Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
HIV Transmission Through Transfusion --- Missouri and Colorado, 2008
Transmission of human immunodeficiency virus (HIV) through transfusion of contaminated blood components was documented in the United States in 1982 (1). Since then, the risk for transfusion-transmitted HIV infection has been almost eliminated by the use of questionnaires to exclude donors at higher risk for HIV infection and the use of highly sensitive laboratory screening tests to identify infected blood donations. The risk for acquiring HIV infection through blood transfusion today is estimated conservatively to be one in 1.5 million, based on 2007--2008 data (2). This report describes the first U.S. case of transfusion-transmitted HIV infection reported to CDC since 2002 (3). A blood center in Missouri discovered that blood components from a donation in November 2008 tested positive for HIV infection. A lookback investigation determined that this donor had last donated in June 2008, at which time he incorrectly reported no HIV risk factors and his donation tested negative for the presence of HIV. One of the two recipients of blood components from this donation, a patient undergoing kidney transplantation, was found to be HIV infected, and an investigation determined that the patient's infection was acquired from the donor's blood products. Even though such transmissions are rare, health-care providers should consider the possibility of transfusion-transmitted HIV in HIV-infected transfusion recipients with no other risk factors.
Case Reports
Donor. In June 2008, a man in his forties donated whole blood at a blood center in Missouri (Figure 1). He was a repeat blood donor who reported no HIV risk factors on the routine eligibility screening questionnaire. He was not compensated for his blood donation. His whole blood donation was screened at a reference laboratory for HIV by enzyme immunoassay (EIA) (Genetic Systems HIV-1/HIV-2 Plus O EIA, Bio-Rad Laboratories, Redmond, Washington) and by nucleic acid amplification testing of minipools of plasma specimens (MP-NAT) from 16 donations (Procleix HIV-1 Nucleic Acid Test, Gen Probe, San Diego, California); both tests were negative. Components from this donation later were transfused into two recipients. No specimens from this donation were stored. In November 2008, the man donated blood again at the same blood center and again reported no risk factors on the routine eligibility screening questionnaire. At that time, his blood tested positive for HIV by EIA, MP-NAT, and indirect immunofluorescence assay (Fluorognost HIV-1 IFA, Sanochemia Corporation, Vienna, Austria). The man was placed on the list of donors who are indefinitely ineligible for future donation, all products from this donation were destroyed, and the man was notified by the blood center of his probable HIV infection. The Missouri Department of Health and Senior Services (MDHSS) was notified of this case on December 4, 2008. Because of the rare possibility that the donor might have been infected shortly before his June 2008 donation and donated blood that contained HIV at a concentration too low to be detected, an investigation was initiated to determine whether recipients of the June donation had been infected with HIV, consistent with regulatory requirements to investigate such events.
Initially, the donor declined repeated contacts by MDHSS to be interviewed. In April 2009, he agreed to a brief interview with MDHSS, and an OraQuick rapid HIV test (OraSure Technologies, Bethlehem, Pennsylvania) was performed. This test was reactive and confirmed by a positive Western blot at MDHSS. During his interview, the donor reported he was married but had sex with both men and women outside of his marriage, including just before his June 2008 donation. He indicated that the sex often was anonymous and occurred while he was intoxicated.
Recipients. The investigation initiated by the blood center identified two recipients of blood components (packed red blood cells and fresh frozen plasma) derived from the donor's June 2008 donation. In July 2008, one unit of packed red blood cells from the donor was transfused into a patient in Arkansas during cardiac surgery. This patient died 2 days later from cardiac disease; no premortem or postmortem material was available for testing, and it was unknown whether the patient had been infected with HIV.
In August 2008, one unit of fresh frozen plasma from the donor was transfused into a patient receiving a kidney transplant in Colorado. The recipient's most recent negative serum test for HIV infection (using HIV EIA) was in July 2005. The patient had been receiving regular hemodialysis for management of kidney failure since July 2005. From that date to the date of kidney transplantation, the patient reported no behavioral or health-care--related risk factors for HIV infection and did not received blood components. The kidney donor tested negative for HIV infection by EIA and NAT at the time of organ donation.
In December 2008, MDHSS notified the Colorado Department of Public Health and Environment (CDPHE) that the plasma was from a donor who subsequently tested positive for HIV, and CDPHE notified the recipient's transplant surgeon. When the recipient visited the transplant clinic in December 2008, serum was nonreactive by HIV EIA, but plasma HIV RNA viral load was 7,240 copies/mL, and CD4 cell count was very low (48 cells/µL). At this time, the recipient was placed on antiretroviral therapy. The patient also was receiving mycophenolic acid, a drug used to prevent rejection in organ transplantation that is also a potent inhibitor of both lymphocyte proliferation and HIV replication in CD4+ T cells and macrophages. Physical examination demonstrated no other signs or symptoms of HIV infection. After antiretroviral therapy was initiated, the patient's HIV RNA viral load became undetectable, and CD4 cell count increased to 88 cells/µL in June 2009. HIV EIA repeated in April 2009 was reactive, but the Western blot was indeterminate, with reactivity to the nonviral p38 and p42 bands and weak reactivity to gp120.
HIV DNA from blood specimens collected from the donor and the recipient was amplified and sequenced at CDC. Comparison of these sequences demonstrated that the virus from the donor and recipient were greater than 99% identical, confirming that the donor's 2008 donation was the source of the recipient's HIV infection.
Reported by
B Laffoon, Missouri Dept of Health and Senior Svcs. A Crutchfield, Colorado Dept of Public Health and Environment. M Levi, MD, Univ of Colorado at Denver. WA Bower, MD, M Kuehnert, MD, Office of Blood, Organ, and Other Tissue Safety, Div Health Care Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases; JT Brooks, MD, RM Selik MD, WM Switzer, MPH, W Heneine, PhD, A Shankar, MS, MSc, AD Iuliano, PhD, Div of HIV/AIDS Prevention, National Center for HIV, Hepatitis, STD, and Tuberculosis Prevention, CDC.
Editorial Note
This report describes the first U.S. case of transfusion-transmitted HIV infection reported* to CDC since 2002 (3) (Figure 2). The sequence of events in this case is consistent with transmission by transfusion of HIV-contaminated plasma collected from a donor during the eclipse period of acute infection (i.e., the interval between infection and the development of detectable concentrations of HIV RNA in plasma) to a recipient treated with medication that suppressed HIV replication, reduced the CD4 lymphocyte count, and blunted the humoral response to HIV infection.
In 1999, U.S. blood banks implemented HIV NAT for blood donations to reduce HIV transmission from recently infected donors. NAT can detect the presence of HIV earlier in the course of infection than serologic methods, which only detect antibodies against HIV, thus reducing the window period (i.e., the interval between infection and development of detectable HIV markers in blood) from 22 days to approximately 10--15 days (4,5). However, NAT cannot detect HIV infections during the eclipse period, estimated to average 9 days based on limited data (6).
The Food and Drug Administration (FDA) requires blood centers to assess donor eligibility using a screening questionnaire and to test donations for infections to reduce the risk for transfusion-transmitted disease.† FDA currently requires testing blood donations for HIV using both licensed serologic testing and NAT, which can detect HIV RNA at a minimum concentration of approximately 5.5 copies/mL.§ NAT can be conducted on individual specimens (ID-NAT) or pooled specimens (MP-NAT). The number of specimens pooled for MP-NAT is based on manufacturer's specifications and FDA's test sensitivity requirements.¶ The dilution effect inherent in screening by MP-NAT makes this method slightly less sensitive than ID-NAT (3.8 compared with 6.9 infections prevented per year, respectively); however, ID-NAT is substantially less cost effective (7).
Widespread adoption of effective HIV testing methods to screen donated blood has greatly reduced the risk for transfusion-transmitted HIV infection. The modeled risk for HIV infection from transfusion of blood products in the United States declined from one in 450,000--600,000 donations in 1995 to one in 2,135,000 donations from 1995 to 2001 after the introduction of NAT in 1999 (8) and was recently updated to one in 1,467,000 based on data from 2007--2008, which incorporates the increased incidence of HIV among blood donors (2). However, even the most sensitive screening technologies currently available cannot identify the presence of HIV infection during the first few days after infection, when neither HIV RNA nor HIV-specific antibodies have reached detectable levels.
Transfusion-transmitted HIV infection, although rare, likely is underrecognized, and every case warrants a detailed investigation. Three previous cases of HIV infection attributable to transfusion of infected blood products that tested negative by HIV NAT and EIA because of donation during the eclipse period were identified and reported to CDC in 2000 (9) and 2002 (10). Assuming that 16 million donations occur each year** and using the most conservative estimated risk for HIV infection of one in 1.5 million donations (2), approximately 11 infectious donations and 20 HIV-positive blood components released each year could potentially infect recipients. In this case, eligibility screening questions,†† if answered accurately, would have excluded the donor because of his sexual history. It is the responsibility of persons who donate blood to answer screening questionnaires accurately to ensure the safest blood supply possible.
Blood collection centers conduct investigations of previous donations when a positive antibody or NAT result is identified in a repeat donor. However, fewer than the expected number of cases of transfusion-transmitted HIV infection were reported to CDC from 2002 to 2008, a 6-year period when an estimated 16 million units of blood or blood components were donated annually. Because the number of reported cases is lower than expected, risk estimates might have been too high. Alternatively, transfusion-transmitted HIV infections might have gone unreported either because of 1) recipient death attributed to the underlying condition or some other cause before detection of HIV infection from the receipt of infected blood or blood components, 2) poor recall by infected persons regarding receipt of blood or blood components before their HIV diagnosis, 3) inability to confirm or rule out transfusion as the source of infection because no HIV-infected donors were identified, 4) underrecognition of HIV infections among recipients of potentially infected blood or blood components who recover and might never have been subsequently tested for HIV infection, or 5) misclassification of a transfusion-transmitted HIV infection in a person who also had other risk factors more frequently associated with HIV transmission (e.g., male-to-male sexual contact or injection drug use) to which that infection was attributed. Adoption of CDC's 2006 recommendation for routine opt-out HIV testing recommendations, whereby all persons are tested for HIV as part of routine health care unless they decline, might reduce the possibility of unrecognized transfusion-transmitted infections and possibly reduce donations by HIV-infected persons being made aware of their status.§§ Additionally, blood centers might consider the logistics, costs, and potential benefits of saving specimens of blood so that retrospective testing can be conducted if transfusion-transmitted HIV infection is suspected.
Although the risk for transfusion-transmitted HIV infection is extremely low in the United States, transfusion should be considered along with other possible sources of HIV infection in a patient who has no other HIV risk factors. These investigations are most effective if conducted as soon as they are recognized and in collaboration with the blood center, transfusing health-care facilities, and state and local health departments. The National Healthcare Safety Network (NHSN) is a voluntary, secure, Internet-based surveillance system designed to collect data from a sample of U.S. health-care facilities to permit valid estimation of the magnitude of adverse events among patients. The Hemovigilance Module added this year to the NHSN's Biovigilance Component¶¶ was designed specifically to bolster the collaborative capacity of public health and private industry to detect adverse events (e.g., HIV infections) associated with transfusion. Findings from Hemovigilance Module surveillance data will be used to improve the safety of the blood supply in the United States.
References
- CDC. Possible transfusion-associated acquired immune deficiency syndrome (AIDS)---California. MMWR 1982;31:652--4.
- Zou S, Dorsey KA, Notari EP, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion 2010;50:1495--504.
- Stramer SL. Third reported US case of breakthrough HIV transmission from NAT screened blood. Transmission 2003;43(Supplement):40A.
- Busch MP, Dodd RY. NAT and blood safety: what is the paradigm? Transfusion 2000;40:1157--60.
- Stramer SL, Caglioti S, Strong DM. NAT of the United States and Canadian blood supply. Transfusion 2000;40:1165--8.
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008;105:7552--7.
- Jackson BR, Busch MP, Stramer SL, AuBuchon JP. The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations. Transfusion 2003;43:721--9.
- Dodd RY, Notari EP, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion 2002;42:975--9.
- Delwart EL, Kalmin ND, Jones TS, et al. First report of human immunodeficiency virus transmission via an RNA-screened blood donation. Vox Sang 2004;86:171--7.
- Phelps R, Robbins K, Liberti T, et al. Window-period human immunodeficiency virus transmission to two recipients by an adolescent blood donor. Transfusion 2004;44:929--33.
What is already known on this topic?
Transfusion-transmitted cases of HIV infection are rare, but still might occur despite screening questionnaires for deferral of at-risk donations and improvements in laboratory testing for detecting HIV in blood products.
What is added by this report?
This report describes the first case of transfusion-transmitted HIV infection reported to CDC since 2002.
What are the implications for public health practice?
Although transfusion-transmitted HIV infection is a rare event, clinicians and health departments should evaluate the possibility of such an event in a patient with no other known risk factors for HIV infection. If a case of transfusion-transmitted HIV infection is identified, clinicians should report the case through their public health surveillance system and collaborate with blood collection centers and health departments to conduct an investigation.
FIGURE 1. Sequence of events for a case of transfusion-transmitted HIV infection --- Missouri and Colorado, 2008
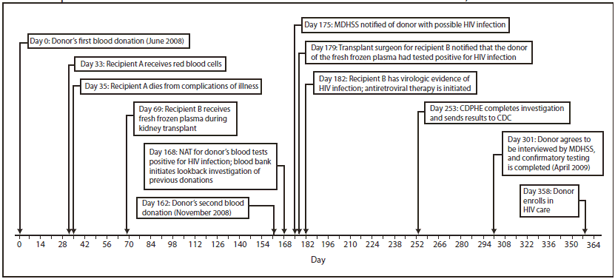
Alternate Text: The figure above shows the sequence of events for a case of transfusion-transmitted HIV infection in 2008. In June 2008, a man donated whole blood at a blood center in Missouri. His donation was screened for HIV; tests were negative. Components from this donation later were infused into two recipients. No specimens were stored. In November 2008, the man donated blood again. At that time, his blood tested positive for HIV. An investigation identified two recipients of blood components derived from the donor's June 2008 donation. In July 2008, one unit from the donor was transfused into a patient in Arkansas during cardiac surgery. This patient died 2 days later from cardiac disease; no premortem or postmortem material was available for testing, and it was unknown whether the patient had been infected with HIV. In August 2008, one unit of fresh frozen plasma from the donor was transfused into a a patient receiving a kidney transplant in Colorado. In December 2008, MDHSS notified the Colorado Department of Public Health and Environment (CDPHE) that the plasma was from a donor who subsequently tested positive for HIV, and CDPHE notified the recipient's transplant surgeon. When the recipient visited the transplant clinic in December 2008, serum was nonreactive by HIV EIA, but plasma HIV RNA viral load was 7,240 copies/mL, and CD4 cell count was very low (48 cells/μL). Later, HIV DNA from blood specimens collected from the donor and the recipient was amplified and sequenced at CDC. Comparison of these sequences demonstrated that the virus from the donor and recipient were greater than 99% identical, confirming that the donor's 2008 donation was the source of the recipient's HIV infection.
FIGURE 2. Number of cases of transfusion-transmitted HIV infection from contaminated blood products, by transfusion year --- United States, 1985--2008
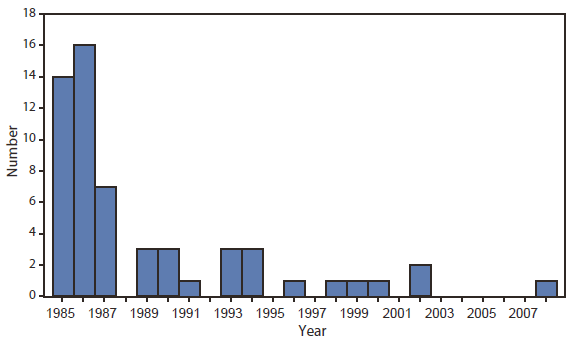
Alternate Text: The figure above shows the number of cases of transfusion-transmitted HIV infection from contaminated blood products, by transfusion year, in the United States during 1985-2008.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.




