FIGURE 1. Number of births affected by a neural tube defect --- worldwide, 1998
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
CDC Grand Rounds: Additional Opportunities to Prevent Neural Tube Defects with Folic Acid Fortification
This is another in a series of occasional CDC Grand Rounds reports. These reports are based on grand rounds presentations at CDC on high-profile issues in public health science, practice, and policy. Information regarding CDC Grand Rounds is available at http://www.cdc.gov/about/grand-rounds.
Neural tube defects (NTDs) are serious birth defects that result from the failure of the neural tube to close in the cranial region (anencephaly) or more caudally along the spine (spina bifida) by the 28th day of gestation. Infants born with anencephaly usually die within a few days of birth, and those with spina bifida have life-long disabilities with varying degrees of paralysis. Currently, identified risk factors for NTDs include a mother who previously had an NTD-affected pregnancy, maternal diabetes, obesity, hyperthermia, certain antiseizure medications, genetic variants, race/ethnicity, and nutrition (particularly folic acid insufficiency). In the United States, during 1995--1996, approximately 4,000 pregnancies were affected by an NTD. This number declined to 3,000 pregnancies in 1999--2000 after fortification of enriched cereal grain products with folic acid was mandated (1). Worldwide, in 1998, approximately 300,000 births were affected by an NTD (Figure 1).
Both observational and intervention studies, including randomized, controlled trials, have demonstrated that adequate consumption of folic acid periconceptionally can prevent 50%---70% of NTDs (2). Three approaches can increase intake of folate/folic acid*: dietary improvement, supplementation, and food fortification. Efforts to improve women's dietary habits so that they consume more foods rich in folate or daily vitamin supplements have had little success because they require behavior change, improved accessibility, affordability, or sustainability (3). Supplementation alone also has not been an effective approach because approximately 50% of pregnancies are unplanned. Fortifying foods with folic acid has been a highly effective and more uniform intervention, because fortification makes folic acid accessible to all women of childbearing age without requiring behavior change.
In 1992, the U.S. Public Health Service (USPHS) recommended that all women of childbearing age capable of becoming pregnant consume 400 µg of folic acid daily for prevention of NTDs. In 1996, the Food and Drug Administration (FDA) established regulations that required that by 1998 all standardized enriched cereal grain products sold in the United States include 140 µg folic acid/100 g and provided for the addition of folic acid to breakfast cereals, corn grits, infant formulas, medical foods, and foods for special dietary use. Also in 1998, the Institute of Medicine (IOM) conducted an independent review, with conclusions supporting the USPHS recommendations for folic acid consumption; in 2009, the U.S. Preventive Services Task Force published updated guidelines reinforcing these recommendations (4).
Impact of Fortification with Folic Acid
U.S. NTD and blood folate trends. The mandatory fortification of standardized enriched cereal grain products in the United States resulted in a substantial increase in blood folate concentrations and a concomitant decrease in NTD prevalence. The percentage of the population with low serum folate (<3 ng/mL) declined from 21% in the period before fortification (1988--1994) to <1% of the total population in the period immediately following fortification (1999--2000) (5). NTD prevalence decreased by 36% after fortification, from 10.8 per 10,000 population during 1995--1996 to 6.9 at the end of 2006 (6).
Health disparities. After mandatory fortification in 1998, NTD prevalence declined 30%--40% among the three largest racial and ethnic groups. Nevertheless, 2005--2007 National Birth Defects Prevention Network (NBDPN) data show that Hispanic women continue to be at significantly greater risk (prevalence ratio = 1.21; 95% confidence interval = 1.11--1.31) for having a baby affected by an NTD than non-Hispanic white women (CDC, unpublished data, 2010) (Figure 2). Non-Hispanic black women have consistently had lower NTD prevalence than Hispanic women and non-Hispanic white women (Figure 2), despite having the lowest folate levels before and after mandatory fortification. Nonfolate risk factors for NTDs might explain this inconsistency between NTD prevalence and folate status and merit further study. Factors that might be contributing to the inconsistency include genetic differences in folate metabolism, maternal diabetes, and obesity, which are known to vary by race and ethnicity; another possibility is intake of nutrients other than folic acid, such as Vitamin B12 (7).
Global NTD and blood folate trends. Successful mandatory fortification programs also have been documented in several other countries, including Canada, Costa Rica, Chile, and South Africa, resulting in significant increases in blood folate concentrations and 25%--50% declines in the prevalence of NTD-affected pregnancies (3). For example, in Chile, fortification of wheat flour for bread at 220 µg folic acid/100 g was associated with a 43% reduction in NTDs from 17.1 per 10,000 population in 1999--2000 to 9.7 in 2001--2002 (8).
Cost. Published economic evaluations have shown that folic acid food fortification is cost saving in the United States and other countries. A 2008 study estimated that current folic acid fortification produces an annual savings of about $300 million, or $100 for each $1 invested in fortification (9). Fortification also has resulted in substantial cost savings globally. Chile has demonstrated a savings of $11 (in international dollars) for each $1 invested in fortification (10).
Potential adverse effects. Concerns have been raised that intake of folic acid might cause harmful effects, including progression of nerve damage in B12-deficient persons; excess intake in children; accumulation of unmetabolized folic acid; blunting of antifolate therapy (methotrexate and phenytoin); accelerated cognitive decline in the elderly; epigenetic hypermethylation; and cancer promotion (11). Most of these concerns are associated with consumption of high levels of folic acid from supplement use rather than fortification. A 2010 study using NHANES 2003--2006 data showed that 6% of the U.S. adult population aged >19 years consumed more than the recommended 400 µg folic acid/day from supplements, and almost half of these persons (2.7% of the U.S. adult population) exceeded the tolerable upper level (UL) of average daily usual folic acid intake of 1,000 µg (12). Conversely, none of the remaining 94% of the U.S. adult population, who consumed ≤400 µg folic acid per day from supplements, exceeded the UL, regardless of folic acid intake levels from enriched cereal grain products and ready-to-eat cereals. No conclusive evidence exists to indicate that folic acid intake at recommended levels contributes to the causation of any of these conditions of concern; however, continued monitoring and research are needed to ensure that folic acid public health recommendations do not have unintended negative consequences.
Current Opportunities and Strategies
Focus on Hispanics. While nonfolate risk factors for NTDs and their contribution to disparities in NTD prevalence must be further considered, prevalence data suggest that Hispanics also might have a need for additional folic acid. Consideration of ways to enhance the intake of folic acid among Hispanics while not contributing to higher folic acid intake in the general population is a high priority. Targeted folic acid awareness and promotion efforts have been successful in increasing the use of folic acid supplements among Spanish-speaking Hispanic women (13), although whether this behavior change is sustained after intensive intervention is concluded has not yet been evaluated. In addition, the possibility of selectively fortifying foods not included in the current fortification regulation that are staples in Hispanic communities, such as corn tortillas or other products made from corn masa flour, is being considered. A recent study suggested that fortifying corn masa flour at the levels currently used for fortified grains (i.e., 140 µg folic acid / 100 g), would increase folic acid consumption by Mexican-American women by 20%, while increasing folic acid consumption among non-Hispanic white and non-Hispanic black women by approximately 5% (14). Currently, FDA regulations do not permit folic acid to be added to corn masa flour. Substantial assessments are needed to address such issues as nutrient composition of corn masa flours; stability, shelf life and consumer acceptance of adding folic acid to such flours; amount of fortification needed to achieve a reduction in risk of NTDs in the target population; and methods for monitoring effectiveness and safety of such proposed fortification.
Expand global fortification. NTDs have been reported on every continent and among diverse populations at all levels of economic development. Currently, 53 countries have regulations for mandatory fortification of wheat flour with folic acid, although many of these programs have not been fully implemented and the existence of regulations does not imply compliance† (Figure 3). Micronutrient fortification programs that include folic acid are only preventing an estimated 9% of total annual cases of folic acid-preventable NTDs (15). Expanding the number of developed and developing countries with mandatory folic acid fortification of high consumption staples has the potential to safely eliminate NTDs that are preventable through folic acid consumption.§
In 2004, CDC, in collaboration with Emory University in Atlanta, Georgia, contributed to formation of the Flour Fortification Initiative (FFI), a network of government and international agencies, wheat and flour industries, and consumer and civic organizations, to promote global flour fortification because none of these sectors can effectively address all the issues alone. Since then, the percentage of the world's wheat flour produced in large roller mills that is fortified has increased from 18% to 30%. By 2015, the target date of the WHO Millennium Development Goals, the FFI goal is for 80% of the world's roller mill wheat flour to be fortified. Future efforts should focus not only on expanding fortification of wheat flour with folic acid but also on fortifying other common staples such as corn and rice.
NTDs are life-threatening and cause life-long disabilities. Fortification of flour and other high-consumption, high-penetration staples with folic acid is a feasible, economical, safe, and effective public health policy to prevent NTDs worldwide. Efforts are needed to evaluate the safety and effectiveness of fortification of corn masa flour in the United States and to expand fortification of staple foods across the globe. Current research and increasing fortification efforts have demonstrated the ability to eliminate those NTDs that are sensitive to folic acid. If 50%--70% of NTDs fall into this category, and assuming an annual prevalence of 300,000 NTDs, worldwide folic acid fortification could lead to the prevention of 150,000--210,000 NTDs per year.
Reported by
A Cordero, MPA, J Mulinare, MD, RJ Berry, MD, C Boyle, PhD, Div of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities; W Dietz, MD, PhD, Div of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, CDC. R Johnston Jr, MD, Univ of Colorado School of Medicine. J Leighton, PhD, Office of the Commissioner, Food and Drug Admin. T Popovic, MD, PhD, Office of the Director, CDC.
References
- CDC. Spina bifida and anencephaly before and after folic acid mandate---United States, 1995--1996 and 1999--2000. MMWR 2004;53:362--5.
- Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol 2010;39(Suppl 1):i110--21.
- Berry RJ, Bailey L, Mulinare J, Bower C, Folic Acid Working Group. Fortification of flour with folic acid. Food Nutr Bull 2010;31(Suppl 1):S22--35.
- US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009;150:626--31.
- Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988--2004. Am J Clin Nutr 2007;86:718--27.
- National Birth Defects Prevention Network. Neural tube defect ascertainment project 2010. Available at http://www.nbdpn.org/current/resources/ntd_fa_info.html. Accessed August 10, 2010.
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995--2002. Pediatrics 2005;116:580--6.
- Hertrampf E, Cortes F. National food-fortification program with folic acid in Chile. Food Nutr Bull 2008;29(Suppl 2):S231--7.
- Grosse SD, Ouyang L, Collins JS, Green D, Dean JH, Stevenson RE. Economic evaluation of a neural tube defect recurrence-prevention program. Am J Prev Med 2008;35:572--7.
- Llanos A, Hertrampf E, Cortes F, Pardo A, Grosse SD, Uauy R. Cost-effectiveness of a folic acid fortification program in Chile. Health Policy 2007;83:295--303.
- Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr 2008;87:517--33.
- Yang Q, Cogswell ME, Hamner HC, et al. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003--2006. Am J Clin Nutr 2010;91:64--72.
- Prue CE, Hamner HC, Flores AL. Effects of folic acid awareness on knowledge and consumption for the prevention of birth defects among Hispanic women in several US communities. J Women's Health 2010;19:689--98.
- Hamner HC, Mulinare J, Cogswell ME, et al. Predicted contribution of folic acid fortification of corn masa flour to the usual folic acid intake for the US population: National Health and Nutrition Examination Survey 2001--2004. Am J Clin Nutr 2009;89:305--15.
- Bell KN, Oakley GP Jr. Update on prevention of folic acid-preventable spina bifida and anencephaly. Birth Defects Res A Clin Mol Teratol 2009;85:102--7.
* Folate often is used as a generic term for two different forms of vitamin B9. One form, folate, is found naturally in foods such as beef liver, green leafy vegetables, some fruits, beans, and whole grains. The other form, folic acid, is the synthetic form found in supplements, ready-to-eat breakfast cereals, and fortified foods.
† Flour Fortification Initiative. Map of global progress, 2010. Available at http://www.sph.emory.edu/wheatflour/ globalmap.php.
§ World Health Organization. Recommendations on wheat and maize flour fortification meeting report: interim consensus statement. Available at http://www.who.int/nutrition/publications/micronutrients/wheat_maize_fortification/en/index.html.
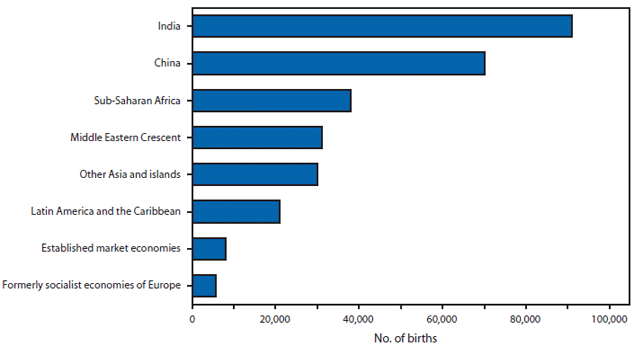
Source: Shibuya K, Murray CJ. Congenital anomalies. In: Health dimensions of sex and reproduction: the global burden of sexually transmitted diseases, HIV, maternal conditions, perinatal disorders, and congenital anomalies. Murray CJ, Lopez AD, eds. Boston, Massachusetts: the Harvard School of Public Health on behalf of the World Health Organization and the World Bank; 1998:455--512.
Alternate Text: The figure above shows the number of births affected by a neural tube defect, worldwide in 1998. Worldwide, in 1998, approximately 300,000 births were affected by an NTD.
FIGURE 2. Neural tube defect rates per 10,000 population, by race/ethnicity and fortification period status --- National Birth Defects Prevention Network,* 1995--2007
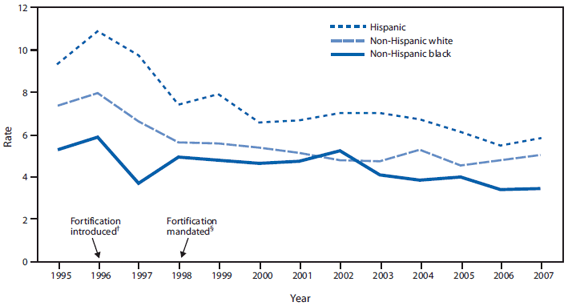
* Data from 25 population-based birth defects programs.
† Food and Drug Administration establishes regulations requiring fortification with 140 µg folic acid/100 g of all standardized enriched cereal grain products sold in the United States by 1998.
§ Mandatory fortification takes effect.
Alternate Text: The figure above shows neural tube defect rates per 10,000 population in the United States, by race/ethnicity and fortification period status from 1995 to 2007. After mandatory fortification began in 1998, NTD prevalence declined 30%–40% among the three largest racial and ethnic groups.
FIGURE 3. Countries (N = 53) with regulations for fortification of wheat flour with folic acid*, by program status --- worldwide, June 2010
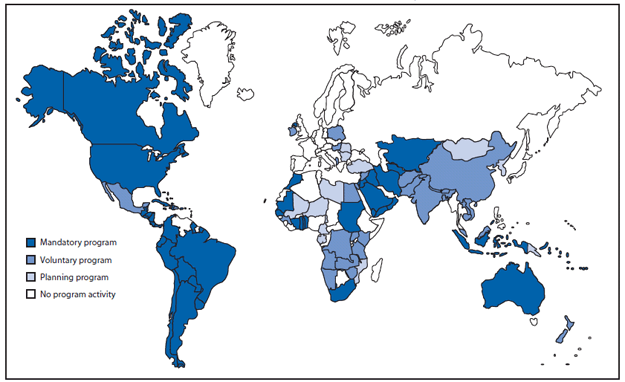
Source: Flour Fortification Initiative. Map of global progess. Available at http://www.sph.emory.edu/wheatflour/globalmap.php.
* The World Health Organization recommends adding 1--5 ppm of folic acid to fortified wheat flour, depending on the average per capita wheat flour availability (g/day). Additional information available at http://www.who.int/nutrition/publications/micronutrients/wheat_maize_fortification/en/index.html.
Alternate Text: The figure above shows countries with regulations for fortification of wheat flour with folic acid, by program status, worldwide as of June 2010. A total of 53 countries had regulations for mandatory fortification of wheat flour with folic acid, although many of these programs had not been fully implemented, and the existence of regulations did not imply compliance.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


