TB NOTES

TB Notes 1, 2023

Dear Colleague,
It has been an exciting first quarter for the Division of Tuberculosis Elimination (DTBE).
Last week, we recognized World TB Day on March 24 to commemorate the date Dr. Robert Koch announced his discovery of the bacillus that causes TB. As CDC Director Dr. Rochelle P. Walensky said in her World TB Day Letter, “While remarkable progress has been made, there is still much to do” towards TB elimination. I am inspired by the stories of CDC’s 2023 U.S. TB Elimination Champions, and want to thank them, as well as the public health and medical professionals continuing to work towards TB elimination.
On March 21st, I participated in a partner-sponsored World TB Day Congressional Briefing where I presented on domestic TB trends, the impact of the COVID-19 pandemic on TB diagnoses, and CDC’s approach to achieve TB elimination in the United States. I also met with staff from the offices of Senators John Hickenlooper (CO) and Bob Casey (PA) to highlight the important work we are doing with our partners to test and treat latent TB infection and TB disease as well as how TB programs within these states are supporting Ukrainians arriving under the Uniting for Ukraine program.
On March 23, CDC published provisional 2022 U.S. TB surveillance data. Reported TB incidence rose 5% during 2022 (2.5 cases per 100,000 persons) compared with 2021 (2.4 cases per 100,000 persons) but was lower than TB incidence in 2019 (2.7 cases per 100,000 persons). Timely diagnosis and treatment for TB disease and latent TB infection remain critical to achieving elimination.
To assist in these efforts, CDC is continuing our efforts to raise awareness of latent TB infection testing and treatment. We launched a new phase of the Think. Test. Treat TB campaign with a new website and digital resources for healthcare providers.
CDC also released new recommendations for the use of video Directly Observed Therapy (vDOT) that can help TB programs meet the U.S. standard of care for patients undergoing treatment, while using resources efficiently.
Additionally, CDC awarded $18.4 million in the second round of supplemental funding to support state and local TB programs’ efforts to screen, evaluate, and treat latent TB infection and TB disease among Ukrainians paroled into the United States under the Uniting for Ukraine program. Several TB programs have shared the impact of this funding with us, and we look forward to learning more about the great work being done to serve communities within the different jurisdictions.
These are just a few highlights from our work over the past quarter. I invite you to read further for additional news, resources, and success stories from across DTBE.
Thank you for your continued efforts towards our goal of TB elimination.
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Symptoms and Systemic Drug Reactions in Persons Receiving Weekly Rifapentine Plus Isoniazid (3HP) Treatment for Latent Tuberculosis Infection
Recently, members of the Clinical Research Branch contributed to a publication in Clinical Infectious Diseases regarding drug reactions in people receiving treatment for latent TB infection. The team analyzed data of symptoms in participants receiving three months of weekly rifapentine and isoniazid (3HP) treatment for latent TB infection in the Tuberculosis Trials Consortium iAdhere clinical trial (TBTC Study 33). This study presents a more complete picture of symptoms and systemic drug reactions in participants taking 3HP.
The team analyzed patterns of symptom development and progression throughout treatment and found that although most participants reported at least one symptom, nearly all were mild and commonly resolved after the first month of treatment without stopping treatment. They also identified participants who met the criteria for systemic drug reactions (SDR). It was found that females, individuals older than 45 years, and individuals using concomitant medications were more likely to meet SDR criteria and discontinue treatment. The results support the safety of 3HP treatment in most patients.
Providers and patients should be aware of common symptoms experienced, especially within the first month of treatment, that will likely resolve. Read the full article in Clinical Infectious Diseases.
Submitted by Claire Sadowski, MPH
Think. Test. Treat TB Campaign
Last year, CDC launched Think. Test. Treat TB, the first national communications campaign to increase testing and treatment for latent TB infection in the United States. CDC is continuing our efforts to raise awareness of latent TB infection testing and treatment, with a focus on healthcare providers.
New Think. Test. Treat TB resources and activities are available to help healthcare providers:

- Know the risk factors for latent TB infection, and recommend testing for patients at increased risk.
- Use TB blood tests (Interferon Gamma Release Assays [IGRAs]) because an IGRA can be done in one visit, and is the most accurate for patients who have been vaccinated for TB (the Bacille Calmette-Guérin, or BCG vaccine) in the past.
- Treat latent TB infection with shorter, rifamycin-based treatment regimens to prevent TB disease.
The Think. Test. Treat TB website has a new page especially for healthcare providers, and new digital resources to help share the Think. Test. Treat TB message. Access free resources in multiple languages, including TB messages, digital and print resources, social media content, patient and provider education materials, and a partner toolkit.
Submitted by John Parmer, PhD
World TB Day Activities

On World TB Day, CDC Director Dr. Rochelle Walensky released a Dear Colleague Letter that highlighted the dedication of TB programs, public health departments, and healthcare facilities to prevent and treat TB. Dr. Walensky recognized the continued work and commitment by CDC and its partners, both domestically and globally, to eliminate TB in the United States and around the world.
CDC U.S. TB Elimination Champions

The 2023 CDC U.S. TB Elimination Champions marked the 8th year of recognizing the hard work and dedication from those working to end TB in their communities. This year, CDC recognized organizations and individuals who have developed innovative strategies to continue preventing and controlling TB in the United States. Congratulations to the 2023 U.S. TB Elimination Champions:
- Amy Painter, South Carolina Department of Health and Environmental Control
- Breathe Easy South Texas (B.E.S.T) TB Elimination Project – San Antonio, Texas
- Breathe Pennsylvania
- Dr. Amy Shen Tang, Director of Immigrant Health, and North East Medical Services (NEMS)
- Dr. Sanchi Malhotra and the TB Infection Team of Children’s Hospital Los Angeles
- Elizabeth Reyes, Coordinator, San Carlos Barromeo
- Erie County Department of Health Tuberculosis Program Staff
- Garfield County Health Department, Oklahoma
- Guam Department of Public Health and Social Services and Todu Guam Foundation (TGF)
- Los Angeles County Refugee Health Program
- Maria Gomez, Florida Department of Health
- Maureen Murphy-Weiss and Luke Celebrezze, Columbus Public Health Department
- New York City (NYC) Bureau of Tuberculosis Control Uniting for Ukraine Work Group
- New York City (NYC) 4 HPMZ Work Group
- Pat Iyer, Massachusetts Department of Public Health
- Tamrin Goldberg with the North American Touring Company of Moulin Rouge! The Musical
- Texas Center for Infectious Disease Hospital inpatient nurses
- Tuberculosis Control Branch, Multidrug-Resistant (MDR) TB Service Team
- Xuan Man, Tacoma-Pierce County Health Department
World TB Day Events Timeline
CDC featured World TB Day activities happening across the country through the 2023 World TB Day Events Timeline. Thank you to everyone who shared a World TB Day activity – there were 19 different events featured on the website!
Submitted by Kevin Crooks, MPH
Mantoux Tuberculin Test Training Video

The new Mantoux Tuberculin Skin Test training video is now available online. The Mantoux Tuberculin Skin Test video is a 27-minute training video that provides step-by-step instructions to healthcare professionals on how to administer the TB skin test and read the results. The video is posted on CDC’s website as well as CDC’s YouTube channel.
This video is part of a set of resources, including a wall chart and a ruler, that can help healthcare professionals administer and read the Mantoux tuberculin skin test. The Mantoux Tuberculin Skin Test training video is available for download from CDC’s website. A DVD version is also available to order for free on the CDC Info on Demand website.
Submitted by Coralis Rodriguez Morales, MPH, and Peri Hopkins, MPH
TB Centers of Excellence for Training, Education and Medical Consultation Summit

In November 2022, DTBE selected four TB Centers of Excellence for Training, Education, and Medical Consultation (TB COEs) for the new project period 2023-2027. The TB COEs are regionally assigned to cover all 50 states and the U.S. territories. They support domestic TB control and prevention efforts with a focus on two major activities: increasing knowledge, skills, and abilities for TB prevention and control through communication, education, and training activities, and improving sustainable evidence-based TB clinical practices and patient care through the provision of expert medical consultation.
The four TB COEs are:
- Curry International Tuberculosis Center
- Global Tuberculosis Institute at Rutgers, The State University of New Jersey
- Mayo Clinic Center for Tuberculosis (MCCT)
- Southeastern National Tuberculosis Center
To kick off the new project period, DTBE held a TB COE Summit from February 6-10, 2023, at the CDC Global Communication Center in Atlanta, Georgia. Key staff members from each TB COE met to discuss administrative issues, annual requirements of the cooperative agreement, and 2023 goals and priorities for training, education, and medical consultation. At the summit, CDC debuted the new medical consultation database system, IDCrowd. Throughout the meeting, COEs shared lessons learned and brainstormed ways to better meet the needs of their regions. They also had the opportunity to hear about upcoming TB events and guidelines, learn about major DTBE initiatives, meet with the field services project officers and laboratory consultants, and hear from key partners, including Global TB Consilium, CDC’s Division of Global Migration and Quarantine (DGMQ), and Office of Refugee Resettlement (ORR). Visit the TB COE webpage to learn more about the centers.
Submitted by Acklynn Byamugisha, MPH, MAIA and Allison Maiuri, MPH
CDC Releases Recommendations for the Use of Video Directly Observed Therapy (vDOT)

On March 23, 2023, CDC released recommendations in Morbidity and Mortality Weekly Report for the use of video directly observed therapy (vDOT) during TB treatment in the United States. CDC recommends vDOT as an equivalent alternative to in-person DOT for people undergoing treatment for diagnosed TB.
The recommendations are based on evidence from published systematic reviews, a published meta-analysis, and a literature search through 2022 that demonstrate that vDOT is associated with a higher proportion of medication doses being observed and similar proportions of treatment completion and microbiologic resolution when compared with in-person DOT.
vDOT is a type of electronic DOT that uses a video-enabled device (e.g., smart phone, tablet, computer) to facilitate the observation of medicine ingestion through a remote interaction between patients and health care workers. vDOT can be conducted through real-time (synchronous) or recorded (asynchronous) videos.
vDOT can help people diagnosed with TB complete treatment. vDOT can also assist health department TB programs meet the U.S. standard of care for patients undergoing tuberculosis treatment, while using resources efficiently.
To find supplementary information that addresses additional considerations, concerns, and limitations of vDOT, please visit the CDC website.
The State TB Control Offices and TB Centers of Excellence for Training, Education, and Medical Consultation can provide additional assistance and support in treating people with diagnosed TB.
Submitted by Carissa Sera-Josef, MS
Provisional TB Surveillance Data for 2022
On March 23, 2023, DTBE published provisional 2022 U.S. TB data in Morbidity and Mortality Weekly Report.
Compared with 2020 and 2021, U.S. TB incidence increased during 2022 but remained lower than during prepandemic years. After a substantial decline in 2020 and partial rebound in 2021, incidence appears to be returning to the pre-pandemic trajectory among U.S.-born and non–U.S.-born populations.
TB epidemiology in 2022 was characterized by more cases among persons newly arrived in the United States, higher TB incidence among American Indian or Alaska Native and Native Hawaiian/other Pacific Islander persons and persons aged less than 4 and 15–24 years, and slightly lower incidence among persons over 65 years old. TB disparities persist and addressing these disparities requires timely TB diagnosis and treatment to interrupt transmission, as well as prevention of TB through treatment of latent TB infection.
Submitted by Kimberly Schildknecht, RN, MPH and Julie Self, PhD
2021 State and City Tuberculosis Report
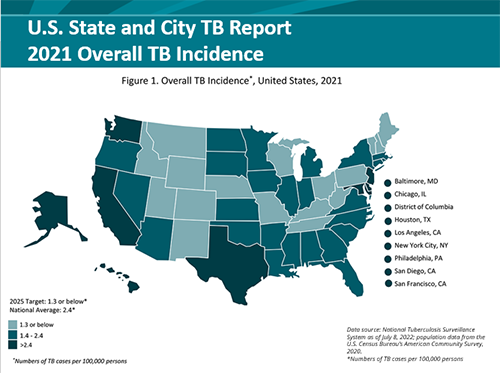
The State and City Tuberculosis Report provides key process and outcomes measures that are used to monitor progress towards TB elimination in the United States. Data for calculating these performance measures are derived from the National Tuberculosis Surveillance System (NTSS) and the Aggregate Reports for Program Evaluation (ARPE). The 2021 report contains a new section on Sputum Culture Conversion (a patient had a positive sputum culture before being treated for TB disease but after starting treatment their sputum culture became negative). This conversion from positive to negative is the best way to measure how well a patient is responding to treatment and can help to:
- Detect failure to respond to therapy or early warning signs of relapse of TB disease
- Monitor patients with multidrug-resistant TB disease to ensure they continue to respond to therapy
- Determine length of treatment (the longer it takes to convert, the longer the length of treatment)
- Document that the patient no longer has TB disease at the end of therapy
For more information, visit the 2021 State and City TB Report.
Submitted by Rachel Woodruff, MPH, and Ashley Rodriguez
Diagnosis, Treatment, and Prevention of TB Among Persons Experiencing Homelessness in the United States
On January 27, 2023, Public Health Reports released “Diagnosis, Treatment, and Prevention of Tuberculosis Among People Experiencing Homelessness in the United States.”
TB is a public health problem, especially among people experiencing homelessness (PEH). The Advisory Council for the Elimination of Tuberculosis issued recommendations in 1992 for TB prevention and control among PEH. The goal of the recent publication is to provide current guidelines and information in one place to inform medical and public health providers and TB programs on TB incidence, diagnosis, and treatment among PEH.
The current U.S. guidelines and recommendations relevant to TB diagnosis, treatment, and prevention call for health care providers serving PEH to:
- Assess the magnitude of homelessness in their jurisdictions
- Test PEH and shelter staff for TB disease and latent TB infection and treat those diagnosed with TB using short-course treatment regimens
- Identify TB among PEH by using TB diagnostics that can detect TB within 24 to 48 hours
- Immediately report possible TB among PEH to the local public health department
- Screen routinely for HIV among all PEH who begin treatment for TB
- Provide temporary housing and link PEH to permanent housing opportunities
- Perform location-based contact investigations with PEH who are infected with TB
This publication found that direct observation resulted in increased adherence to treatment among PEH, as did involvement of social workers in the provision of housing, food, safety, and comorbidity treatment, especially for HIV and substance abuse disorders. Testing and treating latent TB infection helps prevent the progression of latent TB infection to TB disease and shorter latent TB infection regimens improve adherence. By following existing recommendations and using client-centered approaches, providers serving PEH can improve TB diagnosis, treatment, and prevention among PEH.
To eliminate TB in the United States, we must diagnose, treat, and prevent TB, especially among groups at increased risk of TB. Timely diagnoses of TB save lives and prevent spread in our communities.
Submitted by Suzanne Marks, MPH, MA
Service Enhancements for CDC’s TB Molecular Detection of Drug Resistance (MDDR) Service
The Division of Tuberculosis Elimination Laboratory Branch has implemented a new targeted next generation sequencing (tNGS) assay as the primary method for CDC’s Molecular Detection of Drug Resistance (MDDR) service. The new assay enhances early detection of mutations associated with drug resistance and allows for examination of genetic loci associated with resistance to rifampin, isoniazid, ethambutol, pyrazinamide, fluoroquinolones, second-line injectables, clofazimine, linezolid, and bedaquiline. Growth-based drug susceptibility testing will also be performed concurrently but will not yet include testing for clofazimine, linezolid, and bedaquiline. Turnaround time for molecular results is 7-to-10-days; turnaround time for growth-based susceptibility test results is 35 days.
Additionally, as part of the service enhancements, reporting of tNGS results will be sent through CDC’s laboratory information management system (LIMS) with reports going primarily to state public health laboratories. This is a change in that MDDR reports will no longer be provided via secure fax. The report format has been simplified to aid review of results. Public health laboratory submitters are encouraged to explore use of CDC’s Specimen Test Order and Reporting (CSTOR) web portal.
The MDDR User’s Guide provides additional details regarding the tNGS assay and MDDR service with instructions for sample submission including the use of the MDDR Request Form.
An informational webinar was hosted to discuss these changes to the MDDR Service: Service Enhancements for CDCs TB Molecular Detection of Drug Resistance Service. The webinar included an overview of the MDDR Service, described the new tNGS assay, provided a description of the bioinformatic pipeline and outputs, reviewed the new laboratory report format and reporting through CDC’s LIMS, reviewed specimen acceptance criteria and sample submission information, and provided information about the CSTOR portal.
For any questions regarding this new tNGS assay, please contact tblab@cdc.gov.
Submitted by Angela Starks, PhD and Monica Youngblood, MPH, M(ASCP)
27th Annual Conference of the Union-North America Region (NAR)
The annual NAR Conference was held from February 22 – 25, 2023, at the Sheraton Wall Centre Hotel in Vancouver, BC, Canada. DTBE staff members presented and participated in a variety of forums during the conference, including:
- Glenn Morlock: Determination of Minimal Inhibitory Concentrations (MICs) of drug-resistant and susceptible Mycobacterium tuberculosis isolates to the novel antibiotic Teixobactin
- Kimberly Schildknecht, Molly Deutsch-Feldman, Divia Forbes MPH2, Maryam Haddad PhD, Jonathan Wortham: Tuberculosis and End-Stage Renal Disease, United States, 2010–2019

Wenming Zhu, Morlock, G.P, Burns, S., Cowan L., Posey, J. E.: Determination of MICs for Rifampin (RIF), Rifabutin (RFB) and Rifapentine (RFP) in Mycobacterium tuberculosis strains possessing various missense mutations in rpoΒ
- Mari Galvis: Challenges to Program Implementation of the Four-Month Rifapentine-Moxifloxacin Regimen for the treatment of Drug-Susceptible Tuberculosis in New York City
- Elise Caruso: Evaluation of the Think. Test. Treat TB National Latent Tuberculosis Infection Communications Campaign
- Joan Mangan: Community Engagement to Build Trust and Transform Health Systems and presented the Lifetime Achievement Award to Dr. Max Salfinger
- Carla Winston: Updates on CDC Recommendations for Tuberculosis Treatment
- Thomas (Dan) Filardo: Epidemiology and Clinical Characteristics of People with Ocular Tuberculosis in the United States, 1993‒2019
Oeltmann J, Vohra D, Matulewicz HH, DeLuca N, Smith JP, Couzens C, Lash RR, Harvey B, Boyette M, Edwards A, Talboy PM, Dubose O, Regan P, Loosier P, Caruso E, Katz DJ, Taylor MM, Moonan PK. Isolation and quarantine for COVID-19 in the United States, 2020–2022, Clin Infect Dis 2023.
DeLuca N, Caruso E, Gupta R, Kemmerer C, Coughlin R, Chan O, Vohra D, Oeltmann J, Taylor M, Moonan P, Thorpe P, Loosier P, Haile G, CDC COVID-19 Case Investigation and Contact Tracing Task Force. Experiences with COVID-19 case investigation and contact tracing: A qualitative analysis, June 2023. SSM – Qualitative Research in Health.
Li R, Deutsch-Feldman M, Adams T, Law M, Biak C, Pitcher E, Drees M, Hernandez-Romieu AC, Filardo TD, Cropper T, Martinez A, Wilson WW, Althomsons SP, Morris SB, Wortham JM, Benowitz I, Schwartz NG, White K, Haddad MB, Glowicz JB; Bone Allograft Tuberculosis Investigators. Transmission of Mycobacterium tuberculosis to healthcare personnel resulting from contaminated bone graft material, United States, June 2021–August 2022. Clin Infect Dis 2023. Epub ahead of print.
Marks SM, Self JL, Venkatappa T, Wolff MB, Hopkins PB, Augustine RJ, Khan A, Schwartz NG, Schmit KM, Morris SB. Diagnosis, treatment, and prevention of tuberculosis among people experiencing homelessness in the United States: current recommendations. Public Health Rep 2023. Epub ahead of print.
Woodruff RC, Garg S, George MG, Patel K, Jackson SL, Loustalot F, Wortham JM, Taylor CA, Whitaker M, Reingold A, Alden NB, Meek J, Anderson EJ, Weigel A, Henderson J, Bye E, Davis SS, Barney G, Bennett NM, Shiltz E, Sutton M, Talbot HK, Price A, Sperling LS, Havers FP; COVID-19-Associated Hospitalization Surveillance Network. Acute cardiac events during COVID-19–associated hospitalizations. J Am Coll Cardiol 2023;81(6):557–69.
Tsang CA, Patel NN, Stout JE, Fernando R, Pratt R, Goswami ND. Factors associated with receiving longer than recommended therapy among culture-negative pulmonary tuberculosis patients. Open Forum Infect Dis 2022;9(12):ofac630.
Chuey MR, Stewart RJ, Walters M, Curren EJ, Hills SL; COVID-19 Miramar Response Team Working Group, Moser KS, Staples JE, Braden CR, McDonald E. COVID-19 case investigations among federally quarantined evacuees from Wuhan, China and exposed personnel at a US military base, United States, February 5–21, 2020. Public Health Rep 2022;137(2):203–7.
Thomas EV, Jennings MA, Kidder DP, Fechter-Leggett ED, Bautista GJ, Johns MM; Ally Training Committee. Development and evaluation of the Ally Sexual and Gender Minority Diversity and Inclusion Training at the Centers for Disease Control and Prevention. J Public Health Manag Pract 2023;29(1):56–63.
Walker WL, Schmit KM, Welch EC, Vonnahme LA, Talwar A, Nguyen M, Stojanovic D, Langer AJ, Cocoros NM. Using the Food and Drug Administration´s Sentinel System for surveillance of TB infection. Int J Tuberc Lung Dis 2022;26(12):1170–6.
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email Kevin Crooks at qyd7@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates through Adobe Campaign.