PROTECT Safety Improvements
The PROTECT collaborative approach has led to specific improvements in each key activity area.
PROTECT Initiative activities have demonstrated that enhanced safety packaging can prevent unsupervised medication ingestions, or at least limit the amount of medication that young children can access on their own. PROTECT participants have developed and refined the concept of pediatric exposure-limiting packaging, assessed efficacy of specific innovative packaging designs in limiting access by young children, and encouraged adoption of innovative safety packaging.
Safety improvements include:
- Implementation of innovative safety packaging for oral liquid medications. Flow restrictors are adapters added to the necks of liquid medicine bottles to limit the amount of liquid that can come out of the bottle, even when turned upside down, shaken, or squeezed. In 2011, manufacturers of over-the-counter (OTC) medications committed to incorporating new product enhancements for infants’ liquid acetaminophen products, including adding flow restrictors to infants’ and children’s acetaminophen [PDF – 1 Page].
- Demonstrated efficacy of enhanced safety packaging. In collaboration with the Georgia Poison Center, a multi-site randomized trial demonstrated how well flow restrictors prevent preschool-aged children from getting liquid out of medicine bottles, even when the child-resistant caps were not fully secured on the bottles.
Clearly and consistently showing volumetric measures (e.g., mL) on liquid medication packaging, labels, and dosing devices (e.g., oral syringes, dosing cups) could minimize errors when measuring and giving doses. PROTECT partners have initiated and led a number of activities to improve labeling on medication bottles and dosing devices and have participated in related activities led by partner organizations.
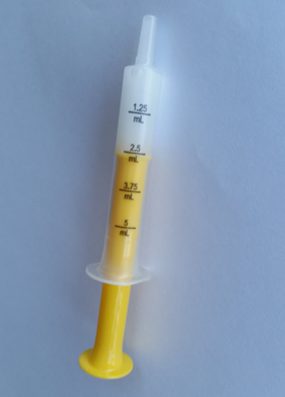
Safety improvements include:
- Development of a Voluntary Guideline for standardizing dosing measures for OTC liquid medicines with dosing for children. All members of the Consumer Healthcare Products Association (CHPA) participate in developing the association’s voluntary guidelines. The recommendations in the CHPA Guideline are consistent with recommendations in the FDA Guidance for industry on OTC dosage delivery devices, and encourage even further dosing standardization on product labels to reduce caregiver confusion. The CHPA Guideline was updated in 2014 to reflect growing support for use of a single volumetric unit (mL) when dosing liquid medications to prevent errors.
- Evaluation of guideline adherence. A CDC-led study assessed adherence to recommendations in the CHPA Guideline and FDA Guidance for national brand OTC medications intended for use by children and infants. Product-specific findings were shared with respective manufacturers so that any areas needing improvement could be addressed.
- Creation of standards for safe use. The National Council for Prescription Drug Programs (NCPDP) developed recommendations for standardizing how doses are displayed on prescription medication containers. The White Paper [PDF – 82 pages] “NCPDP Recommendations and Guidance for Standardizing the Dosing Designations on Prescription Container Labels of Oral Liquid Medications” recommends that:
- Milliliter (mL) should be the standard unit of measure used on prescription container labels for oral liquid medications
- Dose amounts should always use leading zeros before decimal amounts less than one, and should NOT use trailing zeros after a decimal on prescription container labels for oral liquid medications
- Dosing devices with numeric graduations and units that correspond to the container labeling should be made easily and universally available each time oral liquid prescription medicines are dispensed

Education about the importance of safe medication use and storage can complement improvements made in safety packaging to protect children from unsupervised ingestions. Curious young children can act quickly, and it is important to know how to store medicines safely around young children and to be prepared in case of an emergency.
Safety improvements include:
- Development of an education campaign. The Up and Away and Out of Sight campaign was developed by a broad range of PROTECT partners with support from the CHPA Educational Foundation. The Up and Away campaign is centered around several simple, data-driven actions that parents and caregivers can take to prevent medication overdoses in the children they care about and care for.
- Up and Away materials (tip sheets, brochures, posters)
- Continued dissemination of safe storage messages. Participating in poison prevention activities of partner organizations to extend the reach of messages about safe medication use and storage using a variety of media channels (print and online articles, social media, radio, video, etc.).
Up and Away and Out of Sight Campaign, CDC
Protect Kids from the Dangers in Our Medicine Cabinets, (video), Coffee with America
American Association of Poison Control Centers
Medication Safety, American Academy of Pediatrics
The Healthy Children Show: Giving Liquid Medicine Safely [Video – 2:41] American Academy of Pediatrics
Healthline on Medication Safety [Video – 5:40] Safe Kids Worldwide
Medication Safety, Safe Kids Worldwide
Safe Medicine Dosing Photo Gallery, CHPA Educational Foundation
