Background D. Water
Guidelines for Environmental Infection Control in Health-Care Facilities (2003)
- 1. Modes of Transmission of Waterborne Diseases
- 2. Waterborne Infectious Diseases in Health-Care Facilities
- Table 11. Clinical and epidemiologic characteristics of Legionellosis/Legionnaires disease
- Table 12. Pseudomonas aeruginosa infections
- Table 13. Other gram-negative bacteria associated with water and moist environments
- Table 14. Nontuberculous mycobacteria—environmental vehicles
- 3. Water Systems in Health-Care Facilities
- Table 15. Water and point-of-use fixtures as sources and reservoirs of waterborne pathogens*
- Table 16. Water demand in health-care facilities during water disruption emergencies
- Box 9. Recovery and remediation measures for water-related emergencies*
- Box 10. Contingency planning for flooding
- 4. Strategies for Controlling Waterborne Microbial Contamination
- Box 11. Steps in an epidemiologic investigation for legionellosis
- Table 17. Additional infection-control measures to prevent exposure of high-risk patients to waterborne pathogens
- 5. Cooling Towers and Evaporative Condensers
- Figure 5. Diagram of a typical air conditioning (induced draft) cooling tower*
- 6. Dialysis Water Quality and Dialysate
- Figure 6. Dialysis water treatment system*
- Table 18. Microbiologic limits for hemodialysis fluids
- Table 18a. Present standard
- Table 18b. Proposed standard**
- Table 19. Factors influencing microbial contamination in hemodialysis systems
- 7. Ice Machines and Ice
- Table 20. Microorganisms and their sources in ice and ice machines
- Box 12. General steps for cleaning and maintaining ice machines, dispensers, and storage chests*+
- 8. Hydrotherapy Tanks and Pools
- Table 21. Infections associated with use of hydrotherapy equipment
- 9. Miscellaneous Medical/Dental Equipment Connected to Main Water Systems
Moist environments and aqueous solutions in health-care settings have the potential to serve as reservoirs for waterborne microorganisms. Under favorable environmental circumstances (e.g., warm temperature and the presence of a source of nutrition), many bacterial and some protozoal microorganisms can either proliferate in active growth or remain for long periods in highly stable, environmentally resistant (yet infectious) forms. Modes of transmission for waterborne infections include
- direct contact [e.g., that required for hydrotherapy];
- ingestion of water [e.g., through consuming contaminated ice];
- indirect-contact transmission [e.g., from an improperly reprocessed medical device];6
- inhalation of aerosols dispersed from water sources;3 and
- aspiration of contaminated water.
The first three modes of transmission are commonly associated with infections caused by gram-negative bacteria and nontuberculous mycobacteria (NTM). Aerosols generated from water sources contaminated with Legionella spp. often serve as the vehicle for introducing legionellae to the respiratory tract.394
a. Legionellosis
Legionellosis is a collective term describing infection produced by Legionella spp., whereas Legionnaires disease is a multi-system illness with pneumonia.395 The clinical and epidemiologic aspects of these diseases (Table 11) are discussed extensively in another guideline.3 Although Legionnaires disease is a respiratory infection, infection-control measures intended to prevent healthcare–associated cases center on the quality of water—the principal reservoir for Legionella spp.
Modes of transmission
- Aspiration of water, direct inhalation or water aerosols. 3, 394–398, 400
Causative agent
- Legionella pneumophila (90% of infections); L. micdadei, L. bozemanii, L. dumoffii, L. longbeachii, (14 additional species can cause infection in humans). 395–399
Source of exposure
- Exposure to environmental sources of Legionella spp. (i.e., water or water aerosols). 31, 33, 401–414
Clinical syndromes and diseases
- Two distinct illnesses: 397–399, 415–422
- Pontiac fever [a milder, influenza-like illness]; and
- progressive pneumonia that may be accompanied by cardiac, renal, and gastrointestinal involvement 3
Patient populations at greatest risk
- Immunosuppressed patients (e.g., transplant patients, cancer patients, and patients receiving corticosteroid therapy);
- Immunocompromised patients (e.g., surgical patients, patients with underlying chronic lung disease, and dialysis patients);
- Elderly persons; and
- Patients who smoke. 395–397, 423–433
Occurrence
- Proportion of community-acquired pneumonia caused by Legionella spp. ranges from 1%–5%; estimated annual incidence among the general population is 8,000–18,000 cases in the United States; the incidence of healthcare-associated pneumonia (0%–14%) may be underestimated if appropriate laboratory diagnostic methods are unavailable. 396, 397, 434–444
Mortality rate
- Mortality declined markedly during 1980–1998, from 34% to 12% for all cases; the mortality rate is higher among persons with health-care associated pneumonia compared with the rate among community-acquired pneumonia patients (14% for health-care associated pneumonia versus 10% for community-acquired pneumonia [1998 data]). 395–397, 445
Legionella spp. are commonly found in various natural and man-made aquatic environments446, 447 and can enter health-care facility water systems in low or undetectable numbers.448, 449 Cooling towers, evaporative condensers, heated potable water distribution systems, and locally-produced distilled water can provide environments for multiplication of legionellae.450–454 In several hospital outbreaks, patients have been infected through exposure to contaminated aerosols generated by cooling towers, showers, faucets, respiratory therapy equipment, and room-air humidifiers.401–410, 455 Factors that enhance colonization and amplification of legionellae in man-made water environments include
- temperatures of 77°F–107.6°F [25°C–42°C],456–460
- stagnation,461
- scale and sediment, 462 and
- presence of certain free-living aquatic amoebae that can support intracellular growth of legionellae.462, 463
The bacteria multiply within single-cell protozoa in the environment and within alveolar macrophages in humans.
b. Other Gram-Negative Bacterial Infections
Other gram-negative bacteria present in potable water also can cause health-care associated infections. Clinically important, opportunistic organisms in tap water include Pseudomonas aeruginosa, Pseudomonas spp., Burkholderia cepacia, Ralstonia pickettii, Stenotrophomonas maltophilia, and Sphingomonas spp. (Tables 12 and 13). Immunocompromised patients are at greatest risk of developing infection. Medical conditions associated with these bacterial agents range from colonization of the respiratory and urinary tracts to deep, disseminated infections that can result in pneumonia and bloodstream bacteremia. Colonization by any of these organisms often precedes the development of infection. The use of tap water in medical care (e.g., in direct patient care, as a diluent for solutions, as a water source for medical instruments and equipment, and during the final stages of instrument disinfection) therefore presents a potential risk for exposure. Colonized patients also can serve as a source of contamination, particularly for moist environments of medical equipment (e.g., ventilators).
In addition to Legionella spp., Pseudomonas aeruginosa and Pseudomonas spp. are among the most clinically relevant, gram-negative, health-care associated pathogens identified from water. These and other gram-negative, non-fermentative bacteria have minimal nutritional requirements (i.e., these organisms can grow in distilled water) and can tolerate a variety of physical conditions. These attributes are critical to the success of these organisms as health-care associated pathogens. Measures to prevent the spread of these organisms and other waterborne, gram-negative bacteria include hand hygiene, glove use, barrier precautions, and eliminating potentially contaminated environmental reservoirs.464, 465
Modes of transmission
- Direct contact with water, aerosols; aspiration of water and inhalation of water aerosols; and indirect transfer from moist environmental surfaces via hands of health-care workers. 28, 502–506
Clinical syndromes and diseases
- Septicemia, pneumonia (particularly ventilator-associated), chronic respiratory infections among cystic fibrosis patients, urinary tract infections, skin and soft-tissue infections (e.g., tissue necrosis and hemorrhage), burn-wound infections, folliculitis, endocarditis, central nervous system infections (e.g., meningitis and abscess), eye infections, and bone and joint infections. 466–503
Environmental sources of pseudomonads in healthcare settings
- Potable (tap) water, distilled water, antiseptic solutions contaminated with tap water, sinks, hydrotherapy pools, whirlpools and whirlpool spas, water baths, lithotripsy therapy tanks, dialysis water, eyewash stations, flower vases, and endoscopes with residual moisture in the channels. 28, 29, 466, 468, 507–520
Environmental sources of pseudomonads in the community
- Fomites (e.g., drug injection equipment stored in contaminated water). 494, 495
Patient populations at greatest risk
- Intensive care unit (ICU) patients (including neonatal ICU), transplant patients (organ and hematopoietic stem cell), neutropenic patients, burn therapy and hydrotherapy patients, patients with malignancies, cystic fibrosis patients, patients with underlying medical conditions, and dialysis patients. 28, 466, 467, 472, 477, 493, 506–508, 511, 512, 521–526
| Bacteria | Implicated contaminated environmental vehicle |
|---|---|
| Burkholderia cepacia |
|
| Stenotrophomonas maltophlia, Sphingomonas spp. |
|
| Ralstonia pickettii |
|
| Serratia marcescens |
|
| Acinetobacter spp. |
|
| Enterobacter spp. |
|
Two additional gram-negative bacterial pathogens that can proliferate in moist environments are Acinetobacter spp. and Enterobacter spp. 571, 572 Members of both genera are responsible for healthcare–associated episodes of colonization, bloodstream infections, pneumonia, and urinary tract infections among medically compromised patients, especially those in ICUs and burn therapy units.566, 572–583
Infections caused by Acinetobacter spp. represent a significant clinical problem. Average infection rates are higher from July through October compared with rates from November through June.584 Mortality rates associated with Acinetobacterbacteremia are 17%–52%, and rates as high as 71% have been reported for pneumonia caused by infection with either Acinetobacter spp. or Pseudomonas spp.Multi-drug resistance, especially in third generation cephalosporins for Enterobacter spp., contributes to increased morbidity and mortality.569, 572
Patients and health-care workers contribute significantly to the environmental contamination of surfaces and equipment with Acinetobacter spp. and Enterobacter spp., especially in intensive care areas, because of the nature of the medical equipment (e.g., ventilators) and the moisture associated with this equipment.549, 571, 572, 585
Hand carriage and hand transfer are commonly associated with health-care– associated transmission of these organisms and for S. marcescens. 586 Enterobacter spp. are primarily spread in this manner among patients by the hands of health-care workers.567, 587 Acinetobacter spp. have been isolated from the hands of 4%–33% of health-care workers in some studies,585–590 and transfer of an epidemic strain of Acinetobacter from patients’ skin to health-care workers’ hands has been demonstrated experimentally.591 Acinetobacter infections and outbreaks have also been attributed to medical equipment and materials (e.g., ventilators, cool mist humidifiers, vaporizers, and mist tents) that may have contact with water of uncertain quality (e.g., rinsing a ventilator circuit in tap water).549– 556 Strict adherence to hand hygiene helps prevent the spread of both Acinetobacter spp. and Enterobacter spp.577, 592
Acinetobacter spp. have also been detected on dry environmental surfaces (e.g., bed rails, counters, sinks, bed cupboards, bedding, floors, telephones, and medical charts) in the vicinity of colonized or infected patients; such contamination is especially problematic for surfaces that are frequently touched.557–564 In two studies, the survival periods of Acinetobacter baumannii and Acinetobacter calcoaceticus on dry surfaces approximated that for S. aureus (e.g., 26–27 days).593, 594Because Acinetobacter spp. may come from numerous sources at any given time, laboratory investigation of health-care associated Acinetobacter infections should involve techniques to determine biotype, antibiotype, plasmid profile, and genomic fingerprinting (i.e., macrorestriction analysis) to accurately identify sources and modes of transmission of the organism(s).595
c. Infections and Pseudo-Infections Due to Nontuberculous Mycobacteria
NTM are acid-fast bacilli (AFB) commonly found in potable water. NTM include both saprophytic and opportunistic organisms. Many NTM are of low pathogenicity, and some measure of host impairment is necessary to enhance clinical disease.596 The four most common forms of human disease associated with NTM are
- pulmonary disease in adults;
- cervical lymph node disease in children;
- skin, soft tissue, and bone infections; and
- disseminated disease in immunocompromised patients.596, 597
Person-to-person acquisition of NTM infection, especially among immunocompetent persons, does not appear to occur, and close contacts of patients are not readily infected, despite the high numbers of organisms harbored by such patients.596, 598–600 NTM are spread via all modes of transmission associated with water. In addition to health-care associated outbreaks of clinical disease, NTM can colonize patients in health-care facilities through consumption of contaminated water or ice or through inhalation of aerosols.601–605 Colonization following NTM exposure, particularly of the respiratory tract, occurs when a patient’s local defense mechanisms are impaired; overt clinical disease does not develop.606 Patients may have positive sputum cultures in the absence of clinical disease.
Using tap water during patient procedures and specimen collection and in the final steps of instrument reprocessing can result in pseudo-outbreaks of NTM contamination.607– 609 NTM pseudo-outbreaks of Mycobacterium chelonae, M. gordonae,and M. xenopi have been associated with both bronchoscopy and gastrointestinal endoscopy when
- tap water is used to provide irrigation to the site or to rinse off the viewing tip in situ or
- the instruments are inappropriately reprocessed with tap water in the final steps.610– 612
Table 14a. Infections or colonizations
| Pathogen | Vehicles associated with infections or colonizations |
|---|---|
| Mycobacterium abscessus |
|
| Mycobacterium avium complex (MAC) |
|
| Mycobacterium chelonae |
|
| Mycobacterium fortuitum |
|
| Mycobacterium marinum |
|
| Mycobacterium ulcerans |
|
Table 14b. Pseudo-outbreaks
| Pathogen | Vehicles associated with pseudo-outbreaks |
|---|---|
| Mycobacterium chelonae |
|
| Mycobacterium fortuitum |
|
| Mycobacterium gordonae |
|
| Mycobacterium kansasii |
|
| Mycobacterium terrae |
|
| Mycobacterium xenopi |
|
NTM can be isolated from both natural and man-made environments. Numerous studies have identified 632 Some NTM species (e.g., Mycobacterium xenopi) can survive in water at 113°F (45°C), and can be isolated from hot water taps, which can pose a problem for hospitals that lower the temperature of their hot water systems.627 Other NTM (e.g., Mycobacterium kansasii, M. gordonae, M. fortuitum, and M. chelonae) cannot tolerate high temperatures and are associated more often with cold water lines and taps.629
NTM have a high resistance to chlorine; they can tolerate free chlorine concentrations of 0.05–0.2 mg/L (0.05–0.2 ppm) found at the tap.598, 633, 634 They are 20–100 times more resistant to chlorine compared with coliforms; slow-growing strains of NTM (e.g., Mycobacterium avium and M. kanasii) appear to be more resistant to chorine inactivation compared to fast-growing NTM.635 Slow-growing NTM species have also demonstrated some resistance to formaldehyde and glutaraldehyde, which has posed problems for reuse of hemodialyzers.31 The ability of NTM to form biofilms at fluid-surface interfaces (e.g., interior surfaces of water pipes) contributes to the organisms’ resistance to chemical inactivation and provides a microenvironment for growth and proliferation.636, 637
d. Cryptosporidiosis
Cryptosporidium parvum is a protozoan parasite that causes self-limiting gastroenteritis in normal hosts but can cause severe, life-threatening disease in immunocompromised patients. First recognized as a human pathogen in 1976, C. parvumcan be present in natural and finished waters after fecal contamination from either human or animal sources.638–641
The health risks associated with drinking potable water contaminated with minimal numbers of C. parvum oocysts are unknown.642 It remains to be determined if immunosuppressed persons are more susceptible to lower doses of oocysts than are immunocompetent persons. One study demonstrated that a median 50% infectious dose (ID50) of 132 oocysts of calf origin was sufficient to cause infection among healthy volunteers.643 In a second study, the same researchers found that oocysts obtained from infected foals (newborn horses) were infectious for human volunteers at median ID50 of 10 oocysts, indicating that different strains or species of Cryptosporidium may vary in their infectivity for humans.644 In a small study population of 17 healthy adults with pre-existing antibody to C. parvum, the ID50 was determined to be 1,880 oocysts, more than 20-fold higher than in seronegative persons.645 These data suggest that pre-existing immunity derived from previous exposures to Cryptosporidium offers some protection from infection and illness that ordinarily would result from exposure to low numbers of oocysts.645, 646
Oocysts, particularly those with thick walls, are environmentally resistant, but their survival under natural water conditions is poorly understood. Under laboratory conditions, some oocysts remain viable and infectious in cold (41°F [5°C]) for months.641 The prevalence of Cryptosporidium in the U.S. drinking water supply is notable. Two surveys of approximately 300 surface water supplies revealed that 55%–77% of the water samples contained Cryptosporidium oocysts.647, 648 Because the oocysts are highly resistant to common disinfectants (e.g., chlorine) used to treat drinking water, filtration of the water is important in reducing the risk of waterborne transmission. Coagulation-floculation and sedimentation, when used with filtration, can collectively achieve approximately a 2.5 log10 reduction in the number of oocysts.649 However, outbreaks have been associated with both filtered and unfiltered drinking water systems (e.g., the 1993 outbreak in Milwaukee, Wisconsin that affected 400,000 people).641, 650–652 The presence of oocysts in the water is not an absolute indicator that infection will occur when the water is consumed, nor does the absence of detectable oocysts guarantee that infection will not occur. Health-care associated outbreaks of cryptosporidiosis primarily have been described among groups of elderly patients and immunocompromised persons.653
a. Basic Components and Point-of-Use Fixtures
Treated municipal water enters a health-care facility via the water mains and is distributed throughout the building(s) by a network of pipes constructed of galvanized iron, copper, and polyvinylchloride (PVC). The pipe runs should be as short as is practical. Where recirculation is employed, the pipe runs should be insulated and long dead legs avoided in efforts to minimize the potential for water stagnation, which favors the proliferation of Legionella spp. and NTM. In high-risk applications (e.g., PE areas for severely immunosuppressed patients), insulated recirculation loops should be incorporated as a design minimal loss.
Each water service main, branch main, riser, and branch (to a group of fixtures) has a valve and a means to reach the valves via an access panel.120 Each fixture has a stop valve. Valves permit the isolation of a portion of the water system within a facility during repairs or maintenance. Vacuum breakers and other similar devices in the lines prevent water from back-flowing into the system. All systems that supply water should be evaluated to determine risk for potential back siphonage and cross connections.
Health-care facilities generate hot water from municipal water using a boiler system. Hot water heaters and storage vessels for such systems should have a drainage facility at the lowest point, and the heating element should be located as close as possible to the bottom of the vessel to facilitate mixing and to prevent water temperature stratification. Those hot or cold water systems that incorporate an elevated holding tank should be inspected and cleaned annually. Lids should fit securely to exclude foreign materials.
The most common point-of-use fixtures for water in patient-care areas are sinks, faucets, aerators, showers, and toilets; eye-wash stations are found primarily in laboratories. The potential for these fixtures to serve as a reservoir for pathogenic microorganisms has long been recognized (Table 15).509, 654–656
Wet surfaces and the production of aerosols facilitate the multiplication and dispersion of microbes. The level of risk associated with aerosol production from point-of-use fixtures varies. Aerosols from shower heads and aerators have been linked to a limited number of clusters of gram-negative bacterial colonizations and infections, including Legionnaires disease, especially in areas where immunocompromised patients are present (e.g., surgical ICUs, transplant units, and oncology units).412, 415, 656–659
In one report, clinical infection was not evident among immunocompetent persons (e.g., hospital staff) who used hospital showers when Legionella pneumophila was present in the water system.660 Given the infrequency of reported outbreaks associated with faucet aerators, consensus has not been reached regarding the disinfection of or removal of these devices from general use. If additional clusters of infections or colonizations occur in high-risk patient-care areas, it may be prudent to clean and decontaminate the aerators or to remove them.658, 659 ASHRAE recommends cleaning and monthly disinfection of aerators in high-risk patient-care areas as part of Legionella control measures.661 Although aerosols are produced with toilet flushing,662, 663 no epidemiologic evidence suggests that these aerosols pose a direct infection hazard.
Although not considered a standard point-of-use fixture, decorative fountains are being installed in increasing numbers in health-care facilities and other public buildings. Aerosols from a decorative fountain have been associated with transmission of Legionella pneumophila serogroup 1 infection to a small cluster of older adults.664 This hotel lobby fountain had been irregularly maintained, and water in the fountain may have been heated by submersed lighting, all of which favored the proliferation of Legionella in the system.664 Because of the potential for generations of infectious aerosols, a prudent prevention measure is to avoid locating these fixtures in or near high-risk patient-care areas and to adhere to written policies for routine fountain maintenance.120
| Reservoir | Associated pathogens | Transmission | Strength of evidence+ | Prevention and control | References |
|---|---|---|---|---|---|
| Potable water | Pseudomonas, gram-negative bacteria, NTM | Contact | Moderate: occasional well-described outbreaks. | Follow public health guidelines. | (See Tables 12–14) |
| Potable water | Legionella | Aerosol inhalation | Moderate: occasional well-described outbreaks. | Provide supplemental treatment for water. | (See Table 11) |
| Holy water | Gram-negative bacteria | Contact | Low: few well-described outbreaks | Avoid contact with severe burn injuries. Minimize use among immunocompromised patients. | 665 |
| Dialysis water | Gram-negative bacteria | Contact | Moderate: occasional well-described outbreaks. | Dialysate should be ≤2,000 cfu/mL; water should be ≤200 cfu/mL. | 2, 527, 666–668 |
| Automated endoscope reprocessors and rinse water | Gram-negative bacteria | Contact | Moderate: occasional well-described outbreaks. | Use and maintain equipment according to instructions; eliminate residual moisture by drying the channels (e.g., through alcohol rinse and forced air drying). | 669–675 |
| Water baths | Pseudomonas, Burkholderia, Acinetobacter | Contact | Moderate: occasional well-described outbreaks. | Add germicide to the water; wrap transfusion products in protective plastic wrap if using the bath to modulate the temperature of these products. | 29, 533, 676, 677 |
| Tub immersion | Pseudomonas, Enterobacter, Acinetobacter | Contact | Moderate: occasional well-described outbreaks. | Drain and disinfect tub after each use; consider adding germicide to the water; water in large hydrotherapy pools should be properly disinfected and filtered. | 678–683 |
| Ice and ice machines | NTM, Enterobacter, Pseudomonas, Cryptosporidium Legionella | Ingestion, contact | Moderate: occasional well-described outbreaks.
Low: few well-described outbreaks |
Clean periodically; use automatic dispenser (avoid open chest storage compartments in patient areas). | 601, 684–687 |
| Faucet aerators | Legionella | Aerosol inhalation | Moderate: occasional well-described outbreaks. | Clean and disinfect monthly in high-risk patient areas; consider removing if additional infections occur. | 415, 661 |
| Faucet aerators | Pseudomonas, Acinetobacter, Stenotrophomonas, Chryseobacterium | Contact, droplet | Low: few well-described outbreaks | No precautions are necessary at present in immunocompetent patient-care areas. | 658, 659, 688, 689 |
| Sinks | Pseudomonas | Contact, droplet | Moderate: occasional well-described outbreaks. | Use separate sinks for handwashing and disposal of contaminated fluids. | 509, 653, 685–693 |
| Showers | Legionella | Aerosol inhalation | Low: few well-described outbreaks | Provide sponge baths for hematopoietic stem cell transplant patients; avoid shower use for immunocompromised patients when Legionella is detected in facility water. | 656 |
| Dental unit water lines | Pseudomonas, Legionella, Sphingomonas, Acinetobacter | Contact | Low: few well-described outbreaks | Clean water systems according to system manufacturer’s instructions. | 636, 694–696 |
| Ice baths for thermodilution catheters | Ewingella, Staphylococcus | Contact | Low: few well-described outbreaks | Use sterile water. | 697, 698 |
| Decorative fountains | Legionella | Aerosol inhalation | Low: few well-described outbreaks | Perform regular maintenance, including water disinfection; avoid use in or near high-risk patient-care areas. | 664 |
| Eyewash stations | Pseudomonas, amoebae, Legionella | Contact | Low: few well-described outbreaks
Minimal: actual infections not demonstrated. |
Flush eyewash stations weekly; have sterile water available for eye flushes. | 518, 699, 700 |
| Toilets | Gram-negative bacteria | n/a | Minimal: actual infections not demonstrated. | Clean regularly; use good hand hygiene. | 662 |
| Flowers | Gram-negative bacteria, Aspergillus | n/a | Minimal: actual infections not demonstrated. | Avoid use in intensive care units and in immunocompromised patient-care settings. | 515, 701, 702 |
* Modified from reference 654 and used with permission of the publisher (Slack, Inc.)
b. Water Temperature and Pressure
Hot water temperature is usually measured at the point of use or at the point at which the water line enters equipment requiring hot water for proper operation.120 Generally, the hot water temperature in hospital patient-care areas is no greater than a temperature within the range of 105°F–120°F (40.6°C– 49°C), depending on the AIA guidance issued at the year in which the facility was built.120 Hot water temperature in patient-care areas of skilled nursing-care facilities is set within a slightly lower range of 95°F–110°F (35°C–43.3°C) depending on the AIA guidance at the time of facility construction.120 Many states have adopted a temperature setting in these ranges into their health-care regulations and building codes. ASHRAE, however, has recommended higher settings.661 Steam jets or booster heaters are usually needed to meet the hot water temperature requirements in certain service areas of the hospital (e.g., the kitchen [120°F (49°C)] or the laundry [160°F (71°C)]).120 Additionally, water lines may need to be heated to a particular temperature specified by manufacturers of specific hospital equipment. Hot-water distribution systems serving patient-care areas are generally operated under constant recirculation to provide continuous hot water at each hot-water outlet.120 If a facility is or has a hemodialysis unit, then continuously circulated, cold treated water is provided to that unit.120
To minimize the growth and persistence of gram-negative waterborne bacteria (e.g., thermophilic NTM and Legionellaspp.),627, 703–709 cold water in health-care facilities should be stored and distributed at temperatures below 68°F (20°C); hot water should be stored above 140°F (60°C) and circulated with a minimum return temperature of 124°F (51°C),661 or the highest temperature specified in state regulations and building codes. If the return temperature setting of 124°F (51°C) is permitted, then installation of preset thermostatic mixing valves near the point-of-use can help to prevent scalding. Valve maintenance is especially important in preventing valve failure, which can result in scalding. New shower systems in large buildings, hospitals, and nursing homes should be designed to permit mixing of hot and cold water near the shower head. The warm water section of pipe between the control valve and shower head should be self-draining. Where buildings can not be retrofitted, other approaches to minimize the growth of Legionella spp. include
- periodically increasing the temperature to at least 150°F [66°C] at the point of use [i.e., faucets] and
- adding additional chlorine and flushing the water. 661, 710, 711
Systems should be inspected annually to ensure that thermostats are functioning properly.
Adequate water pressure ensures sufficient water supplies for
- direct patient care;
- operation of water-cooled instruments and equipment [e.g., lasers, computer systems, telecommunications systems, and automated endoscope reprocessors712 ];
- proper function of vacuum suctioning systems;
- indoor climate control; and
- fire-protection systems.
Maintaining adequate pressure also helps to ensure the integrity of the piping system.
c. Infection-Control Impact of Water System Maintenance and Repair
Corrective measures for water-system failures have not been studied in well-designed experiments; these measures are instead based on empiric engineering and infection-control principles. Health-care facilities can occasionally sustain both intentional cut-offs by the municipal water authority to permit new construction project tie-ins and unintentional disruptions in service when a water main breaks as a result of aging infrastructure or a construction accident. Vacuum breakers or other similar devices can prevent backflow of water in the facility’s distribution system during water-disruption emergencies.11 To be prepared for such an emergency, all health-care facilities need contingency plans that identify
- the total demand for potable water,
- the quantity of replacement water [e.g., bottled water] required for a minimum of 24 hours when the water system is down,
- mechanisms for emergency water distribution, and
- procedures for correcting drops in water pressure that affect operation of essential devices and equipment that are driven or cooled by a water system [Table 16].713
| Potable water use | Bottled, sterile water use |
|---|---|
|
|
Detailed, up-to-date plans for hot and cold water piping systems should be readily available for maintenance and repair purposes in case of system problems. Opening potable water systems for repair or construction and subjecting systems to water-pressure changes can result in water discoloration and dramatic increases in the concentrations of Legionella spp. and other gram-negative bacteria. The maintenance of a chlorine residual at all points within the piping system also offers some protection from entry of contamination to the pipes in the event of inadvertent cross-connection between potable and non-potable water lines. As a minimum preventive measure, ASHRAE recommends a thorough flushing of the system.661High-temperature flushing or hyperchlorination may also be appropriate strategies to decrease potentially high concentrations of waterborne organisms. The decision to pursue either of these remediation strategies, however, should be made on a case-by-case basis. If only a portion of the system is involved, high temperature flushing or chlorination can be used on only that portion of the system.661
When shock decontamination of hot water systems is necessary (e.g., after disruption caused by construction and after cross-connections), the hot water temperature should be raised to 160°F–170°F (71°C–77°C) and maintained at that level while each outlet around the system is progressively flushed. A minimum flush time of 5 minutes has been recommended;3the optimal flush time is not known, however, and longer flush times may be necessary.714 The number of outlets that can be flushed simultaneously depends on the capacity of the water heater and the flow capability of the system. Appropriate safety procedures to prevent scalding are essential. When possible, flushing should be performed when the fewest building occupants are present (e.g., during nights and weekends).
When thermal shock treatment is not possible, shock chlorination may serve as an alternative method.661 Experience with this method of decontamination is limited, however, and high levels of free chlorine can corrode metals. Chlorine should be added, preferably overnight, to achieve a free chlorine residual of at least 2 mg/L (2 ppm) throughout the system.661 This may require chlorination of the water heater or tank to levels of 20–50 mg/L (20–50 ppm). The pH of the water should be maintained at 7.0–8.0.661 After completion of the decontamination, recolonization of the hot water system is likely to occur unless proper temperatures are maintained or a procedure such as continuous supplemental chlorination is continued.
Interruptions of the water supply and sewage spills are situations that require immediate recovery and remediation measures to ensure the health and safety of patients and staff.715 When delivery of potable water through the municipal distribution system has been disrupted, the public water supplier must issue a “boil water” advisory if microbial contamination presents an immediate public health risk to customers. The hospital engineer should oversee the restoration of the water system in the facility and clear it for use when appropriate. Hospitals must maintain a high level of surveillance for waterborne disease among patients and staff after the advisory is lifted.642
Flooding from either external (e.g., from a hurricane) or internal sources (e.g., a water system break) usually results in property damage and a temporary loss of water and sanitation.716–718 JCAHO requires all hospitals to have plans that address facility response for recovery from both internal and external disasters.713, 719 The plans are required to discuss
- general emergency preparedness,
- staffing,
- regional planning among area hospitals,
- emergency supply of potable water,
- infection control and medical services needs,
- climate control, and
- remediation.
The basic principles of structural recovery from flooding are similar to those for recovery from sewage contamination (Box 9 and 10). Following a major event (e.g., flooding), facilities may elect to conduct microbial sampling of water after the system is restored to verify that water quality has been returned to safe levels (<500 CFU/mL, heterotrophic plate count). This approach may help identify point-of-use fixtures that may harbor contamination as a result of design or engineering features.720 Medical records should be allowed to dry and then either photocopied or placed in plastic covers before returning them to the record.
Moisture meters can be used to assess water-damaged structural materials. If porous structural materials for walls have a moisture content of >20% after 72 hours, the affected material should be removed.266, 278, 313 The management of water-damaged structural materials is not strictly limited to major water catastrophes (e.g., flooding and sewage intrusions); the same principles are used to evaluate the damage from leaking roofs, point-of-use fixtures, and equipment. Additional sources of moisture include condensate on walls from boilers and poorly engineered humidification in HVAC systems.
Potable water disruptions
- Contingency plan items
- Ensure access to plumbing network so that repairs can be easily made.
- Provide sufficient potable water, either from bottled sources or truck delivery.
- Post advisory notices against consuming tap water, ice, or beverages made with water.
- Rope off or bag drinking fountains to designate these as being “out of service” until further notice.
- Rinse raw foods as needed in disinfected water.
- Disconnect ice machines whenever possible. (Ice machines should always be disconnected from the water source in advance of planned water disruptions.)
- Postpone laundry services until after the water system is restored.
- Water treatment
- Heat water to a rolling boil for ≥1 minute.
- Remediation of the water system after the “boil water” advisory is rescinded
- Flush fixtures (e.g., faucets and drinking fountains) and equipment for several minutes and restart.
- Run water softeners through a regeneration cycle.
- Drain, disinfect, and refill water storage tanks, if needed.
- Change pretreatment filters and disinfect the dialysis water system.
Sewage spills/malfunction
- Overall strategy
- Move patients and clean/sterile supplies out of the area.
- Redirect traffic away from the area.
- Close the doors or use plastic sheeting to isolate the area prior to clean-up.
- Restore sewage system function first, then the potable water system (if both are malfunctioning).
- Remove sewage solids, drain the area, and let dry.
- Remediation of the structure
- Hard surfaces: clean with detergent/disinfectant after the area has been drained.
- Carpeting, loose tiles, buckled flooring: remove and allow the support surface to dry; replace the items; wet down carpeting with a low-level disinfectant or sanitizer prior to removal to minimize dust dispersion to the air.
- Wallboard and other porous structural materials: remove and replace if they cannot be cleaned and dried within 72 hours. (Moisture meter readings should be <20% moisture content.)
- Furniture
- Hard surface furniture (e.g., metal or plastic furniture): clean and allow to dry.
- Wood furniture: let dry, sand the wood surface, and reapply varnish.
- Cloth furniture: replace.
- Electrical equipment
- Replace if the item cannot be easily dismantled, cleaned, and reassembled.
* Material in this box is compiled from references 266, 278, 315, 713, 716–719, 721–729.
An exception to these recommendations is made for hemodialysis units where water is further treated either by portable water treatment or large-scale water treatment systems usually involving reverse osmosis (RO). In the United States, >97% of dialysis facilities use RO treatment for their water.721 However, changing pre-treatment filters and disinfecting the system to prevent colonization of the RO membrane and microbial contamination down-stream of the pre-treatment filter are prudent measures.
General emergency preparedness
- Ensure that emergency electrical generators are not located in flood-prone areas of the facility.
- Develop alternative strategies for moving patients, water containers, medical records, equipment, and supplies in the event that the elevators are inoperable.
- Establish in advance a centralized base of operations with batteries, flashlights, and cellular phones.
- Ensure sufficient supplies of sandbags to place at the entrances and the area around boilers, incinerators, and generators.
- Establish alternative strategies for bringing core employees to the facility if high water prevents travel.
Staffing Patterns
- Temporarily reassign licensed staff as needed to critical care areas to provide manual ventilation and to perform vital assessments on patients.
- Designate a core group of employees to remain on site to keep all services operational if the facility remains open.
- Train all employees in emergency preparedness procedures.
Regional planning among are facilities for disaster management
- Incorporate community support and involvement (e.g., media alerts, news, and transportation).
- Develop in advance strategies for transferring patients, as needed.
- Develop strategies for sharing supplies and providing essential services among participating facilities (e.g., central sterile department services, and laundry services).
- Identify sources for emergency provisions (e.g., blood, emergency vehicles, and bottled water).
Medical services and infection control
- Use alcohol-based hand rubs in general patient-care areas.
- Postpone elective surgeries until full services are restored, or transfer these patients to other facilities.
- Consider using portable dialysis machines. (Portable dialysis machines require less water compared to the larger units situated in dialysis settings.)
- Provide an adequate supply of tetanus and hepatitis A immunizations for patients and staff.
Climate control
- Provide adequate water for cooling towers. (Water for cooling towers may need to be trucked in, especially if the tower uses a potable water source.)
* Material in this box was compiled from references 713, 716–719.
a. Supplemental Treatment of Water with Heat and/or Chemicals
In addition to using supplemental treatment methods as remediation measures after inadvertent contamination of water systems, health-care facilities sometimes use special measures to control waterborne microorganisms on a sustained basis. This decision is most often associated with outbreaks of legionellosis and subsequent efforts to control legionellae,722although some facilities have tried supplemental measures to better control thermophilic NTM.627
The primary disinfectant for both cold and hot water systems is chlorine. However, chlorine residuals are expected to be low, and possibly nonexistent, in hot water tanks because of extended retention time in the tank and elevated water temperature. Flushing, especially that which removes sludge from the bottom of the tank, probably provides the most effective treatment of water systems. Unlike the situation for disinfecting cooling towers, no equivalent recommendations have been made for potable water systems, although specific intervention strategies have been published.403, 723 The principal approaches to disinfection of potable systems are heat flushing using temperatures 160°F–170°F (71°– 77°C), hyperchlorination, and physical cleaning of hot-water tanks.3, 403, 661 Potable systems are easily recolonized and may require continuous intervention (e.g., raising of hot water temperatures or continuous chlorination).403, 711 Chlorine solutions lose potency over time, thereby rendering the stocking of large quantities of chlorine impractical.
Some hospitals with hot water systems identified as the source of Legionella spp. have performed emergency decontamination of their systems by pulse (i.e., one-time) thermal disinfection/superheating or hyperchlorination.711, 714, 724, 725 After either of these procedures, hospitals either maintain their heated water with a minimum return temperature of 124°F (51°C) and cold water at <68°F (<20°C) or chlorinate their hot water to achieve 1–2 mg/L (1–2 ppm) of free residual chlorine at the tap.26, 437, 709–711, 726, 727
Additional measures (e.g., physical cleaning or replacement of hot-water storage tanks, water heaters, faucets, and shower heads) may be required to help eliminate accumulations of scale and sediment that protect organisms from the biocidal effects of heat and chlorine.457, 711 Alternative methods for controlling and eradicating legionellae in water systems (e.g., treating water with chlorine dioxide, heavy metal ions [i.e., copper/silver ions], ozone, and UV light) have limited the growth of legionellae under laboratory and operating conditions.728–742 Further studies on the long-term efficacy of these treatments are needed before these methods can be considered standard applications.
Renewed interest in the use of chloramines stems from concerns about adverse health effects associated with disinfectants and disinfection by-products.743 Monochloramine usage minimizes the formation of disinfection by-products, including trihalomethanes and haloacetic acids. Monochloramine can also reach distal points in a water system and can penetrate into bacterial biofilms more effectively than free chlorine.744 However, monochloramine use is limited to municipal water treatment plants and is currently not available to health-care facilities as a supplemental water-treatment approach. A recent study indicated that 90% of Legionnaires disease outbreaks associated with drinking water could have been prevented if monochloramine rather than free chlorine has been used for residual disinfection.745 In a retrospective comparison of health-care associated Legionnaires disease incidence in central Texas hospitals, the same research group documented an absence of cases in facilities located in communities with monochloramine-treated municipal water.746Additional data are needed regarding the effectiveness of using monochloramine before its routine use as a disinfectant in water systems can be recommended. No data have been published regarding the effectiveness of monochloramine installed at the level of the health-care facility.
Additional filtration of potable water systems is not routinely necessary. Filters are used in water lines in dialysis units, however, and may be inserted into the lines for specific equipment (e.g., endoscope washers and disinfectors) for the purpose of providing bacteria-free water for instrument reprocessing. Additionally, an RO unit is usually added to the distribution system leading to PE areas.
b. Primary Prevention of Legionnaires Disease (No Cases Identified)
The primary and secondary environmental infection-control strategies described in this section on the guideline pertain to health-care facilities without transplant units. Infection-control measures specific to PE or transplant units (i.e., patient-care areas housing patients at the highest risk for morbidity and mortality from Legionella spp. infection) are described in the subsection titled Preventing Legionnaires Disease in Protective Environments.
Health-care facilities use at least two general strategies to prevent health-care associated legionellosis when no cases or only sporadic cases have been detected. The first is an environmental surveillance approach involving periodic culturing of water samples from the hospital’s potable water system to monitor for Legionella spp. 747–750 If any sample is culture-positive, diagnostic testing is recommended for all patients with health-care associated pneumonia.748, 749 In-house testing is recommended for facilities with transplant programs as part of a comprehensive treatment/management program. If ≥30% of the samples are culture-positive for Legionella spp., decontamination of the facility’s potable water system is warranted.748 The premise for this approach is that no cases of health-care associated legionellosis can occur if Legionellaspp. are not present in the potable water system, and, conversely, cases of health-care associated legionellosis could potentially occur if Legionella spp. are cultured from the water.26, 751 Physicians who are informed that the hospital’s potable water system is culture-positive for Legionella spp. are more likely to order diagnostic tests for legionellosis.
A potential advantage of the environmental surveillance approach is that periodic culturing of water is less costly than routine laboratory diagnostic testing for all patients who have health-care associated pneumonia. The primary argument against this approach is that, in the absence of cases, the relationship between water-culture results and legionellosis risk remains undefined.3 Legionnella spp. can be present in the water systems of buildings752 without being associated with known cases of disease.437, 707, 753 In a study of 84 hospitals in Québec, 68% of the water systems were found to be colonized with Legionella spp., and 26% were colonized at >30% of sites sampled; cases of Legionnaires disease, however, were infrequently reported from these hospitals.707
Other factors also argue against environmental surveillance. Interpretation of results from periodic water culturing might be confounded by differing results among the sites sampled in a single water system and by fluctuations in the concentration of Legionella spp. at the same site.709, 754 In addition, the risk for illness after exposure to a given source might be influenced by several factors other than the presence or concentration of organisms, including
- the degree to which contaminated water is aerosolized into respirable droplets,
- the proximity of the infectious aerosol to the potential host,
- the susceptibility of the host, and
- the virulence properties of the contaminating strain.755–757
Thus, data are insufficient to assign a level of disease risk even on the basis of the number of colony-forming units detected in samples from areas for immunocompetent patients. Conducting environmental surveillance would obligate hospital administrators to initiate water-decontamination programs if Legionella spp. are identified. Therefore, periodic monitoring of water from the hospital’s potable water system and from aerosol-producing devices is not widely recommended in facilities that have not experienced cases of health-care associated legionellosis.661, 758
The second strategy to prevent and control health-care associated legionellosis is a clinical approach, in which providers maintain a high index of suspicion for legionellosis and order appropriate diagnostic tests (i.e., culture, urine antigen, and direct fluorescent antibody [DFA] serology) for patients with health-care associated pneumonia who are at high risk for legionellosis and its complications.437, 759, 760 The testing of autopsy specimens can be included in this strategy should a death resulting from healthcare–associated pneumonia occur. Identification of one case of definite or two cases of possible healthcare–associated Legionnaires disease should prompt an epidemiologic investigation for a hospital source of Legionella spp., which may involve culturing the facility’s water for Legionella. Routine maintenance of cooling towers, and use of sterile water for the filling and terminal rinsing of nebulization devices and ventilation equipment can help to minimize potential sources of contamination. Circulating potable water temperatures should match those outlined in the subsection titled Water Temperature and Pressure, as permitted by state code.
c. Secondary Prevention of Legionnaires Disease (With Identified Cases)
The indications for a full-scale environmental investigation to search for and subsequently decontaminate identified sources of Legionella spp. in health-care facilities without transplant units have not been clarified; these indications would likely differ depending on the facility. Case categories for health-care associated Legionnaires disease in facilities without transplant units include definite cases (i.e., laboratory-confirmed cases of legionellosis that occur in patients who have been hospitalized continuously for ≥10 days before the onset of illness) and possible cases (i.e., laboratory-confirmed infections that occur 2–9 days after hospital admission).3 In settings in which as few as one to three health-care associated cases are recognized over several months, intensified surveillance for Legionnaires disease has frequently identified numerous additional cases.405, 408, 432, 453, 739, 759, 760 This finding suggests the need for a low threshold for initiating an investigation after laboratory confirmation of cases of health-care associated legionellosis. When developing a strategy for responding to such a finding, however, infection-control personnel should consider the level of risk for health-care– associated acquisition of, and mortality from, Legionella spp. infection at their particular facility.
An epidemiologic investigation conducted to determine the source of Legionella spp. involves several important steps (Box 11). Laboratory assessment is crucial in supporting epidemiologic evidence of a link between human illness and a specific environmental source.761 Strain determination from subtype analysis is most frequently used in these investigations.410, 762–764 Once the environmental source is established and confirmed with laboratory support, supplemental water treatment strategies can be initiated as appropriate.
- Review medical and microbiologic records.
- Initiate active surveillance to identify all recent or ongoing cases.
- Develop a line listing of cases by time, place, and person.
- Determine the type of epidemiologic investigation needed for assessing risk factors:
- Case-control study,
- Cohort study.
- Gather and analyze epidemiologic information:
- Evaluate risk factors associated with potential environmental exposures (e.g., showers, cooling towers, and respiratory-therapy equipment).
- Collect water samples:
- Sample environmental sources implicated by epidemiologic investigation,
- Sample other potential source of water aerosols.
- Subtype strains of Legionella spp. cultured from both patients and environmental sources.
- Review autopsy records and include autopsy specimens in diagnostic testing.
The decision to search for hospital environmental sources of Legionella spp. and the choice of procedures to eradicate such contamination are based on several considerations, as follows:
- the hospital’s patient population
- the cost of an environmental investigation and institution of control measures to eradicate Legionella spp. from the water supply;765–768 and
- the differential risk, based on host factors, for acquiring health-care associated legionellosis and developing severe and fatal infection.
d. Preventing Legionnaires Disease in Protective Environments
This subsection outlines infection-control measures applicable to those health-care facilities providing care to severely immunocompromised patients. Indigenous microorganisms in the tap water of these facilities may pose problems for such patients. These measures are designed to prevent the generation of potentially infectious aerosols from water and the subsequent exposure of PE patients or other immunocompromised patients (e.g., transplant patients) (Table 17). Infection-control measures that address the use of water with medical equipment (e.g., ventilators, nebulizers, and equipment humidifiers) are described in other guidelines and publications.3, 455
If one case of laboratory-confirmed, health-care associated Legionnaires disease is identified in a patient in a solid-organ transplant program or in PE (i.e., an inpatient in PE for all or part of the 2–10 days prior to onset of illness) or if two or more laboratory-confirmed cases occur among patients who had visited an outpatient PE setting, the hospital should report the cases to the local and state health departments. The hospital should then initiate a thorough epidemiologic and environmental investigation to determine the likely environmental sources of Legionella spp.9 The source of Legionellashould be decontaminated or removed. Isolated cases may be difficult to investigate. Because transplant recipients are at substantially higher risk for disease and death from legionellosis compared with other hospitalized patients, periodic culturing for Legionella spp. in water samples from the potable water in the solid-organ transplant and/or PE unit can be performed as part of an overall strategy to prevent Legionnaires disease in PE units.9, 431, 710, 769 The optimal methodology (i.e., frequency and number of sites) for environmental surveillance cultures in PE units has not been determined, and the cost-effectiveness of this strategy has not been evaluated. Because transplant recipients are at high risk for Legionnaires disease and because no data are available to determine a safe concentration of legionellae organisms in potable water, the goal of environmental surveillance for Legionella spp. should be to maintain water systems with no detectable organisms.9, 431 Culturing for legionellae may be used to assess the effectiveness of water treatment or decontamination methods, a practice that provides benefits to both patients and health-care workers.767, 770
- Restrict patients from taking showers if the water is contaminated with Legionella spp. 407, 412, 654, 655, 658
- Use water that is not contaminated with Legionella spp. for patients’ sponge baths. 9
- Provide sterile water for drinking, tooth brushing, or for flushing nasogastric tubes. 9, 412
- Perform supplemental treatment of the water for the unit. 732
- Consider periodic monitoring (i.e., culturing) of the unit water supply for Legionella spp. 9, 431
- Remove shower heads and faucet aerators monthly for cleaning. (These measures can be considered in settings where legionellosis cases have occurred. These measures are not generally recommended in routine patient-care setting.) 661
- Use a 500–600 ppm (1:100 v/v dilution) solution of sodium hypochlorite to disinfect shower heads and faucet aerators. (These measures can be considered in settings where legionellosis cases have occurred. These measures are not generally recommended in routine patient-care setting.) 661
- Do not use large-volume room air humidifiers that create aerosols unless these are subjected to cleaning and high-level disinfection daily and filled with distilled water. 3
- Eliminate water-containing bath toys. (These items have been associated with outbreaks of Pseudomonas.) 30
Protecting patient-care devices and instruments from inadvertent tap water contamination during room cleaning procedures is also important in any immunocompromised patient-care area. In a recent outbreak of gram-negative bacteremias among open-heart-surgery patients, pressure-monitoring equipment that was assembled and left uncovered overnight prior to the next day’s surgeries was inadvertently contaminated with mists and splashing water from a hose-disinfectant system used for cleaning.771
Modern health-care facilities maintain indoor climate control during warm weather by use of cooling towers (large facilities) or evaporative condensers (smaller buildings). A cooling tower is a wet-type, evaporative heat transfer device used to discharge to the atmosphere waste heat from a building’s air conditioning condensers (Figure 5).772, 773 Warm water from air-conditioning condensers is piped to the cooling tower where it is sprayed downward into a counter- or cross-current air flow. To accelerate heat transfer to the air, the water passes over the fill, which either breaks water into droplets or causes it to spread into a thin film.772, 773 Most systems use fans to move air through the tower, although some large industrial cooling towers rely on natural draft circulation of air. The cooled water from the tower is piped back to the condenser, where it again picks up heat generated during the process of chilling the system’s refrigerant. The water is cycled back to the cooling tower to be cooled. Closed-circuit cooling towers and evaporative condensers are also evaporative heat-transfer devices. In these systems, the process fluid (e.g., water, ethylene glycol/water mixture, oil, or a condensing refrigerant) does not directly contact the cooling air, but is contained inside a coil assembly.661
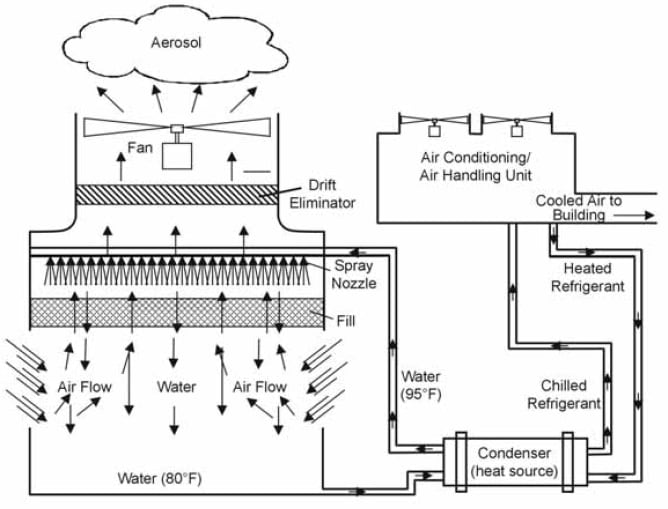
* This figure is reprinted with permission of the publisher of reference 773 (Plenum Medical).
Water temperatures are approximate and may differ substantially according to system use and design. Warm water from the condenser (or chiller) is sprayed downward into a counter- or cross-current air flow. Water passes over the fill (a component of the system designed to increase the surface area of the water exposed to air), and heat from the water is transferred to the air. Some of the water becomes aerosolized during this process, although the volume of aerosol discharged to the air can be reduced by the placement of a drift eliminator. Water cooled in the tower returns to the heat source to cool refrigerant from the air conditioning unit.
Cooling towers and evaporative condensers incorporate inertial stripping devices called drift eliminators to remove water droplets generated within the unit. Although the effectiveness of these eliminators varies substantially depending on design and condition, some water droplets in the size range of <5 μm will likely leave the unit, and some larger droplets leaving the unit may be reduced to ≤5 μm by evaporation. Thus, even with proper operation, a cooling tower or evaporative condenser can generate and expel respirable water aerosols. If either the water in the unit’s basin or the make-up water (added to replace water lost to evaporation) contains Legionella spp. or other waterborne microorganisms, these organisms can be aerosolized and dispersed from the unit.774 Clusters of both Legionnaires disease and Pontiac fever have been traced to exposure to infectious water aerosols originating from cooling towers and evaporative condensers contaminated with Legionella spp. Although most of these outbreaks have been community-acquired episodes of pneumonia,775–782 health-care associated Legionnaires disease has been linked to cooling tower aerosol exposure.404, 405 Contaminated aerosols from cooling towers on hospital premises gained entry to the buildings either through open windows or via air handling system intakes located near the tower equipment.
Cooling towers and evaporative condensers provide ideal ecological niches for Legionella spp. The typical temperature of the water in cooling towers ranges from 85°F–95°F (29°C–35°C), although temperatures can be above 120°F (49°C) and below 70°F (21°C) depending on system heat load, ambient temperature, and operating strategy.661 An Australian study of cooling towers found that legionellae colonized or multiplied in towers with basin temperatures above 60.8°F (16°C), and multiplication became explosive at temperatures above 73.4°F (23°C).783 Water temperature in closed-circuit cooling towers and evaporative condensers is similar to that in cooling towers. Considerable variation in the piping arrangement occurs. In addition, stagnant areas or dead legs may be difficult to clean or penetrate with biocides.
Several documents address the routine maintenance of cooling towers, evaporative condensers, and whirlpool spas.661, 784–787 They suggest following manufacturer’s recommendations for cleaning and biocide treatment of these devices; all health-care facilities should ensure proper maintenance for their cooling towers and evaporative condensers, even in the absence of Legionella spp (Appendix C). Because cooling towers and evaporative condensers can be shut down during periods when air conditioning is not needed, this maintenance cleaning and treatment should be performed before starting up the system for the first time in the warm season.782 Emergency decontamination protocols describing cleaning procedures and hyperchlorination for cooling towers have been developed for towers implicated in the transmission of legionellosis.786, 787
a. Rationale for Water Treatment in Hemodialysis
Hemodialysis, hemofiltration, and hemodiafiltration require special water-treatment processes to prevent adverse patient outcomes of dialysis therapy resulting from improper formulation of dialysate with water containing high levels of certain chemical or biological contaminants. The Association for the Advancement of Medical Instrumentation (AAMI) has established chemical and microbiologic standards for the water used to prepare dialysate, substitution fluid, or to reprocess hemodialyzers for renal replacement therapy.788–792 The AAMI standards address:
- equipment and processes used to purify water for the preparation of concentrates and dialysate and the reprocessing of dialyzers for multiple use and
- the devices used to store and distribute this water. Future revisions to these standards may include hemofiltration and hemodiafiltration.
Water treatment systems used in hemodialysis employ several physical and/or chemical processes either singly or in combination (Figure 6). These systems may be portable units or large systems that feed several rooms. In the United States, >97% of maintenance hemodialysis facilities use RO alone or in combination with deionization.793 Many acute-care facilities use portable hemodialysis machines with attached portable water treatment systems that use either deionization or RO. These machines were exempted from earlier versions of AAMI recommendations, but given current knowledge about toxic exposures to and inflammatory processes in patients new to dialysis, these machines should now come into compliance with current AAMI recommendations for hemodialysis water and dialysate quality.788, 789 Previous recommendations were based on the assumption that acute-care patients did not experience the same degree of adverse effects from short-term, cumulative exposures to either chemicals or microbiologic agents present in hemodialysis fluids compared with the effects encountered by patients during chronic, maintenance dialysis.788, 789 Additionally, JCAHO is reviewing inpatient practices and record-keeping for dialysis (acute and maintenance) for adherence to AAMI standards and recommended practices.
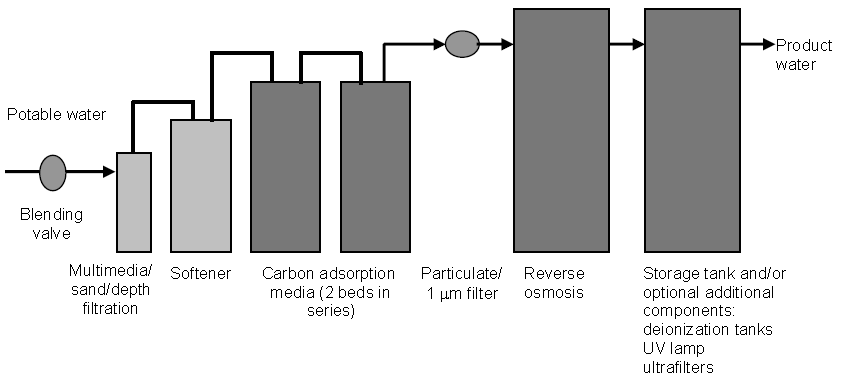
* See text for description of the placement and function of these components.
Neither the water used to prepare dialysate nor the dialysate itself needs to be sterile, but tap water can not be used without additional treatment. Infections caused by rapid-growing NTM (e.g., Mycobacterium chelonae and M. abscessus) present a potential risk to hemodialysis patients (especially those in hemodialyzer reuse programs) if disinfection procedures to inactivate mycobacteria in the water (low-level disinfection) and the hemodialyzers (high-level disinfection) are inadequate.31, 32, 633 Other factors associated with microbial contamination in dialysis systems could involve the water treatment system, the water and dialysate distribution systems, and the type of hemodialyzer.666, 667, 794–799 Understanding the various factors and their influence on contamination levels is the key to preventing high levels of microbial contamination in dialysis therapy.
In several studies, pyrogenic reactions were demonstrated to have been caused by lipopolysaccharide or endotoxin associated with gram-negative bacteria.794, 800–803 Early studies demonstrated that parenteral exposure to endotoxin at a concentration of 1 ng/kg body weight/hour was the threshold dose for producing pyrogenic reactions in humans, and that the relative potencies of endotoxin differ by bacterial species.804, 805 Gram-negative water bacteria (e.g., Pseudomonas spp.) have been shown to multiply rapidly in a variety of hospital-associated fluids that can be used as supply water for hemodialysis (e.g., distilled water, deionized water, RO water, and softened water) and in dialysate (a balanced salt solution made with this water).806 Several studies have demonstrated that the attack rates of pyrogenic reactions are directly associated with the number of bacteria in dialysate.666, 667, 807 These studies provided the rationale for setting the heterotrophic bacteria standards in the first AAMI hemodialysis guideline at ≤2,000 CFU/mL in dialysate and one log lower (≤200 CFU/mL) for the water used to prepare dialysate.668, 788 If the level of bacterial contamination exceeded 200 CFU/mL in water, this level could be amplified in the system and effectively constitute a high inoculum for dialysate at the start of a dialysis treatment.807, 808 Pyrogenic reactions did not appear to occur when the level of contamination was below 2,000 CFU/mL in dialysate unless the source of the endotoxin was exogenous to the dialysis system (i.e., present in the community water supply). Endotoxins in a community water supply have been linked to the development of pyrogenic reactions among dialysis patients.794
Whether endotoxin actually crosses the dialyzer membrane is controversial. Several investigators have shown that bacteria growing in dialysate-generated products that could cross the dialyzer membrane.809, 810 Gram-negative bacteria growing in dialysate have produced endotoxins that in turn stimulated the production of anti-endotoxin antibodies in hemodialysis patients;801, 811 these data suggest that bacterial endotoxins, although large molecules, cross dialyzer membranes either intact or as fragments. The use of the very permeable membranes known as high-flux membranes (which allow large molecules [e.g., β2 microglobulin] to traverse the membrane) increases the potential for passage of endotoxins into the blood path. Several studies support this contention. In one such study, an increase in plasma endotoxin concentrations during dialysis was observed when patients were dialyzed against dialysate containing 103 –104 CFU/mL Pseudomonasspp.812 In vitro studies using both radiolabeled lipopolysaccharide and biologic assays have demonstrated that biologically active substances derived from bacteria found in dialysate can cross a variety of dialyzer membranes.802, 813–816 Patients treated with high-flux membranes have had higher levels of anti-endotoxin antibodies than subjects or patients treated with conventional membranes.817 Finally, since 1989, 19%–22% of dialysis centers have reported pyrogenic reactions in the absence of septicemia.818, 819
Investigations of adverse outcomes among patients using reprocessed dialyzers have demonstrated a greater risk for developing pyrogenic reactions when the water used to reprocess these devices contained >6 ng/mL endotoxin and >104CFU/mL bacteria.820 In addition to the variability in endotoxin assays, host factors also are involved in determining whether a patient will mount a response to endotoxin.803 Outbreak investigations of pyrogenic reactions and bacteremias associated with hemodialyzer reuse have demonstrated that pyrogenic reactions are prevented once the endotoxin level in the water used to reprocess the dialyzers is returned to below the AAMI standard level.821
Reuse of dialyzers and use of bicarbonate dialysate, high-flux dialyzer membranes, or high-flux dialysis may increase the potential for pyrogenic reactions if the water in the dialysis setting does not meet standards.796–798 Although investigators have been unable to demonstrate endotoxin transfer across dialyzer membranes,803, 822, 823 the preponderance of reports now supports the ability of endotoxin to transfer across at least some high-flux membranes under some operating conditions. In addition to the acute risk of pyrogenic reactions, indirect evidence in increasingly demonstrating that chronic exposure to low amounts of endotoxin may play a role in some of the long-term complications of hemodialysis therapy. Patients treated with ultrafiltered dialysate for 5–6 months have demonstrated a decrease in serum β2 microglobulin concentrations and a decrease in markers of an inflammatory response.824–826 In studies of longer duration, use of microbiologically ultrapure dialysate has been associated with a decreased incidence of β2 microglobulin-associated amyloidosis.827, 828
Although patient benefit likely is associated with the use of ultrapure dialysate, no consensus has been reached regarding the potential adoption of this as standard in the United States. Debate continues regarding the bacterial and endotoxin limits for dialysate. As advances in water treatment and hemodialysis processes occur, efforts are underway to move improved technology from the manufacturer out into the user community. Cost-benefit studies, however, have not been done, and substantially increased costs to implement newer water treatment modalities are anticipated.
To reconcile AAMI documents with current International Organization for Standardization (ISO) format, AAMI has determined that its hemodialysis standards will be discussed in the following four installments: RD 5 for hemodialysis equipment, RD 62 for product water quality, RD 47 for dialyzer reprocessing, and RD 52 for dialysate quality. The Renal Diseases and Dialysis Committee of AAMI is expected to finalize and promulgated the dialysate standard pertinent to the user community (RD 52), adopting by reference the bacterial and endotoxin limits in product water as currently outlined in the AAMI standard that applies to systems manufacturers (RD 62). At present, the user community should continue to observe water quality and dialysate standards as outlined in AAMI RD 5 (Hemodialysis Systems, 1992) and AAMI RD 47 (Reuse of Hemodialyzers, 1993) until the new RD 52 standard becomes available (Table 18).789, 791
| Hemodialysis fluid | Maximum total heterotrophs (CFU/mL)
(colony forming units per milliliter) |
Maximum endotoxin level (EU/mL)
(endotoxin units per milliliter) |
|---|---|---|
| Product water – Used to prepare dialysate | 200 | No standard |
| Product water – Used to reprocess dialyzers | 200 | 5 |
| Dialysate | 2,000 | No standard |
| Hemodialysis fluid | Maximum total heterotrophs (CFU/mL)
(colony forming units per milliliter) |
Maximum endotoxin level (EU/mL)
(endotoxin units per milliliter) |
|---|---|---|
| Product water | 200 | 2 |
| Dialysate | 200 | 2 |
** Dialysate for hemodialysis, RD 52, under development, American National Standards Institute, Association for the Advancement of Medical Instrumentation (AAMI).
* The material in this table was compiled from references 789 and 791 (ANSI/AAMI standards RD 5-1992 and ANSI/AAMI RD 47-1993).
The current AAMI standard directed at systems manufacturers (RD 62 [Water Treatment Equipment for Hemodialysis Applications, 2001]) now specifies that all product water used to prepare dialysate or to reprocess dialyzers for multiple use should contain <2 endotoxin units per milliliter (EU/mL).792 A level of 2 EU/mL was chosen as the upper limit for endotoxin because this level is easily achieved with contemporary water treatment systems using RO and/or ultrafiltration. CDC has advocated monthly endotoxin testing along with microbiologic assays of water, because endotoxin activity may not correspond to the total heterotrophic plate counts.829 Additionally, the current AAMI standard RD 62 for manufacturers includes action levels for product water. Because 48 hours can elapse between the time of sampling water for microbial contamination and the time when results are received, and because bacterial proliferation can be rapid, action levels for microbial counts and endotoxin concentrations are reported as 50 CFU/mL and 1 EU/mL, respectively, in this revision of the standard.792 These recommendations will allow users to initiate corrective action before levels exceed the maximum levels established by the standard.
In hemodialysis, the net movement of water is from the blood to the dialysate, although within the dialyzer, local movement of water from the dialysate to the blood through the phenomenon of back-filtration may occur, particularly in dialyzers with highly permeable membranes.830 In contrast, hemofiltration and hemodiaflltration feature infusion of large volumes of electrolyte solution (20–70 L) into the blood. Increasingly, this electrolyte solution is being prepared on-line from water and concentrate. Because of the large volumes of fluid infused, AAMI considered the necessity of setting more stringent requirements for water to be used in this application, but this organization has not yet established these because of lack of expert consensus and insufficient experience with on-line therapies in the United States. On-line hemofiltration and hemodiafiltration systems use sequential ultrafiltration as the final step in the preparation of infusion fluid. Several experts from AAMI concur that these point-of-use ultrafiltration systems should be capable of further reducing the bacteria and endotoxin burden of solutions prepared from water meeting the requirements of the AAMI standard to a safe level for infusion.
b. Microbial Control Strategies
The strategy for controlling massive accumulations of gram-negative water bacteria and NTM in dialysis systems primarily involves preventing their growth through proper disinfection of water-treatment systems and hemodialysis machines. Gram-negative water bacteria, their associated lipopolysaccharides (bacterial endotoxins), and NTM ultimately come from the community water supply, and levels of these bacteria can be amplified depending on the water treatment system, dialysate distribution system, type of dialysis machine, and method of disinfection (Table 19).634, 794, 831 Control strategies are designed to reduce levels of microbial contamination in water and dialysis fluid to relatively low levels but not to completely eradicate it.
Water supply—Source of community water
- Ground water
Contains endotoxin and bacteria - Surface water
Contains high levels of endotoxin and bacteria
Water treatment at the dialysis center
- None
Not recommended - Filtration: Prefilter
Particulate filter to protect equipment; does not remove microorganisms - Filtration:Absolute filter (depth or membrane filter)
Removes bacteria, however, unless the filter is changed frequently or disinfected, bacteria will accumulate and grow through the filter; acts as a significant reservoir of bacteria and endotoxin - Filtration:Activated carbon filter
Removes organics and available chlorine or chloramines; acts as a significant reservoir of bacteria and endotoxin
Water treatment devices
- Deionization/ion-exchange softener
Both softeners and deionizers are significant reservoirs of bacteria and do not remove endotoxin. - Reverse osmosis (RO)
Removes bacteria and endotoxin, but must be disinfected; operates at high water pressure - Ultraviolet light
Kills some bacteria, but there is no residual; ultraviolet-resistant bacteria can develop if the unit is not properly maintained - Ultrafilter
Removes bacteria and endotoxin; operates on normal line pressure; can be positioned distal to deionizer; must be disinfected
Water and dialysate distribution system (Distribution pipes)
- Size
Oversized diameter and length decrease fluid flow and increase bacterial reservoir for both treated water and centrally-prepared dialysate. - Construction
Rough joints, dead ends, unused branches, and polyvinyl chloride (PVC) piping can act as bacterial reservoirs. - Elevation
Outlet taps should be located at the highest elevation to prevent loss of disinfectant; keep a recirculation loop in the system; flush unused ports routinely. - Storage tanks
Tanks are undesirable because they act as a reservoir for water bacteria; if tanks are present, they must be routinely scrubbed and disinfected.
Dialysis machines
- Single-pass
Disinfectant should have contact with all parts of the machine that are exposed to water or dialysis fluid. - Recirculating single-pass or recirculating (batch)
Recirculating pumps and machine design allow for massive contamination levels if not properly disinfected; overnight chemical germicide treatment is recommended.
Two components of hemodialysis water distribution systems – pipes (particularly those made of polyvinyl chloride [PVC]) and storage tanks – can serve as reservoirs of microbial contamination. Hemodialysis systems frequently use pipes that are wider and longer than are needed to handle the required flow, which slows the fluid velocity and increases both the total fluid volume and the wetted surface area of the system. Gram-negative bacteria in fluids remaining in pipes overnight multiply rapidly and colonize the wet surfaces, producing bacterial populations and endotoxin quantities in proportion to the volume and surface area. Such colonization results in formation of protective biofilm that is difficult to remove and protects the bacteria from disinfection.832 Routine (i.e., monthly), low-level disinfection of the pipes can help to control bacterial contamination of the distribution system. Additional measures to protect pipes from contaminations include
- situating all outlet taps at equal elevation and at the highest point of the system so that the disinfectant cannot drain from pipes by gravity before adequate contact time has elapsed and
- eliminating rough joints, dead-end pipes, and unused branches and taps that can trap fluid and serve as reservoirs of bacteria capable of continuously inoculating the entire volume of the system.800
Maintain a flow velocity of 3–5 ft/sec.
A storage tank in the distribution system greatly increases the volume of fluid and surface area available and can serve as a niche for water bacteria. Storage tanks are therefore not recommended for use in dialysis systems unless they are frequently drained and adequately disinfected, including scrubbing the sides of the tank to remove bacterial biofilm. An ultrafilter should be used distal to the storage tank.808, 833
Microbiologic sampling of dialysis fluids is recommended because gram-negative bacteria can proliferate rapidly in water and dialysate in hemodialysis systems; high levels of these organisms place patients at risk for pyrogenic reactions or health-care associated infection.667, 668, 808
Health-care facilities are advised to sample dialysis fluids at least monthly using standard microbiologic assay methods for waterborne microorganisms.788, 793, 799, 834–836 Product water used to prepare dialysate and to reprocess hemodialyzers for reuse on the same patient should also be tested for bacterial endotoxin on a monthly basis.792, 829, 837 (See Appendix C for information about water sampling methods for dialysis.)
Cross-contamination of dialysis machines and inadequate disinfection measures can facilitate the spread of waterborne organisms to patients. Steps should be taken to ensure that dialysis equipment is performing correctly and that all connectors, lines, and other components are specific for the equipment, in good repair, and properly in place. A recent outbreak of gram-negative bacteremias among dialysis patients was attributed to faulty valves in a drain port of the machine that allowed backflow of saline used to flush the dialyzer before patient use.838, 839 This backflow contaminated the drain priming connectors, which contaminated the blood lines and exposed the patients to high concentrations of gram-negative bacteria. Environmental infection control in dialysis settings also includes low-level disinfection of housekeeping surfaces and spot decontamination of spills of blood (see Environmental Services in Part I of this guideline for further information).
c. Infection-Control Issues in Peritoneal Dialysis
Peritoneal dialysis (PD), most commonly administered as continuous ambulatory peritoneal dialysis (CAPD) and continual cycling peritoneal dialysis (CCPD), is the third most common treatment for end-stage renal disease (ESRD) in the United States, accounting for 12% of all dialysis patients.840 Peritonitis is the primary complication of CAPD, with coagulase-negative staphylococci the most clinically significant causative organisms.841 Other organisms that have been found to produce peritonitis include Staphylococcus aureus, Mycobacterium fortuitum, M. mucogenicum, Stenotrophomonas maltophilia, Burkholderia cepacia, Corynebacterium jeikeium, Candida spp., and other fungi.842–850 Substantial morbidity is associated with peritoneal dialysis infections. Removal of peritoneal dialysis catheters usually is required for treatment of peritonitis caused by fungi, NTM, or other bacteria that are not cleared within the first several days of effective antimicrobial treatment. Furthermore, recurrent episodes of peritonitis may lead to fibrosis and loss of the dialysis membrane.
Many reported episodes of peritonitis are associated with exit-site or tunneled catheter infections. Risk factors for the development of peritonitis in PD patients include
- under dialysis,
- immune suppression,
- prolonged antimicrobial treatment,
- patient age [more infections occur in younger patients and older hospitalized patients],
- length of hospital stay, and
- hypoalbuminemia.844, 851, 852
Concern has been raised about infection risk associated with the use of automated cyclers in both inpatient and outpatient settings; however, studies suggest that PD patients who use automated cyclers have much lower infection rates.853 One study noted that a closed-drainage system reduced the incidence of system-related peritonitis among intermittent peritoneal dialysis (IPD) patients from 3.6 to 1.5 cases/100 patient days.854 The association of peritonitis with management of spent dialysate fluids requires additional study. Therefore, ensuring that the tip of the waste line is not submerged beneath the water level in a toilet or in a drain is prudent.
Microorganisms may be present in ice, ice-storage chests, and ice-making machines. The two main sources of microorganisms in ice are the potable water from which it is made and a transferral of organisms from hands (Table 20). Ice from contaminated ice machines has been associated with patient colonization, blood stream infections, pulmonary and gastrointestinal illnesses, and pseudoinfections.602, 603, 683, 684, 854, 855
Microorganisms in ice can secondarily contaminate clinical specimens and medical solutions that require cold temperatures for either transport or holding.601, 620 An outbreak of surgical-site infections was interrupted when sterile ice was used in place of tap water ice to cool cardioplegia solutions.601
From potable water
- Legionella spp. 684, 685, 857, 858
- Nontuberculous mycobacteria (NTM) 602, 603, 859
- Pseudomonas aeruginosa 859
- Burkholderia cepacia 859, 860
- Stenotrophomonas maltophilia 860
- Flavobacterium spp. 860
From fecally-contaminated water
- Norwalk virus 861–863
- Giardia lamblia 864
- Cryptosporidium parvum 685
From hand-transfer of organisms
- Acinetobacter spp. 859
- Coagulase-negative staphylococci 859
- Salmonella enteriditis 865
- Cryptosporidium parvum 685
In a study comparing the microbial populations of hospital ice machines with organisms recovered from ice samples gathered from the community, samples from 27 hospital ice machines yielded low numbers (<10 CFU/mL) of several potentially opportunistic microorganisms, mainly gram-negative bacilli.859 During the survey period, no health-care associated infections were attributed to the use of ice. Ice from community sources had higher levels of microbial contamination (75%–95% of 194 samples had total heterotrophic plate counts <500 CFU/mL, with the proportion of positive cultures dependent on the incubation temperature) and showed evidence of fecal contamination from the source water.859Thus, ice machines in health-care settings are no more heavily contaminated compared with ice machines in the community. If the source water for ice in a health-care facility is not fecally contaminated, then ice from clean ice machines and chests should pose no increased hazard for immunocompetent patients. Some waterborne bacteria found in ice could potentially be a risk to immunocompromised patients if they consume ice or drink beverages with ice. For example, Burkholderia cepacia in ice could present an infection risk for cystic fibrosis patients.859, 860 Therefore, protecting immunosuppressed and otherwise medically at-risk patients from exposure to tap water and ice potentially contaminated with opportunistic pathogens is prudent.9
No microbiologic standards for ice, ice-making machines, or ice storage equipment have been established, although several investigators have suggested the need for such standards.859, 866 Culturing of ice machines is not routinely recommended, but it may be useful as part of an epidemiologic investigation.867–869 Sampling might also help determine the best schedule for cleaning open ice-storage chests. Recommendations for a regular program of maintenance and disinfection have been published.866–869 Health-care facilities are advised to clean ice-storage chests on a regular basis. Open ice chests may require a more frequent cleaning schedule compared with chests that have covers. Portable ice chests and containers require cleaning and low-level disinfection before the addition of ice intended for consumption. Ice-making machines may require less frequent cleaning, but their maintenance is important to proper performance. The manufacturer’s instructions for both the proper method of cleaning and/or maintenance should be followed. These instructions may also recommend an EPA-registered disinfectant to ensure chemical potency, materials compatibility, and safety. In the event that instructions and suitable EPA-registered disinfectants are not available for this process, then a generic approach to cleaning, disinfecting, and maintaining ice machines and dispensers can be used (Box 12).
Ice and ice-making machines also may be contaminated via improper storage or handling of ice by patients and/or staff.684–686, 855–858, 870 Suggested steps to avoid this means of contamination include
- minimizing or avoiding direct hand contact with ice intended for consumption,
- using a hard-surface scoop to dispense ice, and
- installing machines that dispense ice directly into portable containers at the touch of a control.687, 869
- Disconnect unit from power supply.
- Remove and discard ice from bin or storage chest.
- Allow unit to warm to room temperature.
- Disassemble removable parts of machine that make contact with water to make ice.
- Thoroughly clean machine and parts with water and detergent.
- Dry external surfaces of removable parts before reassembling.
- Check for any needed repair.
- Replace feeder lines, as appropriate (e.g., when damaged, old, or difficult to clean).
- Ensure presence of an air space in tubing leading from water inlet into water distribution system of machine.
- Inspect for rodent or insect infestations under the unit and treat, as needed.
- Check door gaskets (open compartment models) for evidence of leakage or dripping into the storage chest.
- Clean the ice-storage chest or bin with fresh water and detergent; rinse with fresh tap water.
- Sanitize machine by circulating a 50–100 parts per million (ppm) solution of sodium hypochlorite (i.e., 4–8 mL sodium hypochlorite/gallon of water) through the ice-making and storage systems for 2 hours (100 ppm solution), or 4 hours (50 ppm solution).
- Drain sodium hypochlorite solutions and flush with fresh tap water.
- Allow all surfaces of equipment to dry before returning to service.
* Material in this box is adapted from reference 869.
+ These general guidelines should be used only where manufacturer-recommended methods and EPA-registered disinfectants are not available.
a. General Information
Hydrotherapy equipment (e.g., pools, whirlpools, whirlpool spas, hot tubs, and physiotherapy tanks) traditionally has been used to treat patients with certain medical conditions (e.g., burns,871, 872 septic ulcers, lesions, amputations,873 orthopedic impairments and injuries, arthritis,874 and kidney lithotripsy).654 Wound-care medicine is increasingly moving away from hydrotherapy, however, in favor of bedside pulsed-lavage therapy using sterile solutions for cleaning and irrigation.492, 875–878Several episodes of health-care associated infections have been linked to use of hydrotherapy equipment (Table 21). Potential routes of infection include incidental ingestion of the water, sprays and aerosols, and direct contact with wounds and intact skin (folliculitis). Risk factors for infection include
- age and sex of the patient,
- underlying medical conditions,
- length of time spent in the hydrotherapy water, and
- portals of entry.879
| Microorganisms | Medical conditions | References |
|---|---|---|
| Acinetobacter baumanii | Sepsis | 572 |
| Citrobacter freundii | Cellulitis | 880 |
| Enterobacter cloacae | Sepsis | 881 |
| Legionella spp. | Legionellosis | 882 |
| Mycobacterium abscessus, Mycobacterium fortuitum, Mycobacterium marinum | Skin ulcers and soft tissue infections | 621–623, 883 |
| Pseudomonas aeruginosa | Sepsis, soft tissue infections, folliculitis, and wound infections | 492, 493, 506, 679, 884–888 |
| Adenovirus, adeno-associated virus | Conjunctivitis | 889 |
Infection control for hydrotherapy tanks, pools, or birthing tanks presents unique challenges because indigenous microorganisms are always present in the water during treatments. In addition, some studies have found free living amoebae (i.e., Naegleria lovaniensis), which are commonly found in association with Naegleria fowleri, in hospital hydrotherapy pools.890 Although hydrotherapy is at times appropriate for patients with wounds, burns, or other types of non-intact skin conditions (determined on a case-by-case basis), this equipment should not be considered “semi-critical” in accordance with the Spaulding classification.891 Microbial data to evaluate the risk of infection to patients using hydrotherapy pools and birthing tanks are insufficient. Nevertheless, health-care facilities should maintain stringent cleaning and disinfection practices in accordance with the manufacturer’s instructions and with relevant scientific literature until data supporting more rigorous infection-control measures become available. Factors that should be considered in therapy decisions in this situation would include
- availability of alternative aseptic techniques for wound management and
- a risk-benefit analysis of using traditional hydrotherapy.
b. Hydrotherapy Tanks
Hydrotherapy tanks (e.g., whirlpools, Hubbard tanks and whirlpool bath tubs) are shallow tanks constructed of stainless steel, plexiglass, or tile. They are closed-cycle water systems with hydrojets to circulate, aerate, and agitate the water. The maximum water temperature range is 50°F–104°F (10°C–40°C). The warm water temperature, constant agitation and aeration, and design of the hydrotherapy tanks provide ideal conditions for bacterial proliferation if the equipment is not properly maintained, cleaned, and disinfected. The design of the hydrotherapy equipment should be evaluated for potential infection-control problems that can be associated with inaccessible surfaces that can be difficult to clean and/or remain wet in between uses (i.e., recessed drain plates with fixed grill plates).887 Associated equipment (e.g., parallel bars, plinths, Hoyer lifts, and wheelchairs) can also be potential reservoirs of microorganisms, depending on the materials used in these items (i.e., porous vs. non-porous materials) and the surfaces that may become wet during use. Patients with active skin colonizations and wound infections can serve as sources of contamination for the equipment and the water. Contamination from spilled tub water can extend to drains, floors, and walls.680–683 Health-care associated colonization or infection can result from exposure to endogenous sources of microorganisms (autoinoculation) or exogenous sources (via cross-contamination from other patients previously receiving treatment in the unit).
Although some facilities have used tub liners to minimize environmental contamination of the tanks, the use of a tub liner does not eliminate the need for cleaning and disinfection. Draining these small pools and tanks after each patient use, thoroughly cleaning with a detergent, and disinfecting according to manufacturers’ instructions have reduced bacterial contamination levels in the water from 104 CFU/mL to <10 CFU/mL.892 A chlorine residual of 15 ppm in the water should be obtained prior to the patient’s therapy session (e.g., by adding 15 grams of calcium hypochlorite 70% [e.g., HTH®] per 100 gallons of water).892 A study of commercial and residential whirlpools found that superchlorination or draining, cleaning, disinfection, and refilling of whirlpools markedly reduced densities of Pseudomonas aeruginosa in whirlpool water.893 The bacterial populations were rapidly replenished, however, when disinfectant concentrations dropped below recommended levels for recreational use (i.e., chlorine at 3.0 ppm or bromine at 6.0 ppm). When using chlorine, however, knowing whether the community drinking-water system is disinfected with chloramine is important, because municipal utilities adjust the pH of the water to the basic side to enhance chloramine formation. Because chlorine is not very effective at pH levels above 8, it may be necessary to re-adjust the pH of the water to a more acidic level.894
A few reports describe the addition of antiseptic chemicals to hydrotherapy tank water, especially for burn patient therapy.895–897 One study involving a minimal number of participants demonstrated a reduction in the number of Pseudomonas spp. and other gram-negative bacteria from both patients and equipment surfaces when chloramine-T (“chlorazene”) was added to the water.898 Chloramine-T has not, however, been approved for water treatment in the United States.
c. Hydrotherapy Pools
Hydrotherapy pools typically serve large numbers of patients and are usually heated to 91.4°F–98.6°F (31°C–37°C). The temperature range is more narrow (94°F–96.8°F [35°C–36°C]) for pediatric and geriatric patient use.899 Because the size of hydrotherapy pools precludes draining after patient use, proper management is required to maintain the proper balance of water conditioning (i.e., alkalinity, hardness, and temperature) and disinfection. The most widely used chemicals for disinfection of pools are chlorine and chlorine compounds – calcium hypochlorite, sodium hypochlorite, lithium hypochlorite, chloroisocyanurates, and chlorine gas. Solid and liquid formulations of chlorine chemicals are the easiest and safest to use.900 Other halogenated compounds have also been used for pool-water disinfection, albeit on a limited scale. Bromine, which forms bactericidal bromamines in the presence of ammonia, has limited use because of its association with contact dermatitis.901 Iodine does not bleach hair, swim suits, or cause eye irritation, but when introduced at proper concentrations, it gives water a greenish-yellowish cast.892
In practical terms, maintenance of large hydrotherapy pools (e.g., those used for exercise) is similar to that for indoor public pools (i.e., continuous filtration, chlorine residuals no less than 0.4 ppm, and pH of 7.2–7.6).902, 903 Supply pipes and pumps also need to be maintained to eliminate the possibility of this equipment serving as a reservoir for waterborne organisms.904Specific standards for chlorine residual and pH of the water are addressed in local and state regulations. Patients who are fecally incontinent or who have draining wounds should refrain from using these pools until their condition improves.
d. Birthing Tanks and Other Equipment
The use of birthing tanks, whirlpool spas, and whirlpools is a recent addition to obstetrical practice.905 Few studies on the potential risks associated with these pieces of equipment have been conducted. In one study of 32 women, a newborn contracted a Pseudomonas infection after being birthed in such a tank, the strain of which was identical to the organism isolated from the tank water.906 Another report documented identical strains of P. aeruginosa isolates from a newborn with sepsis and on the environmental surfaces of a tub that the mother used for relaxation while in labor.907 Other studies have shown no significant increases in the rates of post-immersion infections among mothers and infants.908, 909
Because the water and the tub surfaces routinely become contaminated with the mother’s skin flora and blood during labor and delivery, birthing tanks and other tub equipment must be drained after each patient use and the surfaces thoroughly cleaned and disinfected. Health-care facilities are advised to follow the manufacturer’s instructions for selection of disinfection method and chemical germicide. The range of chlorine residuals for public whirlpools and whirlpool spas is 2–5 ppm.910 Use of an inflatable tub is an alternative solution, but this item must be cleaned and disinfected between patients if it is not considered a single-use unit.
Recreational tanks and whirlpool spas are increasingly being used as hydrotherapy equipment. Although such home equipment appears to be suitable for hydrotherapy, they are neither designed nor constructed to function in this capacity. Additionally, manufacturers generally are not obligated to provide the health-care facility with cleaning and disinfecting instructions appropriate for medical equipment use, and the U.S. Food and Drug Administration (FDA) does not evaluate recreational equipment. Health-care facilities should therefore carefully evaluate this “off-label” use of home equipment before proceeding with a purchase.
a. Automated Endoscope Reprocessors
The automated endoscopic reprocessor (AER) is classified by the FDA as an accessory for the flexible endoscope.654 A properly operating AER can provide a more consistent, reliable method of decontaminating and terminal reprocessing for endoscopes between patient procedures than manual reprocessing methods alone.911 An endoscope is generally subjected to high-level disinfection using a liquid chemical sterilant or a high-level disinfectant. Because the instrument is a semi-critical device, the optimal rinse fluid for a disinfected endoscope would be sterile water.3 Sterile water, however, is expensive and difficult to produce in sufficient quantities and with adequate quality assurance for instrument rinsing in an AER.912, 913 Therefore, one option to be used for AERs is rinse water that has been passed through filters with a pore size of 0.1–0.2 μm to render the water “bacteria-free.” These filters usually are located in the water line at or near the port where the mains water enters the equipment. The product water (i.e., tap water passing through these filters) in these applications is not considered equivalent in microbial quality to that for membrane-filtered water as produced by pharmaceutical firms. Membrane filtration in pharmaceutical applications is intended to ensure the microbial quality of polished product water.
Water has been linked to the contamination of flexible fiberoptic endoscopes in the following two scenarios:
- rinsing a disinfected endoscope with unfiltered tap water, followed by storage of the instrument without drying out the internal channels and
- contamination of AERs from tap water inadvertently introduced into the equipment.
In the latter instance, the machine’s water reservoirs and fluid circuitry become contaminated with waterborne, heterotrophic bacteria (e.g., Pseudomonas aeruginosa and NTM), which can survive and persist in biofilms attached to these components.914–917 Colonization of the reservoirs and water lines of the AER becomes problematic if the required cleaning, disinfection, and maintenance are not performed on the equipment as recommended by the manufacturer.669, 916, 917 Use of the 0.1–0.2-μm filter in the water line helps to keep bacterial contamination to a minimum,670, 911, 917 but filters may fail and allow bacteria to pass through to the equipment and then to the instrument undergoing reprocessing.671–674, 913, 918 Filters also require maintenance for proper performance.670, 911, 912, 918, 919 Heightened awareness of the proper disinfection of the connectors that hook the instrument to the AER may help to further reduce the potential for contaminating endoscopes during reprocessing.920 An emerging issue in the field of endoscopy is that of the possible role of rinse water monitoring and its potential to help reduce endoscopy/bronchoscopyassociated infections.918
Studies have linked deficiencies in endoscope cleaning and/or disinfecting processes to the incidence of post-endoscopic adverse outcomes.921–924 Several clusters have been traced to AERs of older designs and these were associated with water quality.675, 914–916 Regardless of whether manual or automated terminal reprocessing is used for endoscopes, the internal channels of the instrument should be dried before storage.925 The presence of residual moisture in the internal channels encourages the proliferation of waterborne microorganisms, some of which may be pathogenic. One of the most frequently used methods employs 70% isopropyl alcohol to flush the internal channels, followed by forced air drying of these channels and hanging the endoscope vertically in a protected cabinet; this method ensures internal drying of the endoscope, lessens the potential for proliferation of waterborne microorganisms,669, 913, 917, 922, 926, 927 and is consistent with professional organization guidance for endoscope reprocessing.928
An additional problem with waterborne microbial contamination of AERs centers on increased microbial resistance to alkaline glutaraldehyde, a widely used liquid chemical sterilant/high-level disinfectant.669, 929 Opportunistic waterborne microorganisms (e.g., Mycobacterium chelonae, Methylobacterium spp.) have been associated with pseudo-outbreaks and colonization; infection caused by these organisms has been associated with procedures conducted in clinical settings (e.g., bronchoscopy).669, 913, 929–931 Increasing microbial resistance to glutaraldehyde has been attributed to improper use of the disinfectant in the equipment, allowing the dilution of glutaraldehyde to fall below the manufacturer’s recommended minimal use concentration.929
b. Dental Unit Water Lines
Dental unit water lines (DUWLs) consist of small-bore plastic tubing that delivers water used for general, non-surgical irrigation and as a coolant to dental handpieces, sonic and ultrasonic scalers, and air-water syringes; municipal tap water is the source water for these lines. The presence of biofilms of waterborne bacteria and fungi (e.g., Legionella spp., Pseudomonas aeruginosa, and NTM) in DUWLs has been established.636, 637, 694, 695, 932– 934 Biofilms continually release planktonic microorganisms into the water, the titers of which can exceed 1H106 CFU/mL.694 However, scientific evidence indicates that immunocompetent persons are only at minimal risk for substantial adverse health effects after contact with water from a dental unit. Nonetheless, exposing patients or dental personnel to water of uncertain microbiological quality is not consistent with universally accepted infection-control principles.935
In 1993, CDC issued guidelines relative to water quality in a dental setting. These guidelines recommend that all dental instruments that use water (including high-speed handpieces) should be run to discharge water for 20–30 seconds after each patient and for several minutes before the start of each clinic day.936 This practice can help to flush out any patient materials that many have entered the turbine, air, or waterlines.937, 938 The 1993 guidance also indicated that waterlines be flushed at the beginning of the clinic day. Although these guidelines are designed to help reduce the number of microorganisms present in treatment water, they do not address the issue of reducing or preventing biofilm formation in the waterlines. Research published subsequent to the 1993 dental infection control guideline suggests that flushing the lines at the beginning of the day has only minimal effect on the status of the biofilm in the lines and does not reliably improve the quality of water during dental treatment.939–941 Updated recommendations on infection-control practices for water line use in dentistry will be available in late 2003.942
The numbers of microorganisms in water used as coolant or irrigant for non-surgical dental treatment should be as low as reasonably achievable and, at a minimum, should meet nationally recognized standards for safe drinking water.935, 943 Only minimal evidence suggests that water meeting drinking water standards poses a health hazard for immunocompetent persons. The EPA, the American Public Health Association (APHA), and the American Water Works Association (AWWA) have set a maximum limit of 500 CFU/mL for aerobic, heterotrophic, mesophilic bacteria in drinking water in municipal distribution systems.944, 945 This standard is achievable, given improvements in water-line technology. Dentists should consult with the manufacturer of their dental unit to determine the best equipment and method for maintaining and monitoring good water quality.935, 946
