FIGURE. Time from hospital emergancy department (ED) exposure and rash onset until laboratory confirmation of measles among six patients — Pennsylvania, March–April 2009
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Hospital-Associated Measles Outbreak — Pennsylvania, March–April 2009
Although endemic measles transmission has been interrupted in the United States, importations of this highly infectious virus continue (1,2). On March 28, 2009, a physician notified the Pennsylvania Department of Health (PADOH) of a measles case involving an unvaccinated child. Within 5 days, four additional cases were reported to PADOH and the Allegheny County Health Department. All five infected persons had been in the same hospital emergency department (ED) on March 10; one of them was a physician who worked in the ED. To find the source patient, PADOH reviewed electronic records of patients evaluated in the ED on March 10 for fever and rash. This identified a child who arrived recently from India, was treated for viral exanthema, and discharged. On April 3, PADOH obtained serum from this child and confirmed a diagnosis of measles. After an extensive regional search and investigation of the six patients' 4,000 contacts, no additional cases were identified. The hospital reviewed employee health records to identify any exposed personnel who did not have serologic evidence of measles immunity. Among 168 potentially exposed employees, 72 (43%) had no documented measles immunity, thus requiring serologic testing and subsequent vaccination if they lacked serologic evidence of immunity. This outbreak highlights the potential for measles transmission in health-care settings. To decrease transmission, clinicians should know the signs and symptoms of measles, request travel histories of patients suspected of any infectious disease, and isolate potentially infectious patients. Hospital employees should have documented immunity to measles, and employees without evidence of measles immunity should be offered vaccination in accordance with Advisory Committee on Immunization Practices (ACIP) and Hospital Infection Control Practices Advisory Committee (HICPAC) recommendations.
Initial Investigation
On March 28, 2009, a previously healthy child aged 23 months (patient A, index patient) was brought to a community hospital with a fever of 102.5°F (39.2°C), cough, coryza, and a generalized maculopapular rash that had developed on March 26. He was recognized as possibly having measles, was transported to a referral hospital (hospital A) ED, and placed in airborne isolation. Serum, nasopharyngeal, and urine specimens were collected and sent to PADOH's public health laboratory. All three specimens subsequently were forwarded to CDC for serologic confirmation and virologic testing; serology performed on March 30 was measles immunoglobulin M (IgM)-positive, indicating acute infection.
The index patient's brother (patient B), aged 4 years, had onset of fever, cough, coryza, and rash on March 23; the boys' father (patient C) had onset of similar symptoms on March 26. Serum from the brother and father tested measles IgM-positive from specimens collected after patient A's diagnosis. The parents had elected not to vaccinate either child; the father had received a single vaccine dose during childhood. All three family members met the standard measles surveillance case definition.*
The incubation period for measles is 7–18 days from exposure until rash onset, and persons are considered contagious from 4 days before to 4 days after rash onset. Because all three family members exhibited rash onset within 3 days of one another, a point-source exposure was suspected. All had been in hospital A's ED together on March 10 for one child's unrelated illness; none had traveled internationally. On April 2, CDC established that the measles virus isolated from the index patient's nasopharyngeal specimen was genotype D8, which is endemic in India (3).
Additional Cases
Two additional cases subsequently were reported to PADOH by hospital staff members. One was in a physician (patient D) who worked in hospital A's ED and had fever and rash onset on March 26. The physician had not sought medical attention but previously had received 3 doses of measles-containing vaccine. Serum obtained April 1 tested measles IgM-positive. The other case involved an infant (patient E), aged 11 months, who had fever and rash onset on March 27 and had been evaluated in hospital A's infectious disease clinic on April 1 to rule out Kawasaki disease. His serum was drawn April 2 and was measles IgM-positive. Both patients had been in the hospital's ED on March 10; neither had traveled internationally.
Source Patient
On April 3, review of electronic medical records of the 200 patients evaluated in the ED on March 10 focused on patients with a chief complaint of fever and rash and those who had reported recent international travel; this search identified a child (patient F, source patient), aged 10 years, with unknown vaccination history, who had moved to Pennsylvania from India on March 8. Onset of fever, coryza, and conjunctivitis occurred on March 7, and a generalized maculopapular rash began on March 9. He was examined at a pediatrician's office on March 10 and sent to hospital A's ED to rule out Kawasaki disease. He was evaluated in a room adjacent to the examination rooms of patients A and E, overlapping with them by 4 hours. The ED physician with the positive measles IgM titer had examined this patient and provided a diagnosis of viral exanthema. Serum collected on April 3 from the suspected source patient was measles IgM-positive (Figure).
Control Measures
Contact investigations were based on the six patients' locations during their contagious periods (Table). None of the children attended school or child care during that time. Because the source patient traveled on a commercial aircraft while contagious, CDC's Division of Global Migration and Quarantine obtained contact information for exposed passengers on his flight from India and provided this information to health departments in contact passengers' home states. No secondary cases of measles were reported among these passengers. Alerts to the public and health-care providers were distributed through the Pennsylvania Health Alert Network, Epi-X, press releases, and fliers posted at exposure sites and bus routes. Staff members from hospital A, other health-care facilities, PADOH, the Allegheny County Health Department, and CDC telephoned 4,000 potentially exposed persons. During a 2-week period, PADOH's laboratory processed 70 serum samples from persons with suspected measles. Contact tracing within the hospital was facilitated by its electronic medical record-keeping. No other confirmed cases were identified.
Since 2007, hospital A has required documentation of measles serologic immunity among new employees, but previously hired employees had been tested inconsistently. During the outbreak, the hospital reviewed serologic records of all potentially exposed employees, including employees without clinical responsibilities. If no serology was on record, serum was drawn, and if immunoglobulin G (IgG)-negative, measles vaccine was administered and employees furloughed from their duties for 18 days after exposure to measles patients. Of 168 hospital employees, 72 (43%) did not have measles IgG titers on record. Of the 69 employees who subsequently were tested, eight (12%) did not have measles IgG antibodies; of these, five were furloughed until 18 days had elapsed. Except for the ED physician, no employees became symptomatic.
Reported by
Michael Green, MD, James Levin, MD, Marian Michaels, MD, Sheila Vasbinder, MSN, Children's Hospital of Pittsburgh; Ronald Voorhees, MD, Allegheny County Health Dept; James Lute, PhD, Virginia Dato, MD, Perrianne Lurie, MD, Veronica Urdaneta, MD, Pennsylvania Dept of Health. Gregory Armstrong, MD, Gregory Wallace, MD, William Bellini, PhD, Preeta Kutty, MD, Paul Rota, PhD, Jennifer Rota, MPH, Luis Lowe, MS, Lauren Stockman, MPH, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases; James Lando, MD, Career Epidemiology Field Officer Program, Office of Public Health Preparedness and Response; George Han, MD, EIS Officer, CDC. Corresponding contributor: George Han, ghan@cdc.gov, 787-679-8108.
Editorial Note
Because the measles-mumps-rubella vaccine is highly efficacious and U.S. vaccination coverage levels are high (4), U.S. clinicians have limited experience with measles. Of these six cases, only the index patient initially was suspected of having measles; therefore, he was the only patient for whom isolation precautions were taken. HICPAC's 2007 guidelines recommend precautions against airborne transmission for any patient who has a maculopapular rash accompanied by cough, coryza, and fever.†
Health-care–associated measles outbreaks are costly (5). Electronic medical records facilitated the search for the source patient and identification of potentially exposed patients in the ED and hospital A's other clinics, eliminating the need for a more time-consuming review of hundreds of paper records (6). Extensive contact tracing was necessary for nonisolated cases, placing a substantial burden on public health resources and health-care facilities.
This outbreak continues the trend of measles outbreaks that have been linked to importation; although measles has been eliminated in the Americas, it continues to circulate in all other regions (5,7–9). The measles virus isolated from the index patient was determined by CDC to be genotype D8, a genotype common in India, where measles remains endemic (3). A history of recent international travel should increase clinical suspicion for diseases rare in the United States but common elsewhere.
Despite delays in diagnoses and lack of isolation precautions, measles transmission during this outbreak was limited, possibly because of the high rates of measles immunization among members of this community, the fact that the infected children did not attend school or child care, and intense control efforts by public health officials and health-care facilities. Population immunity of 92%–94% is necessary to prevent future measles outbreaks because importations are likely (10). None of the three secondarily infected children had been vaccinated for measles; the child aged 11 months was too young for routine vaccination, and the index patient and his brother were unvaccinated by parental choice.
During this outbreak, hospital A tested all of its remaining employees who did not have measles serologic results documented; those few who did not have serologic evidence of immunity were vaccinated. All health-care facilities should follow ACIP and HICPAC guidelines that health-care facilities should ensure that their employees are fully vaccinated for measles or have laboratory evidence of immunity.§ This can minimize the need for emergency testing and furlough of employees exposed to measles and associated outbreaks.
Acknowledgments
Rose Moon, Barry Minkel, Children's Hospital of Pittsburgh; Sharon Silvestri, Shirley Slagy, Charles O'Brien, James Davidson, Allegheny County Health Dept; Kirsten Waller, Tiffany Marchbanks, Mària Moll, Stephen Ostroff, Nancy Rea, Carol Teacher, Judi Sedivy, Chandra Marriott, Stanley Reynolds, Heather Stafford, Alexandra McFall, Patricia Matlock, John Bart, Alice Gray, Pennsylvania Dept of Health. Paul Edelson, Div of Global Migration and Quarantine, National Center for Emerging and Zoonotic Diseases, CDC.
References
- Papania MJ, Seward JF, Redd SB, Lievano F, Harpaz R, Wharton ME. Epidemiology of measles in the United States, 1997–2001. J Infect Dis 2004;189(Suppl 1):S61–8.
- Parker Fiebelkorn A, Redd SB, Gallagher K, et al. Measles in the United States during the postelimination era. J Infect Dis 2010;202:1520–8.
- Rota PA, Bellini WJ. Update on the global distribution of genotypes of wild type measles viruses. J Infect Dis 2003;187(Suppl 1):S270–6.
- Orenstein WA, Papania MJ, Wharton M. Measles elimination in the United States. J Infect Dis 2004;189(Suppl 1):S1–3.
- Chen SY, Anderson S, Kutty PK, et al. Health care-associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis 2011;203:1517–25.
- Bonebrake AL, Silkaitis C, Monga G, et al. Effects of mumps outbreak in hospital, Chicago, Illinois, USA, 2006. Emerg Infect Dis 2010;16:426–32.
- CDC. Measles—United States, January–May 20, 2011. MMWR 2011;60:666–8.
- CDC. Import-associated measles outbreak—Indiana, May–June 2005. MMWR 2005;54:1073–5.
- CDC. Preventable measles among U.S. residents, 2001–2004. MMWR 2005;54:817–20.
- Fine PEM, Mulholland K. Community immunity. In: Plotkin S, Orenstein W, Offit P, eds. Vaccines. 5th ed. Philadelphia, PA: WB Sauders; 2008:1573–92.
* Available at http://www.cdc.gov/osels/ph_surveillance/nndss/casedef/measles_2010.htm.
† HICPAC guidelines are available at http://www.cdc.gov/hicpac/2007ip/2007ip_table2.html.
§ ACIP recommendations are available at http://www.cdc.gov/mmwr/pdf/rr/rr6007.pdf.
What is already known on this topic?
Measles outbreaks in the United States frequently occur as a result of importations.
What is added by this report?
A child who had arrived recently in Pennsylvania from India was brought to a hospital emergency department (ED) with a rash that was diagnosed as a viral exanthema. The child was not isolated. Subsequently, one ED physician and four visitors to the ED, including three unvaccinated children, were diagnosed with measles.
What are the implications for public health practice?
All health-care settings should ensure that employees have documented immunity to measles, and clinicians should include measles in the differential diagnosis of patients with fever and rash, especially among patients with recent international travel.
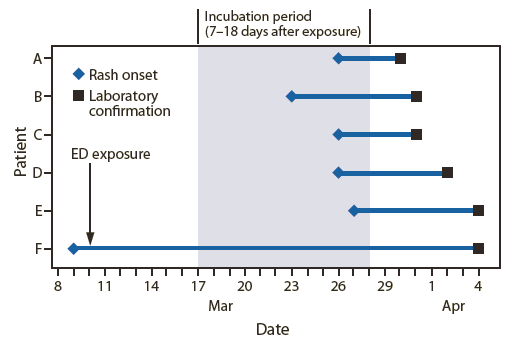
Alternate Text: The figure above shows time from hospital emergency department exposure on March 10 and rash onset until laboratory confirmation of measles among six patients in Pennsylvania during march-April 2009. Serum collected on April 3 from the suspected source patient, who had traveled from India, was measles immunoglobulin M-positive.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


