Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Recommended Immunization Schedules for Persons Aged 0 Through 18 Years — United States, 2012
Please note: An erratum has been
published for this article. To view the erratum, please click
here.
Each year, the Advisory Committee on Immunization Practices (ACIP) publishes immunization schedules for persons aged 0 through 18 years. These schedules summarize recommendations for currently licensed vaccines for children aged 0 through 6 years and 7 through 18 years and include recommendations in effect as of December 23, 2011.
Vaccination providers are being advised to use all three schedules (Figure 1, Figure 2, and Figure 3) and their respective footnotes together and not separately.
A parent-friendly schedule for children and adolescents is available online at http://www.cdc.gov/vaccines/recs/schedules/child-schedule.htm#printable.
Changes to the previous schedules include the following:
- Updates to Figure 1 ("Recommended immunization schedule for persons aged 0 through 6 years"):
— Quadrivalent meningococcal conjugate vaccine (MCV4) purple bar has been extended to reflect licensure of MCV4-D (Menactra) use in children as young as age 9 months.
— A wording change has been introduced in the hepatitis A (HepA) vaccine yellow bar; wording now states, "Dose 1." A new yellow and purple bar has been added to reflect HepA vaccine recommendations for children aged 2 years and older.
- Guidance is provided for administration of hepatitis B (HepB) vaccine in infants with birthweights <2,000 grams and ≥2,000 grams. Clarification is provided for doses after administration of the birth dose of HepB vaccine.
- Rotavirus (RV) vaccine footnotes have been condensed.
- Haemophilus influenzae type b (Hib) conjugate vaccine footnotes have been condensed, and use of Hiberix for the booster (final) dose has been clarified. Guidance for use of Hib vaccine in persons aged 5 years and older in the catch-up schedule has been updated.
- Pneumococcal vaccine footnotes have been condensed.
- Guidance is provided for use of measles, mumps, and rubella (MMR) vaccine in infants aged 6 through 11 months. Footnotes in the catch-up schedule have been condensed.
- HepA vaccine footnotes have been updated to clarify that the second dose of HepA vaccine should be administered 6–18 months after dose 1.
- MCV4 footnotes have been updated to reflect recent recommendations published in MMWR.
- Influenza vaccine footnotes have been updated to provide guidance on live, attenuated influenza vaccine (LAIV) contraindications.
- Influenza vaccine footnotes also have been updated to clarify dosing for children aged 6 months through 8 years for the 2011–12 and 2012–13 seasons.
- Figure 2 ("Recommended immunization schedule for persons aged 7 through 18 years") has been updated to include number of doses for each vaccine. Information regarding the recommended age (16 years) for the booster dose of MCV4 has been added.
- Tdap vaccine recommendations for children aged 7 through 10 years have been updated.
- Human papillomavirus (HPV) vaccine footnotes have been updated to include routine recommendations for vaccination of males.
- Varicella (VAR) vaccine footnotes have been condensed.
- Inactivated poliovirus vaccine (IPV) footnotes have been updated to include upper age limit for routine vaccination. IPV footnotes in the catch-up schedule have been condensed, and relevant wording added to Figure 3 ("Catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind").
- In the catch-up immunization schedule, HepA vaccine and HepB vaccine footnotes have been removed. Relevant wording has been added to Figure 3.
- MCV4 vaccine has been added to Figure 3 along with corresponding footnotes.
The recommended immunization schedules for persons aged 0 through 18 years and the catch-up immunization schedule for 2012 are approved by the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians.
Suggested citation: Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0–18 years—United States, 2012. MMWR 2012;61(5).
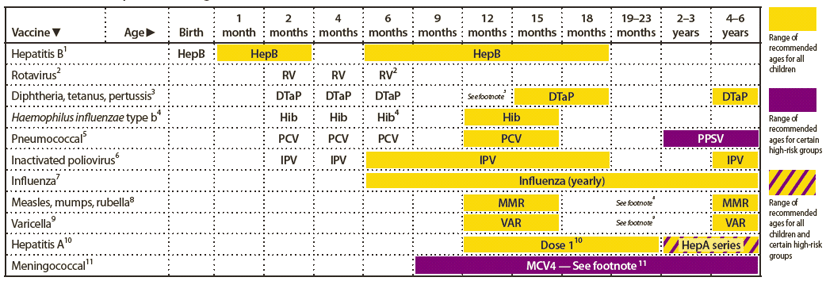
FIGURE 1. Recommended immunization schedule for persons aged 0 through 6 years — United States, 2012 (for those who fall behind or start late, see the catch-up schedule [Figure 3])
Alternate Text: The figure above shows the recommended immunization schedule for persons aged 0 through 6 years in the United States for the year 2012. For persons who fall behind or start late, this schedule and the catch-up schedule (Figure 3) should be consulted.
This schedule includes recommendations in effect as of December 23, 2011. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible. The use of a combination vaccine generally is preferred over separate injections of its equivalent component vaccines. Vaccination providers should consult the relevant Advisory Committee on Immunization Practices (ACIP) statement for detailed recommendations, available online at http://www.cdc.gov/vaccines/pubs/acip-list.htm. Clinically significant adverse events that follow vaccination should be reported to the Vaccine Adverse Event Reporting System (VAERS) online (http://www.vaers.hhs.gov) or by telephone (800-822-7967).
1. Hepatitis B (HepB) vaccine. (Minimum age: birth)
At birth:
- Administer monovalent HepB vaccine to all newborns before hospital discharge.
- For infants born to hepatitis B surface antigen (HBsAg)–positive mothers, administer HepB vaccine and 0.5 mL of hepatitis B immune globulin (HBIG) within 12 hours of birth. These infants should be tested for HBsAg and antibody to HBsAg (anti-HBs) 1 to 2 months after receiving the last dose of the series.
- If mother's HBsAg status is unknown, within 12 hours of birth administer HepB vaccine for infants weighing ≥2,000 grams, and HepB vaccine plus HBIG for infants weighing <2,000 grams. Determine mother's HBsAg status as soon as possible and, if she is HBsAg-positive, administer HBIG for infants weighing ≥2,000 grams (no later than age 1 week).
Doses after the birth dose:
- The second dose should be administered at age 1 to 2 months. Monovalent HepB vaccine should be used for doses administered before age 6 weeks.
- Administration of a total of 4 doses of HepB vaccine is permissible when a combination vaccine containing HepB is administered after the birth dose.
- Infants who did not receive a birth dose should receive 3 doses of a HepB-containing vaccine starting as soon as feasible (Figure 3).
- The minimum interval between dose 1 and dose 2 is 4 weeks, and between dose 2 and 3 is 8 weeks. The final (third or fourth) dose in the HepB vaccine series should be administered no earlier than age 24 weeks and at least 16 weeks after the first dose.
2. Rotavirus (RV) vaccines.(Minimum age: 6 weeks for both RV-1 [Rotarix] and RV-5 [Rota Teq])
- The maximum age for the first dose in the series is 14 weeks, 6 days; and 8 months, 0 days for the final dose in the series. Vaccination should not be initiated for infants aged 15 weeks, 0 days or older.
- If RV-1 (Rotarix) is administered at ages 2 and 4 months, a dose at 6 months is not indicated.
3. Diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine.
(Minimum age: 6 weeks)
- The fourth dose may be administered as early as age 12 months, provided at least 6 months have elapsed since the third dose.
4. Haemophilus influenzae type b (Hib) conjugate vaccine. (Minimum age: 6 weeks)
- If PRP-OMP (PedvaxHIB or Comvax [HepB-Hib]) is administered at ages 2 and 4 months, a dose at age 6 months is not indicated.
- Hiberix should only be used for the booster (final) dose in children aged 12 months through 4 years.
5. Pneumococcal vaccines. (Minimum age: 6 weeks for pneumococcal conjugate vaccine [PCV]; 2 years for pneumococcal polysaccharide vaccine [PPSV])
- Administer 1 dose of PCV to all healthy children aged 24 through 59 months who are not completely vaccinated for their age.
- For children who have received an age-appropriate series of 7-valent PCV (PCV7), a single supplemental dose of 13-valent PCV (PCV13) is recommended for:
— All children aged 14 through 59 months
— Children aged 60 through 71 months with underlying medical conditions.
- Administer PPSV at least 8 weeks after last dose of PCV to children aged 2 years or older with certain underlying medical conditions, including a cochlear implant. See MMWR 2010:59(No. RR-11), available at http://www.cdc.gov/mmwr/pdf/rr/rr5911.pdf.
6. Inactivated poliovirus vaccine (IPV). (Minimum age: 6 weeks)
- If 4 or more doses are administered before age 4 years, an additional dose should be administered at age 4 through 6 years.
- The final dose in the series should be administered on or after the fourth birthday and at least 6 months after the previous dose.
7. Influenza vaccines. (Minimum age: 6 months for trivalent inactivated influenza vaccine [TIV]; 2 years for live, attenuated influenza vaccine [LAIV])
- For most healthy children aged 2 years and older, either LAIV or TIV may be used. However, LAIV should not be administered to some children, including 1) children with asthma, 2) children 2 through 4 years who had wheezing in the past 12 months, or 3) children who have any other underlying medical conditions that predispose them to influenza complications. For all other contraindications to use of LAIV, see MMWR 2010;59(No. RR-8), available at http://www.cdc.gov/mmwr/pdf/rr/rr5908.pdf.
- For children aged 6 months through 8 years:
— For the 2011–12 season, administer 2 doses (separated by at least 4 weeks) to those who did not receive at least 1 dose of the 2010–11 vaccine. Those who received at least 1 dose of the 2010–11 vaccine require 1 dose for the 2011–12 season.
— For the 2012–13 season, follow dosing guidelines in the 2012 ACIP influenza vaccine recommendations.
8. Measles, mumps, and rubella (MMR) vaccine. (Minimum age: 12 months)
- The second dose may be administered before age 4 years, provided at least 4 weeks have elapsed since the first dose.
- Administer MMR vaccine to infants aged 6 through 11 months who are traveling internationally. These children should be revaccinated with 2 doses of MMR vaccine, the first at ages 12 through 15 months and at least 4 weeks after the previous dose, and the second at ages 4 through 6 years.
9. Varicella (VAR) vaccine. (Minimum age: 12 months)
- The second dose may be administered before age 4 years, provided at least 3 months have elapsed since the first dose.
- For children aged 12 months through 12 years, the recommended minimum interval between doses is 3 months. However, if the second dose was administered at least 4 weeks after the first dose, it can be accepted as valid.
10. Hepatitis A (HepA) vaccine. (Minimum age: 12 months)
- Administer the second (final) dose 6 to18 months after the first.
- Unvaccinated children 24 months and older at high risk should be vaccinated. See MMWR 2006;55(No. RR-7), available at http://www.cdc.gov/mmwr/pdf/rr/rr5507.pdf.
- A 2-dose HepA vaccine series is recommended for anyone aged 24 months and older, previously unvaccinated, for whom immunity against hepatitis A virus infection is desired.
11. Meningococcal conjugate vaccines, quadrivalent (MCV4). (Minimum age: 9 months for Menactra [MCV4-D], 2 years for Menveo [MCV4-CRM])
- For children aged 9 through 23 months 1) with persistent complement component deficiency; 2) who are residents of or travelers to countries with hyperendemic or epidemic disease; or 3) who are present during outbreaks caused by a vaccine serogroup, administer 2 primary doses of MCV4-D, ideally at ages 9 months and 12 months or at least 8 weeks apart.
- For children aged 24 months and older with 1) persistent complement component deficiency who have not been previously vaccinated; or 2) anatomic/functional asplenia, administer 2 primary doses of either MCV4 at least 8 weeks apart.
- For children with anatomic/functional asplenia, if MCV4-D (Menactra) is used, administer at a minimum age of 2 years and at least 4 weeks after completion of all PCV doses.
- See MMWR 2011;60:72–6, available at http://www.cdc.gov/mmwr/pdf/wk/mm6003.pdf, and Vaccines for Children Program resolution No. 6/11-1, available at http://www.cdc.gov/vaccines/programs/vfc/downloads/resolutions/06-11mening-mcv.pdf, and MMWR 2011;60:1391–2, available at http://www.cdc.gov/mmwr/pdf/wk/mm6040.pdf, for further guidance, including revaccination guidelines.
This schedule is approved by the Advisory Committee on Immunization Practices (http://www.cdc.gov/vaccines/recs/acip),
the American Academy of Pediatrics (http://www.aap.org), and the American Academy of Family Physicians (http://www.aafp.org).
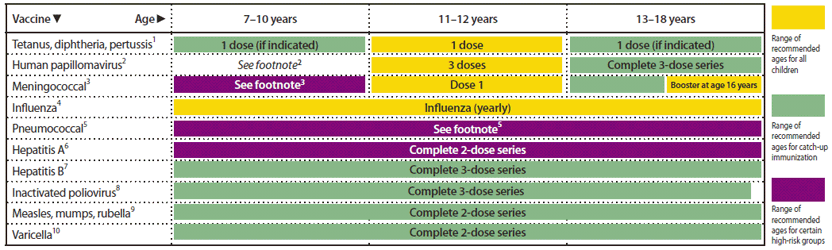
FIGURE 2. Recommended immunization schedule for persons aged 7 through 18 years — United States, 2012 (for those who fall behind or start late, see the schedule below and the catch-up schedule [Figure 3])
Alternate Text: The figure above shows the recommended immunization schedule for persons aged 7 through 18 years in the United States for the year 2012. For persons who fall behind or start late, this schedule and the catch-up schedule (Figure 3) should be consulted.
This schedule includes recommendations in effect as of December 23, 2011. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible. The use of a combination vaccine generally is preferred over separate injections of its equivalent component vaccines. Vaccination providers should consult the relevant Advisory Committee on Immunization Practices (ACIP) statement for detailed recommendations, available online at http://www.cdc.gov/vaccines/pubs/acip-list.htm. Clinically significant adverse events that follow vaccination should be reported to the Vaccine Adverse Event Reporting System (VAERS) online (http://www.vaers.hhs.gov) or by telephone (800-822-7967).
1. Tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccine. (Minimum age: 10 years for Boostrix and 11 years for Adacel)
- Persons aged 11 through 18 years who have not received Tdap vaccine should receive a dose followed by tetanus and diphtheria toxoids (Td) booster doses every 10 years thereafter.
- Tdap vaccine should be substituted for a single dose of Td in the catch-up series for children aged 7 through 10 years. Refer to the catch-up schedule if additional doses of tetanus and diphtheria toxoid–containing vaccine are needed.
- Tdap vaccine can be administered regardless of the interval since the last tetanus and diphtheria toxoid–containing vaccine.
2. Human papillomavirus (HPV) vaccines (HPV4 [Gardasil] and HPV2 [Cervarix]). (Minimum age: 9 years)
- Either HPV4 or HPV2 is recommended in a 3-dose series for females aged 11 or 12 years. HPV4 is recommended in a 3-dose series for males aged 11 or 12 years.
- The vaccine series can be started beginning at age 9 years.
- Administer the second dose 1 to 2 months after the first dose and the third dose 6 months after the first dose (at least 24 weeks after the first dose).
- See MMWR 2010;59:626–32, available at http://www.cdc.gov/mmwr/pdf/wk/mm5920.pdf.
3. Meningococcal conjugate vaccines, quadrivalent (MCV4).
- Administer MCV4 at age 11 through 12 years with a booster dose at age 16 years.
- Administer MCV4 at age 13 through 18 years if patient is not previously vaccinated.
- If the first dose is administered at age 13 through 15 years, a booster dose should be administered at age 16 through 18 years with a minimum interval of at least 8 weeks after the preceding dose.
- If the first dose is administered at age 16 years or older, a booster dose is not needed.
- Administer 2 primary doses at least 8 weeks apart to previously unvaccinated persons with persistent complement component deficiency or anatomic/functional asplenia, and 1 dose every 5 years thereafter.
- Adolescents aged 11 through 18 years with human immunodeficiency virus (HIV) infection should receive a 2-dose primary series of MCV4, at least 8 weeks apart.
- See MMWR 2011;60:72–76, available at http://www.cdc.gov/mmwr/pdf/wk/mm6003.pdf, and Vaccines for Children Program resolution No. 6/11-1, available at http://www.cdc.gov/vaccines/programs/vfc/downloads/resolutions/06-11mening-mcv.pdf, for further guidelines.
4. Influenza vaccines (trivalent inactivated influenza vaccine [TIV] and live, attenuated influenza vaccine [LAIV]).
- For most healthy, nonpregnant persons, either LAIV or TIV may be used, except LAIV should not be used for some persons, including those with asthma or any other underlying medical conditions that predispose them to influenza complications. For all other contraindications to use of LAIV, see MMWR 2010;59(No.RR-8), available at http://www.cdc.gov/mmwr/pdf/rr/rr5908.pdf.
- Administer 1 dose to persons aged 9 years and older.
- For children aged 6 months through 8 years:
— For the 2011–12 season, administer 2 doses (separated by at least 4 weeks) to those who did not receive at least 1 dose of the 2010–11 vaccine. Those who received at least 1 dose of the 2010–11 vaccine require 1 dose for the 2011–12 season.
— For the 2012–13 season, follow dosing guidelines in the 2012 ACIP influenza vaccine recommendations.
5. Pneumococcal vaccines (pneumococcal conjugate vaccine [PCV] and pneumococcal polysaccharide vaccine [PPSV]).
- A single dose of PCV may be administered to children aged 6 through 18 years who have anatomic/functional asplenia, HIV infection or other immunocompromising condition, cochlear implant, or cerebral spinal fluid leak. See MMWR 2010:59(No. RR-11), available at http://www.cdc.gov/mmwr/pdf/rr/rr5911.pdf.
- Administer PPSV at least 8 weeks after the last dose of PCV to children aged 2 years or older with certain underlying medical conditions, including a cochlear implant. A single revaccination should be administered after 5 years to children with anatomic/functional asplenia or an immunocompromising condition.
6. Hepatitis A (HepA) vaccine.
- HepA vaccine is recommended for children older than 23 months who live in areas where vaccination programs target older children, who are at increased risk for infection, or for whom immunity against hepatitis A virus infection is desired. See MMWR 2006;55(No. RR-7), available at http://www.cdc.gov/mmwr/pdf/rr/rr5507.pdf.
- Administer 2 doses at least 6 months apart to unvaccinated persons.
7. Hepatitis B (HepB) vaccine.
- Administer the 3-dose series to those not previously vaccinated.
- For those with incomplete vaccination, follow the catch-up recommendations (Figure 3).
- A 2-dose series (doses separated by at least 4 months) of adult formulation Recombivax HB is licensed for use in children aged 11 through 15 years.
8. Inactivated poliovirus vaccine (IPV).
- The final dose in the series should be administered at least 6 months after the previous dose.
- If both OPV and IPV were administered as part of a series, a total of 4 doses should be administered, regardless of the child's current age.
- IPV is not routinely recommended for U.S. residents aged18 years or older.
9. Measles, mumps, and rubella (MMR) vaccine.
- The minimum interval between the 2 doses of MMR vaccine is 4 weeks.
10. Varicella (VAR) vaccine.
- For persons without evidence of immunity (see MMWR 2007;56[No. RR-4], available at http://www.cdc.gov/mmwr/pdf/rr/rr5604.pdf), administer 2 doses if not previously vaccinated or the second dose if only 1 dose has been administered.
- For persons aged 7 through 12 years, the recommended minimum interval between doses is 3 months. However, if the second dose was administered at least 4 weeks after the first dose, it can be accepted as valid.
- For persons aged 13 years and older, the minimum interval between doses is 4 weeks.
This schedule is approved by the Advisory Committee on Immunization Practices (http://www.cdc.gov/vaccines/recs/acip),
the American Academy of Pediatrics (http://www.aap.org), and the American Academy of Family Physicians (http://www.aafp.org).
FIGURE 3. Catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind — United States, 2012
The figure below provides catch-up schedules and minimum intervals between doses for children whose vaccinations have been delayed. A vaccine series does not need to be restarted, regardless of the time that has elapsed between doses. Use the section appropriate for the child's age. Always use this table in conjunction with the accompanying childhood and adolescent immunization schedules (Figures 1 and 2) and their respective footnotes.
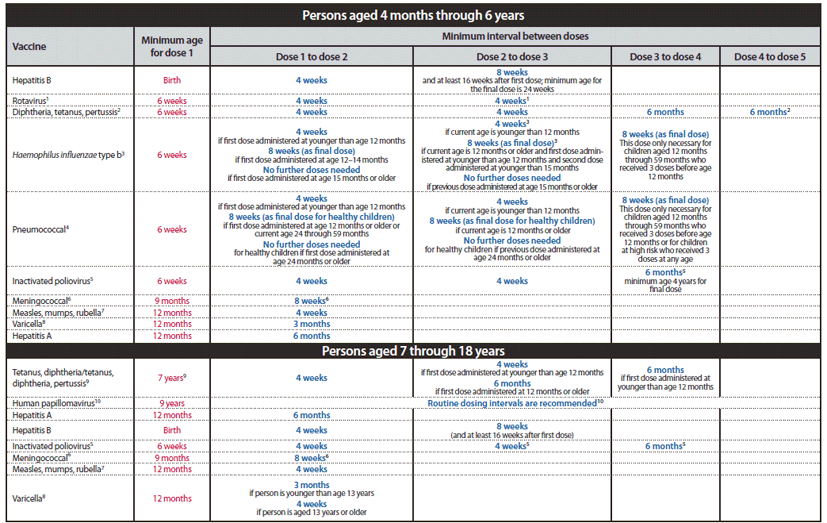
Alternate Text: The figure above shows the 2012 catch-up immunization schedule for persons aged 4 months through 18 years in the United States who start late or who are more than 1 month behind. This figure provides catch-up schedules and minimum intervals between doses for children whose vaccinations have been delayed. A vaccine series does not need to be restarted, regardless of the time that has elapsed between doses. Use the section appropriate for the child’s age. This figure should always be used in conjunction with the accompanying childhood and adolescent immunization schedules (Figures 1 and 2) and their respective footnotes.
1. Rotavirus (RV) vaccines (RV-1 [Rotarix] and RV-5 [Rota Teq]).
- The maximum age for the first dose in the series is 14 weeks, 6 days; and 8 months, 0 days for the final dose in the series. Vaccination should not be initiated for infants aged 15 weeks, 0 days or older.
- If RV-1 was administered for the first and second doses, a third dose is not indicated.
2. Diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine.
- The fifth dose is not necessary if the fourth dose was administered at age 4 years or older.
3. Haemophilus influenzae type b (Hib) conjugate vaccine.
- Hib vaccine should be considered for unvaccinated persons aged 5 years or older who have sickle cell disease, leukemia, human immunodeficiency virus (HIV) infection, or anatomic/functional asplenia.
- If the first 2 doses were PRP-OMP (PedvaxHIB or Comvax) and were administered at age 11 months or younger, the third (and final) dose should be administered at age 12 through 15 months and at least 8 weeks after the second dose.
- If the first dose was administered at age 7 through 11 months, administer the second dose at least 4 weeks later and a final dose at age 12 through 15 months.
4. Pneumococcal vaccines. (Minimum age: 6 weeks for pneumococcal conjugate vaccine [PCV]; 2 years for pneumococcal polysaccharide vaccine [PPSV])
- For children aged 24 through 71 months with underlying medical conditions, administer 1 dose of PCV if 3 doses of PCV were received previously, or administer 2 doses of PCV at least 8 weeks apart if fewer than 3 doses of PCV were received previously.
- A single dose of PCV may be administered to certain children aged 6 through 18 years with underlying medical conditions. See age-specific schedules for details.
- Administer PPSV to children aged 2 years or older with certain underlying medical conditions. See MMWR 2010:59(No. RR-11), available at http://www.cdc.gov/mmwr/pdf/rr/rr5911.pdf.
5. Inactivated poliovirus vaccine (IPV).
- A fourth dose is not necessary if the third dose was administered at age 4 years or older and at least 6 months after the previous dose.
- In the first 6 months of life, minimum age and minimum intervals are only recommended if the person is at risk for imminent exposure to circulating poliovirus (i.e., travel to a polio-endemic region or during an outbreak).
- IPV is not routinely recommended for U.S. residents aged 18 years or older.
6. Meningococcal conjugate vaccines, quadrivalent (MCV4). (Minimum age: 9 months for Menactra [MCV4-D]; 2 years for Menveo [MCV4-CRM])
- See Figure 1 ("Recommended immunization schedule for persons aged 0 through 6 years") and Figure 2 ("Recommended immunization schedule for persons aged 7 through 18 years") for further guidance.
7. Measles, mumps, and rubella (MMR) vaccine.
- Administer the second dose routinely at age 4 through 6 years.
8. Varicella (VAR) vaccine.
- Administer the second dose routinely at age 4 through 6 years. If the second dose was administered at least 4 weeks after the first dose, it can be accepted as valid.
9. Tetanus and diphtheria toxoids (Td) and tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccines.
- For children aged 7 through 10 years who are not fully immunized with the childhood DTaP vaccine series, Tdap vaccine should be substituted for a single dose of Td vaccine in the catch-up series; if additional doses are needed, use Td vaccine. For these children, an adolescent Tdap vaccine dose should not be given.
- An inadvertent dose of DTaP vaccine administered to children aged 7 through 10 years can count as part of the catch-up series. This dose can count as the adolescent Tdap dose, or the child can later receive a Tdap booster dose at age 11–12 years.
10. Human papillomavirus (HPV) vaccines (HPV4 [Gardasil] and HPV2 [Cervarix]).
- Administer the vaccine series to females (either HPV2 or HPV4) and males (HPV4) at age 13 through 18 years if patient is not previously vaccinated.
- Use recommended routine dosing intervals for vaccine series catch-up; see Figure 2 ("Recommended immunization schedule for persons aged 7 through 18 years").
Clinically significant adverse events that follow vaccination should be reported to the Vaccine Adverse Event Reporting System (VAERS) online (http://www.vaers.hhs.gov) or by telephone (800-822-7967). Suspected cases of vaccine-preventable diseases should be reported to the state or local health department. Additional information, including precautions and contraindications for vaccination, is available from CDC online (http://www.cdc.gov/vaccines) or by telephone (800-CDC-INFO [800-232-4636]).