality Assurance Program, which includes all U.S. laboratories involved in newborn screening and >450 international laboratories. This is the only program that addresses quality issues of dried blood spot measurements for all conditions for which newborn screening is available in the United States. The program provides proficiency testing, training, support, technical assistance, and consultation to participating laboratories.
Long-term follow-up. The goal of long-term follow-up is to improve the quality of care for children with diagnosed disorders so that they receive timely, appropriate care (13). Strategies for comprehensive long-term follow-up include coordination of multidisciplinary care through a child's medical home,* monitoring physical and psychosocial outcomes, improving family and provider access to information, establishing evidence-based best practices, and improving quality and timeliness of follow-up, diagnosis, and treatment and management through health information technology. Long-term follow-up is used to assess the needs of patients and families regarding disease management, treatment, and age-appropriate preventive care. Long-term follow-up can provide invaluable data to guide treatment through the development of care guidelines and clinical decision support. In the United States, however, newborn screening resources often are focused on diagnosis and short-term follow-up, and long-term follow-up among state programs varies considerably. A 2005 survey of state newborn screening programs found that only 56% routinely conduct systematic long-term follow-up (14).
Data systems and tracking for follow-up and management of disorders. Improvement of data quality in overall tracking and surveillance systems is needed to track the clinical outcomes of affected children more effectively and to refine protocols for short-term and long-term follow-up of children with conditions identified through newborn screening. For example, newborn screening for hearing loss increased from 46.5% in 1999 to 96.9% in 2008, but data on follow-up testing are lacking. In 2009, nearly 45% of infants who did not pass screens lacked documentation of a follow-up assessment (6). The Indiana newborn hearing screening program is exemplary for its web-based tracking and surveillance system, which includes follow-up reminders and quality improvement activities. Indiana has shown a dramatic decrease in the percentage of infants who are lost to follow-up and documentation, from 35% in 2005 to 7% in 2009 (6) (Figure 2).
Data transmission between clinical care and public health systems is needed to improve follow-up and management. CDC, HRSA, the National Institutes of Health, and the National Library of Medicine are working with state programs and clinicians to describe common variables and standardize data collection procedures to enable different segments of the newborn screening system to share information. Another challenge is that states might differ in the case definitions they use for newborn screening disorders. To create multistate datasets for newborn screening disorders, federal agencies are collaborating with clinicians to develop standardized case definitions for use in state and national newborn screening data collection.
Building partnerships. The need for improvements in long-term follow-up provides opportunities for partnerships at the national, state, and local levels. National partnerships provide a forum for health-care providers, public health professionals, and families to collaborate on newborn screening issues such as data collection, education, laboratory services, and clinical care. At the state and local level, partnerships should be established among state newborn screening programs, Title V programs, professional societies, and health-care providers. Resources to support these partnerships include the HRSA-funded Regional Genetic and Newborn Screening Services Collaboratives, the National Institutes of Health-funded Newborn Screening Translational Research Network, the Genetic Alliance, the National Newborn Screening and Genetics Resource Center, and the National Center on Hearing Assessment and Management. Initiatives designed to improve quality and develop the evidence base for treatment include the Newborn Screening ACTion Quality Improvement Innovation Network and the Newborn Screening Education in Quality Improvement for Pediatric Practice course, which include decision support tools for the clinical practice and education of primary-care providers, assisting them in identifying and closing gaps in care. Learning collaboratives, such as the National Initiative for Children's Healthcare Quality/Maternal Child Health Bureau project, have been developed to help state programs improve hearing screening services and enhance data collection and registries.
Summary
Although newborn screening capabilities have improved and expanded significantly in the past decade, several critical gaps and challenges remain. Clinical challenges include a shortage of experts trained to diagnose and manage conditions detected by newborn screening. Laboratory gaps and opportunities center on detection of multiple conditions using a single test, expansion of automation to reduce testing costs, and extension of new molecular methods to all disorders. General challenges include a lack of public education and understanding about the value of newborn screening that could be improved by deeper engagement of consumers in newborn screening policy and program development. Advocacy organizations can assist with raising awareness of newborn screening and can provide disease-specific education to the public. In 2009, HRSA awarded a cooperative agreement to Genetic Alliance, a consumer advocacy group, to develop a newborn screening clearinghouse. The clearinghouse was launched in September 2011 as an online resource† that provides information on newborn screening, the conditions the tests identify, and screening information for all 50 states, the District of Columbia, Puerto Rico, and U.S. territories. This is a national level resource providing comprehensive education for families. Continued collaboration among partners provides an excellent opportunity to enhance laboratory and data systems through quality assurance, surveillance, tracking, and research to improve screening techniques, better guide follow-up of affected children, and optimize outcomes.
Given all these challenges and opportunities, screening itself clearly is not enough. It is critical to avoid complacency in assuming that every newborn who is screened will receive optimal service and care. Short-term follow-up and management of children with disorders and long-term follow-up activities within the entire newborn screening system are central to realizing the promise of newborn screening.
Reported by
R. Rodney Howell, MD, Univ of Miami, Florida; Secretary's Advisory Committee on Heritable Disorders in Newborns and Children. Sharon Terry, MA, Genetic Alliance, Washington, DC. Vera F. Tait, MD, American Academy of Pediatrics. Richard Olney, MD, Cynthia F. Hinton, PhD, Div of Birth Defects and Developmental Disabilities, Scott Grosse, PhD, Div of Blood Disorders, John Eichwald, MA, Div of Human Development And Disability, National Center on Birth Defects and Developmental Disabilities; Carla Cuthbert, PhD, Div of Laboratory Sciences, National Center for Environmental Health; Tanja Popovic, MD, PhD, Office of the Director; Jill Glidewell, MSN, MPH, EIS officer, CDC. Corresponding contributor: Jill Glidewell, iyp0@cdc.gov, 404-498-3538.
References
- Pass KA, Lane PA, Elsas LJ, et al. US newborn screening system guidelines II: follow-up of children, diagnosis, management, and evaluation. Statement of the Council of Regional Networks for Genetic Services (CORN). Pediatrics 2000;137(4 Suppl):S1–46.
- CDC. Using tandem mass spectrometry for metabolic disease screening among newborns. MMWR 2001;50(No. RR-3).
- CDC. Impact of expanded newborn screening—United States, 2006. MMWR 2008;57:1012–5.
- Watson AS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR. Newborn screening: toward a uniform panel and system. Genet Med 2006;8(Suppl 1):1S–11S.
- Calonge N, Green NS, Rinaldo P, et al. Committee report: method for evaluating conditions nominated for population-based screening of newborns and children. Genet Med 2010;12:153–9.
- CDC. Summary of infants screened for hearing loss, diagnosed, and enrolled in early intervention, United States, 1999–2008. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. Available at http://www.cdc.gov/ncbddd/hearingloss/2008-data/ehdi_1999_2008.pdf. Accessed July 17, 2011.
- Rovet JF. Children with congenital hypothyroidism and their siblings: do they really differ? Pediatrics 2005;115:e52–7.
- Grosse SD, Van Vliet G. Prevention of intellectual disability through screening for congenital hypothyroidism: how much and at what level? Arch Dis Child 2011;96:374–9.
- American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007;120:898–921.
- Azen CG, Kock R, Friedman EG, et al. Intellectual development in 12-year-old children treated for phenylketonuria. Am J Dis Child 1991;145:35–9.
- US General Accounting Office. Newborn screening: characteristics of state programs. Washington, DC: US General Accounting Office; 2003. Available at http://www.gao.gov/new.items/d03449.pdf. Accessed May 24, 2011.
- Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics 2006;117:S287–95.
- Lloyd-Puryear MA, Tonniges T, McPherson M, et al. American Academy of Pediatrics Newborn Screening Task Force recommendations: how far have we come? Pediatrics 2006;117(5 Pt 2):S194–211.
- Hoff T, Hoyt A, Therrell B, Ayoob M. Exploring barriers to long-term follow-up in newborn screening programs. Genet Med 2006;8:563–70.
* Defined as a partnership between a child, a child's family, and the pediatric-care team who oversees the child's health and well-being and works to ensure that all of the medical and nonmedical needs of the patient are met. The medical home is a model of delivering care that is accessible, continuous, and comprehensive.
† Available at http://www.babysfirsttest.org.
FIGURE 1. Number of states screening for the core bloodspot conditions in the Recommended Uniform Screening Panel (RUSP) — United States, 2004–2009
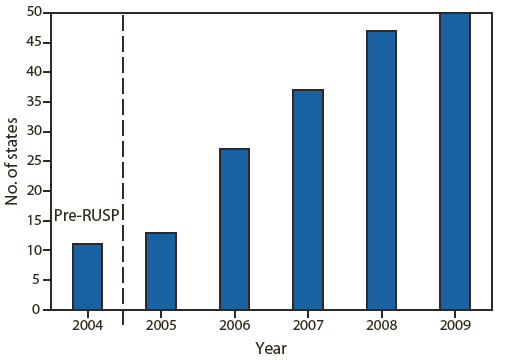
Source: Data reported from National Newborn Screening and Genetics Resource Center. Available at http://genes-r-us.uthscsa.edu.
Alternate Text: The figure above shows the number of states screening for the core bloodspot conditions in the Recommended Uniform Screening Panel in the United States during 2004–2009. In 2006, the American College of Medical Genetics recommended a uniform screening panel of 29 core (or primary) conditions to be included in state newborn screening panels: 20 inborn errors of metabolism, three hemoglobinopathies, and six other conditions. This panel was endorsed by the Advisory Committee on Heritable Disorders in Newborns and Children and designated by the Secretary of the U.S. Department of Health and Human Services as a national standard for newborn screening programs. Its adoption has led to increased uniformity of screening in the United States and its territories. Additional conditions for screening continue to be identified and nominated for inclusion in the panel.
|
TABLE. Estimated number of cases among U.S. children identified in 2006 with disorders listed in the Recommended Uniform Screening Panel,* based on incidence of these disorders in four state newborn screening programs during 2001–2006,† and number diagnosed with hearing loss in 2009§ |
|
|---|---|
|
Disorder |
Estimated no. of cases |
|
Hearing loss |
5,073 |
|
Primary congenital hypothyroidism (excluding secondary, transient, or other) |
2,156 |
|
Cystic fibrosis (including nonclassical) |
1,248 |
|
Hemoglobin SS (sickle cell anemia) |
1,128 |
|
Hemoglobin SC (sickle C disease) |
484 |
|
Medium-chain acyl-CoA dehydrogenase deficiency |
239 |
|
Classical galactosemia (GALT) plus variant (excluding GALK and GALE) |
224 |
|
Phenylketonuria (PKU), including clinically significant hyperphenylalaninemia variants |
215 |
|
Congenital adrenal hyperplasia (excluding non 21-hydroxylase deficiency) |
202 |
|
Hemoglobin S/β thalassemia |
163 |
|
3-Methylcrotonyl-CoA carboxylase deficiency |
100 |
|
Carnitine uptake defect |
85 |
|
Very long-chain acyl-CoA dehydrogenase deficiency |
69 |
|
Biotinidase deficiency (including partial) |
62 |
|
Methylmalonic acidemia (mutase deficiency) |
50 |
|
Glutaric acidemia type I |
38 |
|
Isovaleric acidemia |
32 |
|
Maple syrup urine disease |
26 |
|
Citrullinemia type I |
24 |
|
Propionic acidemia |
15 |
|
Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency |
13 |
|
Methylmalonic acidemia CblA,B |
12 |
|
Homocystinuria |
11 |
|
Argininosuccinic acidemia |
7 |
|
Beta-ketothiolase deficiency |
7 |
|
Hydroxymethylglutaric aciduria |
3 |
|
Multiple carboxylase deficiency |
3 |
|
Trifunctional protein deficiency |
2 |
|
Total |
11, 691 |
|
* One of the 29 disorders listed in the screening panel (tyrosinemia type 1), and two recently approved additions (severe combined immunodeficiency and critical congenital heart disease) are not included in this table. Listing available at http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendedpanel/index.html. † The four states were California, Massachusetts, North Carolina, and Wisconsin. Source: CDC. Impact of expanded newborn screening—United States, 2006. MMWR 2008;57:1012–5. § Estimated number of U.S. cases of hearing loss was obtained from CDC Early Hearing Detection and Intervention Program annual data. Available at http://www.cdc.gov/ncbddd/hearingloss/ehdi-data2009.html. |
|
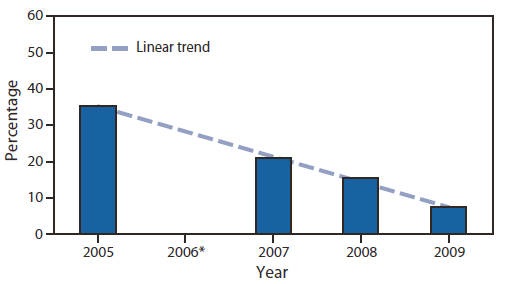
* Data for 2006 were incomplete because of changes in the program.
Alternate Text: The figure above shows the decrease in the percentage of infants lost to follow-up and documentation after hearing screening in Indiana during 2005–2009. The Indiana newborn hearing screening program is exemplary for its web-based tracking and surveillance system, which includes follow-up reminders and quality improvement activities. Indiana has shown a dramatic decrease in the percentage of infants who are lost to follow-up and documentation, from 35% in 2005 to 7% in 2009.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


