Interim Updated Planning Guidance on Allocating and Targeting Pandemic Influenza Vaccine during an Influenza Pandemic
Introduction
Effective allocation and administration of pandemic influenza vaccine will play a critical role in preventing influenza and reducing its effects on health and society during a future pandemic. Although the timing and severity of a future pandemic and characteristics of the next pandemic influenza virus strain are not known, it is important to plan and prepare. The overarching aim of the national pandemic influenza vaccination program is to vaccinate all persons in the United States (U.S.) who choose to be vaccinated, prior to the peak of disease. The U.S. government’s goal is to have sufficient pandemic influenza vaccine available for an effective domestic response within four months of a pandemic declaration. Additionally, plans are to have first doses available within 12 weeks of the President or the Secretary of Health and Human Services declaring a pandemic 1. To meet these timelines, the U.S. government is investing significant resources to create and evaluate new vaccine development approaches and production technologies. Pre-pandemic influenza vaccine stockpiles of bulk vaccine against viruses with pandemic potential are also being established and maintained.
Despite these investments, there are other issues to consider. Stockpiled pandemic vaccine availability will depend on the degree to which they match the circulating pandemic strain and other properties, and manufacturing capacity. In a pandemic, a novel virus has not circulated in humans, and it is assumed that the majority of the population may not have immunity to the virus, causing more people to become ill. Rates of severe illness, complications, and death may be much higher than seasonal flu and more widely distributed. The greater frequency and severity of disease will increase the burden on the health care system, the risk of ongoing transmission in the community, and may increase rates of absenteeism and disruptions in the availability of critical products and services in health care and other sectors. Similarly, homeland and national security and critical infrastructure (e.g., transportation and power supply) could be threatened if illness among critical personnel reduces their capabilities.
Given that influenza vaccine supply will increase incrementally as vaccine is produced during a pandemic, targeting decisions may have to be made. Such decisions should be based on vaccine supply, pandemic severity and impact, potential for disruption of community critical infrastructure, operational considerations, and publicly articulated pandemic vaccination program objectives and principles. The overarching objectives guiding vaccine allocation and use during a pandemic are to reduce the impact of the pandemic on health and minimize disruption to society and the economy. Specifically, the targeting strategy aims to protect those who will: maintain homeland and national security, are essential to the pandemic response and provide care for persons who are ill, maintain essential community services, be at greater risk of infection due to their job, and those who are most medically vulnerable to severe illness such as young children and pregnant women.
Recognizing that demand may exceed supply at the onset of a pandemic, federal, state, tribal, and local governments, communities, and the private sector have asked for updated planning guidance on who should receive vaccination early in a pandemic. This document uses pandemic severity categories based on the current CDC Pandemic Severity Assessment Framework1. Several new elements have been incorporated into the 2018 guidelines to update and provide interim guidance for planning purposes, and to provide the rationale for a new vaccination program during a pandemic allowing for local adjustment where appropriate. These guidelines replace the 2008 Guidance on Allocating and Targeting Pandemic Influenza Vaccine.

To assist state and local health departments in planning for targeting vaccine during an influenza pandemic, the Centers for Disease Control and Prevention (CDC) has developed the following checklist. The items in the checklist are based on the 2018 Interim Updated Planning Guidance on Allocating and Targeting Pandemic Influenza Vaccine During an Influenza Pandemic, and include specific activities public health emergency planners and immunization programs can do to prepare for targeted pandemic influenza vaccination. In many states these activities will require extensive collaboration between state and local public health departments and with neighboring jurisdictions.
This checklist and accompanying guidance are provided for planning purposes and may change as a future influenza pandemic emerges.
Download the Pandemic Influenza Vaccine Targeting Checklist pdf icon[76 KB, 2 Pages]
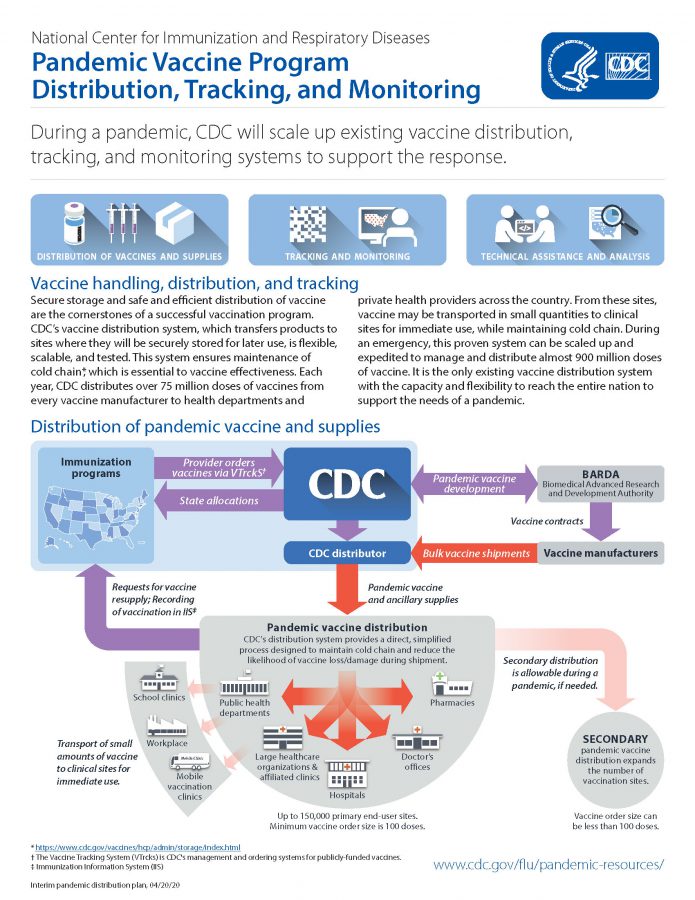
During a pandemic, CDC could scale up scale up existing vaccine distribution, tracking, and monitoring systems in order to support a response. This pdf also provides links to CDC pandemic planning and vaccine resources.
Pandemic Vaccine Program Distribution, Tracking, and Monitoring pdf icon[1.10 MB, 2 pages]

