|
Key Points for the Public |
|
• Over 53,000 U.S. residents die each year from colorectal cancer. • 1,900 deaths could be prevented each year for every 10% increase in colonoscopy screening. • Only 36% of men and women without health insurance are up-to-date with colorectal cancer screening. • Additional information is available at http://www.cdc.gov/vitalsigns. |
Vital Signs: Colorectal Cancer Screening Among Adults Aged 50--75 Years --- United States, 2008
CDC Vital Signs is a new series of MMWR reports that will announce the latest results for key public health indicators.
ABSTRACT
Background: Colorectal cancer (CRC) remains the second leading cause of cancer deaths in the United States and the leading cause of cancer deaths among nonsmokers. Statistical modeling indicates that, if current trends in health behaviors, screening, and treatment continue, U.S. residents can expect to see a 36% decrease in the CRC mortality rate by 2020, compared with 2000.
Methods: Every 2 years, CDC uses Behavioral Risk Factor Surveillance System data to estimate up-to-date CRC screening prevalence in the United States. Adults aged ≥50 years were considered to be up-to-date with CRC screening if they reported having a fecal occult blood test (FOBT) within the past year or lower endoscopy (i.e., sigmoidoscopy or colonoscopy) within the preceding 10 years. Prevalence was calculated for adults aged 50--75 years based on current U.S. Preventive Services Task Force recommendations.
Results: For 2008, the overall age-adjusted CRC screening prevalence for the United States was 62.9% among adult respondents aged 50--75 years, increased from 51.9% in 2002. Among the lowest screening prevalences were those reported by persons aged 50--59 years (53.9%), Hispanics (49.8%), persons with lower income (47.6%), those with less than a high school education (46.1%), and those without health insurance (35.6%).
Conclusions: CRC screening rates continue to increase in the United States. Underscreening persists for certain racial/ethnic groups, lower socioeconomic groups, and the uninsured.
Implications for Public Health Practice: Health reform is anticipated to reduce financial barriers to CRC screening, but many factors influence CRC screening. The public health and medical communities should use methods, including client and provider reminders, to ensure test completion and receipt of follow-up care. Public health surveillance should be expanded and communication efforts enhanced to help the public understand the benefits of CRC screening.
Despite recent declines in both incidence and mortality, colorectal cancer (CRC) remains the second most common cause of cancer deaths after lung cancer in the United States (1) and the leading cause of cancer deaths among nonsmokers. In 2006 (the most recent data available), 139,127 people were diagnosed with colorectal cancer, and 53,196 people died (1). Screening for colorectal cancer is effective in reducing incidence and mortality by removal of premalignant polyps and through early detection and treatment of cancer (2). CRC screening prevalence has improved over the past decade (3); however, in 2006, approximately 30% of eligible U.S. residents had never been screened for CRC (3). This Vital Signs report updates screening prevalence in the United States using data from the 2008 Behavioral Risk Factor Surveillance System (BRFSS) survey for persons aged 50--75 years, based on recommendations for up-to-date CRC screening from the U.S. Preventive Services Task Force (USPSTF) (4).
Methods
BRFSS is a state-based, random-digit dialed telephone survey of the civilian, noninstitutionalized adult population that collects information on health risk behaviors, preventive health practices, and health-care access in the United States (5). Every 2 years (in even numbered years), respondents aged ≥50 years are asked whether they have ever used a "special kit at home to determine whether the stool contains blood (fecal occult blood test [FOBT])," whether they have ever had a "tube inserted into the rectum to view the colon for signs of cancer or other health problems (sigmoidoscopy or colonoscopy)," and when these tests were last performed. CDC calculated the prevalence of adults who reported having had an FOBT within the past year or lower endoscopy (i.e., sigmoidoscopy or colonoscopy) within the preceding 10 years, as was done in previous reports (3). Based on the U.S. Preventive Services Task Force recommended screening age, this analysis was restricted to persons aged 50--75 years (4). Data were aggregated across all 50 states and the District of Columbia. Respondents who refused to answer, had a missing answer, or who answered "don't know/not sure" were excluded from analysis of the question.
The median Council of American Survey and Research Organizations (CASRO) response rate was 53.3%, and the median CASRO cooperation rate was 75.0% (5). Data were weighted to the age, sex, and racial/ethnic distribution of each state's adult population using intercensal estimates and were age-standardized to the 2008 BRFSS population.
Results
The 2008 BRFSS survey was administered to 414,509 respondents, of whom 201,157 were aged 50--75 years. The overall, age-adjusted combined up-to-date CRC screening (FOBT and lower endoscopy) prevalence for the United States was 62.9% among adult respondents aged 50--75 years (Table). Among the lowest screening prevalences were those reported by persons aged 50--59 years (53.9%), Hispanics (49.8%), persons with lower income (47.6%), those with less than a high school education (46.1%), and those without health insurance (35.6%). Similar patterns were noted for FOBT in the preceding year and for lower endoscopy in the preceding 10 years. The percentage of persons up-to-date with CRC screening ranged from 53.2% in Oklahoma to 74.1% in Massachusetts (Figure 1). States with the highest screening prevalence were concentrated in the northeastern United States. CRC screening increased from 51.9% in 2002 to 62.9% in 2008 (Figure 2). During that period, use of endoscopy increased, while FOBT use declined from 20.9% of CRC screening in 2002 to 14.1% in 2008.
Conclusions and Comment
The results in this Vital Signs report indicate that the prevalence of up-to-date CRC screening in the United States is continuing to increase. An increase (from 38% in 2000 to 53% in 2008) also has been reported using National Health Interview Survey data (6). However, in 2008, certain populations in the United States remained underscreened, including those with lower socioeconomic status, Hispanics, and those without health insurance. Multiple factors might explain these differences, including patient education and income, as well as provider and clinical systems factors. As in previous surveys, the 2008 survey indicated notable geographic differences in CRC screening prevalence. The reasons for these geographic differences remain unknown, but screening capacity, lack of physician availability, and patient factors including income, education, and lack of awareness have been proposed as reasons (6).
CRC screening rates continue to increase in the United States. Additional improvements in screening prevalence might have substantive impact on CRC mortality. Statistical modeling indicates that, if current trends in health behaviors, screening, and treatment continue, U.S. residents can expect to see a 36% decrease in the CRC mortality rate by 2020, compared with 2000 (7).
Insufficient evidence exists to recommend "one best" test for CRC screening. Several proven, effective tests exist and are recommended by USPSTF, including annual FOBT, sigmoidoscopy every 5 years, and colonoscopy every 10 years (4). In addition to maximizing prevalence of CRC screening to reduce morbidity and mortality, ensuring proper follow-up of abnormal results is important to maximize the benefits of screening (4).
The findings in this report are subject to at least three limitations. First, because BRFSS is a telephone survey of residential households, only adults in households with landline telephones are represented; therefore, the results might not be representative of the U.S. population. Evidence suggests that adults living in wireless-only households tend to be younger and have lower incomes, and are more likely to be members of minority populations, which might result in either underestimates or overestimates. Second, responses are self-reported and not confirmed by review of medical records. Finally, the survey response rate was low, which increases the risk for response bias.
Policy changes in the Patient Protection and Affordable Care Act are expected to remove financial barriers to CRC screening by expanding insurance coverage and eliminating cost sharing in Medicare and private plans, but additional barriers remain (8). Evidence-based, systems-change interventions, including client and provider reminders to ensure test completion and receipt of follow-up care, have been shown by the Guide to Community Preventive Services* to increase CRC screening; however, these approaches have not been widely adopted in clinical practice. Physician recommendation remains an important but underutilized facilitator of CRC screening. Improving cancer screening benchmarks in clinical practice should be a high priority for new patient-care improvement models such as the patient-centered medical home (9). Case management approaches such as patient navigation models to maximize patient participation and ensure adequate follow-up also appear promising (10). Utah has used multiple approaches to improve its CRC screening prevalence. Reported use of CRC endoscopy increased from 32.1% in 1999 to 51.9% in 2005 through the use of small media (e.g., videos, letters, brochures, and flyers) and large media campaigns and by providing CRC screening tests (mainly FOBT) for those who could not afford it.†
CDC's CRC screening program, funded in 2009, places emphasis on population-based approaches to increase CRC screening.§ The program is based on the recommendations of the Guide to Community Preventive Services, which has identified evidence-based interventions to increase cancer screening in communities by targeting providers and the general population. Full implementation of these recommendations, including a focus on reaching disadvantaged populations, can achieve the goal of more complete population coverage.
Surveillance of cancer screening and diagnostic activities currently is limited to population surveys and is only collected every other year by BRFSS. Additional surveillance efforts might guide population-based outreach, identify and target unscreened populations, and ensure adequate follow-up (10). CDC and state and local health departments should develop and monitor centralized population-based registries of persons eligible for screening, provide appropriate outreach, and ensure adequate follow-up. These registries could be developed to track and promote screening awareness and subsequent utilization through communication media (e.g., telephone, mail, or electronic reminders) or use of peer outreach. Registries of underserved populations, including Medicaid enrollees and those without a regular provider, could be used to promote screening among persons in vulnerable populations at greater risk.
Reported by
LC Richardson, MD, SH Rim, MPH, M Plescia, MD; Div of Cancer Prevention and Control, National Center Chronic Disease Prevention and Health Promotion, CDC.
References
- US Cancer Statistics Working Group. United States cancer statistics: 1999--2006 incidence and mortality web-based report. Atlanta, GA: US Department of Health and Human Services, CDC, and National Cancer Institute; 2010. Available at http://www.cdc.gov/uscs. Accessed June 23, 2010.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977--81.
- CDC. Use of colorectal cancer tests---United States, 2002, 2004, and 2006. MMWR 2008;57:253--8.
- US Preventive Services Task Force. Screening for colorectal cancer. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at http://www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed June 20, 2010.
- CDC. Behavioral Risk Factor Surveillance System. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. Available at http://www.cdc.gov/brfss. Accessed June 20, 2010.
- American Cancer Society. Cancer prevention and early detection facts and figures 2010. Atlanta, GA: American Cancer Society; 2010. Available at http://www.cancer.org/research/cancerfactsfigures/cancerpreventionearlydetectionfactsfigures/index. Accessed June 20, 2010.
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975--2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544--73.
- The Patient Protection and Affordable Care Act. Pub. L. No. 111-148. Available at http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills&docid=f:h3590enr.txt.pdf. Accessed June 20, 2010.
- Wender RC, Altshuler M. Can the medical home reduce cancer morbidity and mortality? Prim Care 2009;36:845--58.
- New York City Department of Health and Mental Hygiene. A practical guide to increasing screening colonoscopy: proven methods for health care facilities to prevent colorectal cancer deaths. New York, NY: New York City Department of Health and Mental Hygiene; 2006. Available at http://www.nyc.gov/html/doh/downloads/pdf/cancer/cancer-colonoscopy-guide.pdf. Accessed June 20, 2010.
* Additional information available at http://www.thecommunityguide.org/index.html.
† Additional information available at http://health.utah.gov/ucan/partners/pub/pdfs/utahcancerplan080206.pdf.
§ Available at http://www.cdc.gov/cancer/crccp.
FIGURE 1. Percentage of respondents aged 50--75 years who reported receiving a fecal occult blood test (FOBT) within 1 year or a lower endoscopy* within 10 years, by state --- Behavioral Risk Factor Surveillance System (BRFSS), United States, 2008†
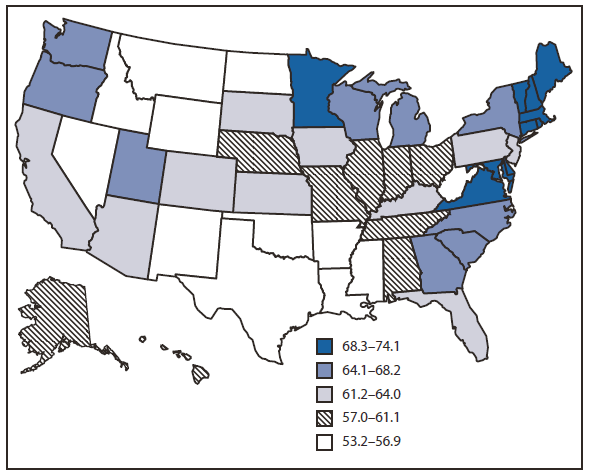
* Sigmoidoscopy or colonoscopy.
† Percentages standardized to the age distribution in the 2008 BRFSS survey.
Alternate Text: The figure above is a U.S. map showing the percentage of respondents aged 50–75 years who reported receiving a fecal occult blood test (FOBT) within 1 year or a lower endoscopy (sigmoidoscopy or colonoscopy) within 10 years, by state, according to the 2008 Behavioral Risk Factor Surveillance System survey. The percentage of persons up-to-date with CRC screening ranged from 53.2% in Oklahoma to 74.1% in Massachusetts. States with the highest screening prevalence were concentrated in the north¬eastern United States
FIGURE 2. Percentage of respondents aged 50--75 years who reported receiving a fecal occult blood test (FOBT) within 1 year or a lower endoscopy* within 10 years --- Behavioral Risk Factor Surveillance System (BRFSS), United States, 2002, 2004, 2006, and 2008†
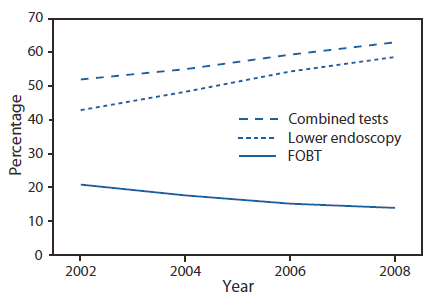
* Sigmoidoscopy or colonoscopy.
†Percentages standardized to the age distribution in the 2008 BRFSS survey.
Alternate Text: The figure above is a line graph showing the percentage of U.S. respondents aged 50–75 years who reported receiving a fecal occult blood test (FOBT) within 1 year or a lower endoscopy (sigmoidoscopy or colonoscopy) within 10 years, according to the Behavioral Risk Factor Surveillance System (BRFSS) surveys for 2002, 2004, 2006, and 2008. CRC screening increased from 51.9% in 2002 to 62.9% in 2008. During that period, use of endoscopy increased, while FOBT use declined from 20.9% of CRC screening in 2002 to 14.1% in 2008.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


