Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Malaria Surveillance — United States, 2011
Karen A. Cullen, PhD
Paul M. Arguin, MD
Division of Parasitic Diseases and Malaria, Center for Global Health, CDC
Corresponding author: Karen Cullen, PhD, Division of Parasitic Diseases, Center for Global Health, CDC. Telephone: 404-718-4702; E-mail: kcullen@cdc.gov.
Abstract
Problem/Condition: Malaria in humans is caused by intraerythrocytic protozoa of the genus Plasmodium. These parasites are transmitted by the bite of an infective female Anopheles mosquito. The majority of malaria infections in the United States occur among persons who have traveled to regions with ongoing malaria transmission. However, malaria is also occasionally acquired by persons who have not traveled out of the country, through exposure to infected blood products, congenital transmission, laboratory exposure, or local mosquitoborne transmission. Malaria surveillance in the United States is conducted to identify episodes of local transmission and to guide prevention recommendations for travelers.
Period Covered: This report summarizes cases in persons with onset of illness in 2011 and summarizes trends during previous years.
Description of System: Malaria cases diagnosed by blood film, polymerase chain reaction, or rapid diagnostic tests are mandated to be reported to local and state health departments by health-care providers or laboratory staff. Case investigations are conducted by local and state health departments, and reports are transmitted to CDC through the National Malaria Surveillance System, National Notifiable Diseases Surveillance System, or direct CDC consults. Data from these reporting systems serve as the basis for this report.
Results: CDC received 1,925 reported cases of malaria with an onset of symptoms in 2011 among persons in the United States, including 1,920 cases classified as imported, one laboratory-acquired case, one transfusion-related case, two congenital cases, and one cryptic case. The total number of cases represents an increase of 14% from the 1,691 cases reported for 2010 and the largest number of reported cases since 1971. Plasmodium falciparum, P. vivax, P. malariae, and P. ovale were identified in 49%, 22%, 3%, and 3% of cases, respectively. Twenty-one (1%) patients were infected by two species. The infecting species was unreported or undetermined in 23% of cases, an increase of 5 percentage points from 2010. Of the 871 patients who reported purpose of travel, 607 (70%) were visiting friends or relatives (VFR). Among the 929 cases in U.S. civilians for whom information on chemoprophylaxis use and travel region was known, 57 (6%) patients reported that they had followed and adhered to a chemoprophylactic drug regimen recommended by CDC for the regions to which they had traveled. Thirty-seven cases were reported in pregnant women, among whom only one adhered to chemoprophylaxis. Among all reported cases, significantly more cases (n=275 [14%]) were classified as severe infections in 2011 compared with 2010 (n=183 [11%]; p=0.0018; chi square). Five persons with malaria died in 2011. After 2 years of improvement in completion of data elements on the malaria case form, higher percentages of incomplete data in 2011 for residential status (from 11% in 2010 to 19% in 2011) and species (from 18% in 2010 to 22% in 2011) were noted.
Interpretation: The number of cases reported in 2011 marked the largest number of cases since 1971 (N = 3,180). Despite progress in reducing the global burden of malaria, the disease remains endemic in many regions, and the use of appropriate prevention measures by travelers is still inadequate.
Public Health Actions: Completion of data elements on the malaria case report form decreased in 2011 compared with 2010. This incomplete reporting compromises efforts to examine trends in malaria cases and prevent infections. VFR travelers continue to be a difficult population to reach with effective malaria prevention strategies. Evidence-based prevention strategies that effectively target VFR travelers need to be developed and implemented to have a substantial impact on the numbers of imported malaria cases in the United States. Although more persons with cases reported taking chemoprophylaxis to prevent malaria, the majority reported not taking it, and adherence was poor among those who did take chemoprophylaxis. Proper use of malaria chemoprophylaxis will prevent the majority of malaria illness and reduce the risk for severe disease (http://www.cdc.gov/malaria/travelers/drugs.html). Malaria infections can be fatal if not diagnosed and treated promptly with antimalarial medications appropriate for the patient's age and medical history, the likely country of malaria acquisition, and previous use of antimalarial chemoprophylaxis. Clinicians should consult the CDC Guidelines for Treatment of Malaria and contact the CDC's Malaria Hotline for case management advice, when needed. Malaria treatment recommendations can be obtained online (http://www.cdc.gov/malaria/diagnosis_treatment) or by calling the Malaria Hotline (770-488-7788 or toll-free at 855-856-4713).
Introduction
Malaria in humans is caused by infection with one or more of several species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, P. malariae, and occasionally other Plasmodium species). The infection is transmitted by the bite of an infective female Anopheles mosquito. P. falciparum and P. vivax species cause the most infections worldwide. P. falciparum is the agent that most commonly causes severe and potentially fatal malaria (see Definitions). According to the most recently available information, an estimated 219 million clinical cases and 660,000 deaths were reported worldwide in 2010, mostly among children aged <5 years living in sub-Saharan Africa (1). P. vivax and P. ovale have dormant liver stages, which can reactivate and cause malaria several months or years after the initial infection. P. malariae can result in long-lasting infections and, if untreated, can persist asymptomatically in the human host for years, even a lifetime (1). Approximately half of the world's population live in regions where malaria is transmitted (i.e., approximately 100 countries in parts of Africa, Asia, the Middle East, Eastern Europe, Central and South America, Caribbean, and Oceania). Before the 1950s, malaria was endemic throughout the southeastern United States; an estimated 600,000 cases occurred in 1914 (2). During the late 1940s, a combination of improved housing and socioeconomic conditions, environmental management, vector-control efforts, and case management was successful at interrupting malaria transmission in the United States (3).* Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate instances of local transmission, to monitor patterns of resistance to antimalarial drugs, and to guide malaria prevention recommendations for international travelers. Malaria vector mosquitoes are still present in the United States.
The majority of reported malaria cases diagnosed each year in the United States are imported from regions where mosquitoborne malaria transmission is known to occur, although congenital infections and infections resulting from exposure to blood or blood products also are reported in the United States. In addition, rare cases of local mosquitoborne transmission have been reported (4). State and local health departments and CDC investigate reported malaria cases in the United States, and CDC analyzes data from imported cases to detect trends in acquisition.
The signs and symptoms of malaria illness are varied, but the majority of patients have fever. Other common symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. A diagnosis of malaria should always be considered for persons with these symptoms who have traveled to an area with known malaria transmission. Malaria also should be considered in the differential diagnosis of persons who have fever of unknown origin, regardless of their travel history. Untreated infections can rapidly progress to coma, renal failure, respiratory distress, and death. This report summarizes malaria cases reported to CDC among persons with onset of symptoms in 2011.
Methods
Data Sources
Malaria case data are reported to the National Malaria Surveillance System (NMSS) and the National Notifiable Diseases Surveillance System (NNDSS) (5). Although both systems rely on passive reporting, the numbers of reported cases might differ because of differences in collection and transmission of data. A substantial difference between the data collected in these two systems is that NMSS receives more detailed clinical and epidemiologic data regarding each case (e.g., information concerning the area to/from which the infected person has traveled). Malaria cases can be reported to CDC through either NMSS or NNDSS or through a direct consultation with CDC malaria staff; therefore, cases reported through these various paths are compared, deduplicated, compiled, and analyzed. The Armed Forces Health Surveillance Center (AFHS) provided information about additional military cases that were not reported to state health departments, and those were added to the NMSS database. This report presents data on the aggregate of cases reported to CDC through all reporting systems.
Malaria cases are categorized by infecting species: Plasmodium falciparum, P. vivax, P. malariae, and P. ovale. When more than a single species is detected, the case is categorized as a mixed infection. All categories are mutually exclusive. Diagnosis of malaria is confirmed by blood film or polymerase chain reaction (PCR). A rapid diagnostic test (RDT) can be used to diagnose malaria; however, it must be confirmed by either microscopy or PCR to be counted as a case. Each confirmed malaria case is reported by health-care providers or laboratories to local or state health departments and to CDC. CDC staff review all reports when received and request additional information from the provider or the state, if necessary (e.g., when no recent travel to or from a malarious country is reported). Reports of other cases are telephoned to CDC directly by health-care providers, usually when they are seeking assistance with diagnosis or treatment. Information regarding cases reported directly to CDC is shared with the relevant state health department. All cases that have been reported as acquired in the United States are investigated further, including all induced, congenital, introduced, and cryptic cases (see Definitions). Information derived from Information derived from uniform case report forms is entered into a database and analyzed annually (http://www.cdc.gov/malaria/resources/pdf/report/malaria_form.pdf).
The chi-square test was used to calculate p values and assess differences between variables reported in 2011 compared with previous years. A p value of <0.05 was considered statistically significant.
Definitions
The following definitions are used in malaria surveillance for the United States:
- U.S. residents — Persons residing in the United States, including both civilian and U.S. military personnel, regardless of legal citizenship.
- U.S. civilians — Any U.S. residents, excluding U.S. military personnel.
- Foreign residents — Persons who hold resident status in a country other than the United States.
- Travelers visiting friends or relatives — Immigrants, ethnically and racially distinct from the major population of the country of residence (a country where malaria is not endemic), who return to their homeland (a country where malaria is endemic) to visit friends or relatives. Included in the VFR category are family members (e.g., spouse or children) who were born in the country of residence.
- Laboratory criteria for diagnosis: Demonstration of malaria parasites on blood film, PCR, or by RDT (followed by blood-film confirmation).
- Confirmed case: Symptomatic or asymptomatic infection that occurs in a person in the United States or one of its territories who has laboratory-confirmed (by microscopy or PCR) malaria parasitemia, regardless of whether the person had previous episodes of malaria while in other countries. A subsequent episode of malaria is counted as an additional case, regardless of indicated Plasmodium species, unless the case is indicated as a treatment failure resulting from drug resistance.
- Suspect case: Symptomatic or asymptomatic infection that occurs in a person in the United States or one of its territories who has Plasmodium species detected by rapid diagnostic antigen testing without confirmation by microscopy or PCR, regardless of whether the person experiences previous episodes of malaria while in other countries.
This report also uses terminology derived from the recommendations of the World Health Organization (6). Definitions of the following terms are included for reference:
- Autochthonous malaria:
— Indigenous. Mosquitoborne transmission of malaria in a geographic area where malaria occurs regularly.
— Introduced. Mosquitoborne transmission of malaria from a person with an imported case in an area where malaria does not occur regularly.
- Imported malaria: Malaria acquired outside a specific area. In this report, imported cases are those acquired outside the United States and its territories.
- Induced malaria: Malaria acquired through artificial means (e.g., blood transfusion, organ transplantation, or by using shared syringes).
- Relapsing malaria: Recurrence of disease after it has been apparently cured. In malaria, true relapses are caused by reactivation of dormant liver-stage parasites (hypnozoites) of P. vivax and P. ovale.
- Severe malaria: A case of malaria with one or more of the following manifestations: neurologic symptoms, renal failure, severe anemia (defined by hemoglobin [Hb] <7g/dL), acute respiratory distress syndrome (ARDS), jaundice, or ≥5% parasitemia (7). To attempt to include severe cases in which clinical criteria were not reported, persons who were treated for severe malaria (i.e., artesunate, quinidine, and/or an exchange blood transfusion) despite having no specific severe manifestations reported also are counted as a severe case in this analysis.
- Cryptic malaria: A case of malaria for which epidemiologic investigations fail to identify a plausible mode of acquisition (this term applies primarily to cases found in countries where malaria is not endemic).
Laboratory Diagnosis of Malaria
To diagnose malaria promptly, physicians must obtain a travel history from every febrile patient. Malaria should be included in the differential diagnosis of every febrile patient who has traveled to a malarious area. If malaria is suspected, a Giemsa-stained film of the patient's peripheral blood should be examined for parasites as soon as possible. Thick and thin blood films must be prepared correctly because diagnostic accuracy depends on blood film quality and examination by experienced laboratory personnel (8). This simple test can quickly detect the presence of malaria parasites and can also be used to determine the species and percentage of red blood cells that are infected, which are all essential pieces of knowledge to have for the appropriate treatment of persons infected with malaria. Some reference laboratories and health departments can diagnose malaria using PCR, although this is generally reserved for cases for which blood-film diagnosis of malaria is inadequate and for confirmation of species. PCR results are also often not available quickly enough to be of use in the initial diagnosis and treatment of a patient with malaria.
In addition, BinaxNOW Malaria, an RDT that detects circulating malaria-specific antigens, is widely available for use by U.S. laboratories. The test is only approved for use by hospital and commercial laboratories, not by individual clinicians or the general public (9,10). In the United States, use of RDTs can decrease the amount of time required to determine whether a patient is infected with malaria but does not eliminate the need for standard tests (9). RDTs are not able to speciate or quantify malaria parasites. Positive and negative RDTs must be confirmed by microscopy (11).
Results
General Surveillance
In 2011, CDC received 1,925 reports concerning cases of malaria among persons in the United States and its territories, representing a 14% increase from the 1,691 cases reported with onset of symptoms in 2010. Additionally, the number of cases reported in 2011 is the largest number of malaria cases that have been reported in the United States since 1971 (N = 3,180). In 2011, a total of 1,189 cases occurred among U.S. residents, 386 cases among foreign residents, and 350 cases among patients with unknown or unreported resident status (Table 1). The proportion of cases with unknown resident status increased 7 percentage points from 2010 to 2011 (11% to 18%, respectively).
Plasmodium Species
Among the 1,925 cases reported in 2011, the infecting species of Plasmodium was identified and reported in 1,490 (77%) cases. This represents a 6% decrease in the proportion of cases with complete reporting of species compared with 2010 (Table 2) (7). P. falciparum and P. vivax comprised the majority of infections and were identified in 64% and 28% of 1,490 infected persons with species reported, respectively. The percentage of identified cases that were P. vivax increased 5 percentage points from 2010. Among 1,379 cases for whom both the region of acquisition and the infecting species were known, P. falciparum accounted for 83% of infections acquired in Africa, 64% in the Americas, 8% in Asia, and 33% in Oceania (Table 3). In the Americas, the proportion of infections that were caused by P. falciparum decreased by 18 percentage points compared with 2010. This was likely a result of a decrease in the number of cases reported from Haiti, from 171 reported cases in 2010 to 72 in 2011. Infections attributed to P. vivax accounted for 8% acquired in Africa, 34% in the Americas, 86% in Asia, and 50% in Oceania.
Region of Acquisition and Diagnosis
Among the 1,925 reported cases, one laboratory-acquired case, one transfusion-related case, two congenital cases, and one cryptic case were reported. A total of 1,920 reported cases were classified as imported. Information on region of acquisition was missing for 265 (14%) imported cases, an increase from 2010 (n=208 [12%]). Of 1,655 imported cases for which the region of acquisition was known, 1,144 (69%) were acquired in Africa, 363 (22%) in Asia, 140 (8%) in the Americas, seven (0.4%) in Oceania, and one in the Middle East (Table 3). Countries in west Africa† accounted for 721 (63%) cases acquired in Africa. The percent of cases acquired in west Africa decreased by 8 percentage points from 2010, likely because of decreases in the number of cases acquired in Ghana (170 in 2010 and 156 in 2011) and Nigeria (240 in 2010 and 213 in 2011) and despite increases in cases acquired in Liberia and Sierra Leone (66.7% and 65.7%, respectively). In Asia, the number of cases that were acquired in south Asia§ increased from 263 in 2010 to 326 in 2011. The majority of those cases (n=223 [68%]) were acquired in India. The number of cases acquired in Afghanistan and Pakistan increased significantly (96 in 2011 compared with 43 in 2010). The increase in cases acquired in Afghanistan was a result of increased cases among U.S, military personnel serving in Afghanistan. The Caribbean region accounted for 53% (n = 74) of the cases in the Americas, of which 97% (n = 72) were from Haiti. There were significant decreases in the number of cases acquired in the Caribbean overall (59%) and in Haiti (58%) from 2010 to 2011 (Table 3).
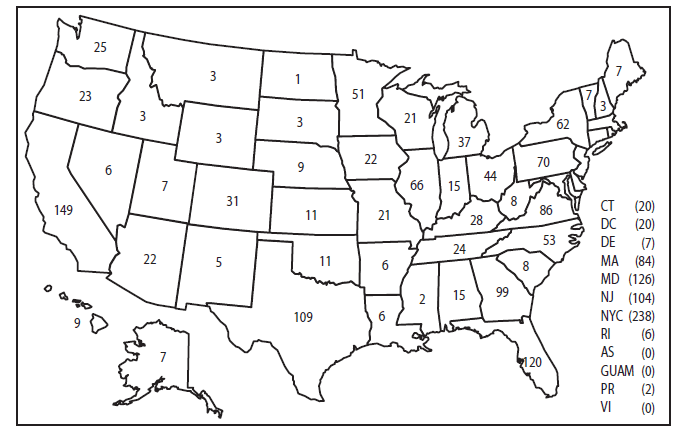
In the United States, seven reporting areas accounted for 50% of the 1,925 reported malaria cases: New York City (n = 238), California (n = 149), Maryland (n = 126), Florida (n = 120), Texas (n = 109), New Jersey (n = 104), and Georgia (n=99) (Figure 1). The state with the greatest change in reported malaria cases in 2011 was Massachusetts, which increased by 133% from 2010 (36 in 2010 compared with 84 in 2011). Cases reported by Kentucky increased from six in 2010 to 29 in 2011, due to an increase in cases among military personnel. Florida reported significantly fewer malaria cases from Haiti in 2011 compared with 2010 (94 cases in 2010 compared with. 25 cases in 2011).
Imported Malaria by Resident Status
Among the 1,571 imported malaria cases of known resident status, 1,186 (75%) occurred among U.S. residents and 385 (25%) among residents of other countries. Among the 1,186 imported malaria cases among U.S. residents, 818 (69%) were acquired in Africa, 216 (18%) in Asia, and 115 (10%) in the Americas (Table 4). This represents a significant increase in cases among U.S. residents who acquired malaria in Africa compared with 2010 (818 in 2011 vs. 724 in 2010). Cases acquired in the Americas among U.S. residents decreased significantly compared with 2010 (115 in 2011 vs. 180 in 2010), likely because of a decrease in cases that were acquired in Haiti that were reported in Florida. No significant change was noted in the cases acquired in Asia or Oceania between 2010 and 2011. Of the 385 imported cases among foreign residents, 239 (62%) were acquired in Africa, 122 (32%) in Asia, and 17 (4%) cases in the Americas. The number of cases of malaria in foreign residents that were acquired in Haiti decreased from 35 cases reported in 2010 to nine cases reported in 2011. Among 303 foreign residents for whom purpose of visit to the United States was known, 80 (26%) were among VFSs and 172 (57%) occurred in recent immigrants or refugees, among whom 71% (n=122) came from Africa.
Seasonality of Malaria Diagnosed in the United States
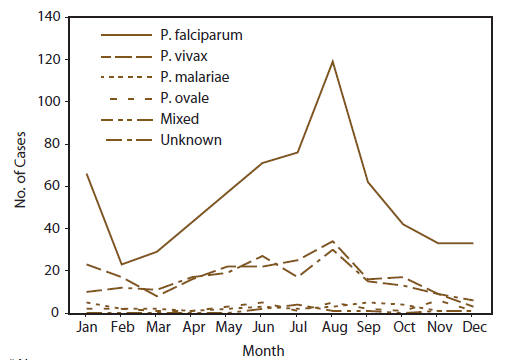
The number of cases of P. falciparum reported in the United States peaked in January and August (Figure 2). Cases occurred primarily among persons who indicated travel to Africa (Figure 2). These peaks likely correlated with peak travel times to African destinations related to winter and summer holidays (12). The number of P. vivax cases reported in the United States also peaked in August, but the peak was much smaller than that for P. falciparum. Most of those cases were in persons who had travelled to Asia, most of who had travelled to India (Table 3).
Interval Between Arrival in the United States and Illness Onset
Among the 1,485 imported malaria cases with an identified Plasmodium species, the interval between both the date of arrival in the United States and onset of illness was known for 1,082 (73%) cases (Table 5). Onset of symptoms began before arrival in the United States in 150 (14%) cases; the remaining 932 (86%) patients experienced malaria symptoms on or after arrival to the United States. Onset of malaria symptoms occurred 0-29 days after arrival in 590 (80%) of the 738 P. falciparum cases and in 111 (41%) of the 270 P. vivax cases.
Imported Malaria Among U.S. Military Personnel
In 2011, a total of 91 cases of imported malaria were reported among U.S. military personnel, a significant increase from the 45 reported in 2010. Region of travel was known for 63 cases and unspecified for 25 cases. Forty-eight military personnel reported travel to Afghanistan and 11 to various regions in Africa. One each reported travel to Guyana, Honduras, Thailand and the Middle East. Compared with 2010, more cases reported travel to Afghanistan and none reported travel to Haiti, likely as a result of changes in U.S. military presence in those regions (13). Information on infecting species was known for 58 cases; 41 cases were identified as P. vivax, 12 as P. falciparum, four as P. ovale, and one as a mixed infection. Among those 58 cases, 32 occurred in persons who reported having taken at least 1 dose of an appropriate drug for primary chemoprophylaxis; only 12 (38%) reported adhering to the regimen. Among the 42 patients infected with P. vivax, 23 reported treatment with primaquine to avoid future relapses. Only four of the 91 cases were classified as severe: two due to P. falciparum and two due to P. vivax infection.
Chemoprophylaxis Use Among U.S. Civilians
Information about chemoprophylaxis use and travel area was known for 997 (84%) of the 1,186 U.S. civilians who had imported malaria. Of these 997 persons, 351 (35%) had taken chemoprophylaxis, a significant increase from 2010 to 2011 (from 257 [28%] to 351 [35%]). Among the 351 persons who reported taking malaria chemoprophylaxis, 93 (26%) did not report specific drug type taken. Of the remaining 258 persons, 213 (83%) had taken a CDC-recommended medication and 45 (17%) had taken a medication that is not recommended by CDC for the area visited. Of the 213 who reported taking CDC-recommended chemoprophylaxis, 69 (32%) had taken mefloquine, 90 (42%) had taken doxycycline, 36 (17%) had taken atovaquone/proguanil, two (1%) had taken chloroquine, and one (0.5%) had taken primaquine. Fifteen additional patients reported taking more than one CDC-recommended malaria chemoprophylaxis medication for the specific travel region. Information about infecting species was available for 177 (83%) patients who had taken a recommended antimalarial drug and was undetermined for the remaining 36 patients. Moreover, among the 184 who reported taking CDC-recommended chemoprophylaxis and for whom adherence was known, 127 (69%) reported nonadherence (i.e., missed doses).
Cases of P. vivax or P. ovale After Recommended Prophylaxis Use. Among the 177 patients who took chemoprophylaxis appropriately and had information on infecting species, 55 (31%) cases were caused by P. vivax, and six (3%) cases were caused by P. ovale. Of the 61 cases of P. vivax or P. ovale, information on 11 cases was insufficient (i.e., missing data regarding symptom onset or return date from travel) to assess if this was an acute infection or a relapse infection. Onset of symptoms for 28 reported cases occurred >45 days after the patient arrived in the United States. The clinical features of these cases are consistent with relapsing infections and do not indicate primary prophylaxis failures. Twenty-two cases occurred ≤45 days after the patient returned to the United States; these cases are consistent with acute infection and could indicate primary prophylaxis failures. Among the 22 cases, 12 patients were nonadherent with their malaria chemoprophylaxis regimen and eight patients did not provide adherence information. The remaining two patients reported adherence with an antimalarial chemoprophylaxis regimen; one was a missionary/dependent who had traveled to Uganda for 3 weeks and had taken mefloquine for malaria chemoprophylaxis and the other was in the U.S. military and stationed in Afghanistan for 8 months who had taken doxycycline for malaria chemoprophylaxis. Possible explanations for infection in these patients cases include inappropriate dosing, unreported nonadherence, malabsorption of the drug, an early relapse from hypnozoites established at the start of the trip, or possibly emerging parasite resistance.
Cases of P. falciparum or P. malariae after Recommended Prophylaxis Use. The 177 cases of malaria reported among persons who had taken a recommended antimalarial drug for chemoprophylaxis included 105 cases of P. falciparum, nine cases of P. malariae, and two cases with mixed infection. Of the 105 P. falciparum cases, 102 (97%) were acquired in Africa and three (3%) in the Americas. Seventy-one (68%) of the 105 P. falciparum patients reported nonadherence to the antimalarial drug regimen, 23 (22%) patients reported adherence, and 11 patients had no adherence information available. All 23 of the cases in which patients reported adherence with antimalarial chemoprophylaxis had traveled to Africa; 13 patients took mefloquine, eight took doxycycline, and two took atovaquone/proguanil. Of the nine P. malariae cases, four reported adherence to the antimalarial drug regimen, all of whom had traveled to Africa (two patients took mefloquine and two took atovaquone/proguanil).
Patients with a Recent History of Malaria
Of the 1,920 imported cases, data on history of malaria was known for 1,286 (67%) cases; 265 (21%) patients reported a history of a malaria infection during the preceding 12 months. Among the 265 cases, 111 (42%) were caused by P. vivax, four (2%) by P. ovale, and 40 (15%) reported no species. A total of 23 probable relapses were identified based on onset date, date of previous infection, and previous infection species type: 22 P. vivax cases and one P. ovale case. Among the 23 relapses, 12 patients (all were P.vivax infections) subsequently received primaquine as part of treatment to avoid future relapses.
Purpose of Travel
Purpose of travel to regions in which malaria is endemic was reported for 871 (80%) of the 1,095 U.S. civilians with imported malaria (Table 6). Of the 871 who reported purpose of travel, 607 (70%) were VFR, 96 (11%) were missionaries, and 78 (9%) were travelling for business. The proportions of purpose for travel in all categories in 2011 were similar to 2010. The proportion of patients who traveled for tourism did not change from 2010 to 2011, ending a downward trend that began in 2007.
Malaria by Age
Among the 1,889 cases among patients for whom age was known, 281 (15%) occurred in persons aged <18 years, 1,508 (80%) in persons aged 18–64 years, and 100 (5%) in persons aged ≥65 years. Although the majority of cases occur in persons aged 18–64 years, pediatric cases are of particular interest because the preventive care of most children is determined by parents or guardians. Among the 281 cases among persons aged <18 years, 142 (51%) occurred among U.S. civilian children, 96 (34%) occurred among children of persons categorized as having a foreign resident status at the time their malaria infection was acquired, and 43 (16%) occurred among children of unknown resident status. Of the 142 cases among U.S. civilian children, nine (6%) were aged <24 months, 26 (18%) were aged 2–4 years, 59 (42%) were aged 5–12 years, and 48 (34%) were aged 13–17 years. One hundred twelve (80%) of the cases among U.S. civilian children for whom country of exposure was known were attributable to travel to Africa. Among the 121 U.S. civilian children for whom reason for travel was known, 104 (86%) were VFRs, 10 (8%) were traveling for missionary work, four (3%) were traveling for educational purposes, and three (2%) were tourists. Of the 122 children for whom chemoprophylaxis information was known, 60 (49%) were reported as having taken chemoprophylaxis, of whom 30 (50%) had taken an appropriate regimen; however, only 12 (40%) of these 30 patients reported adherence.
Hospitalization
Information on hospitalization was reported for 83% (n = 1,596) of all cases. Among those persons, 66% (n = 1,047) were hospitalized. The majority of those cases were P. falciparum (n = 643 [61%]), of which 183 (28%) were considered severe. The second largest proportion of cases was identified as P. vivax (n=188 [18%]) infections. The majority of hospitalized patients with P. vivax cases were uncomplicated malaria infections; however 12% (n = 22) were severe.
Treatment in Uncomplicated Imported Malaria Cases
Of the 1,645 imported cases of uncomplicated malaria in 2011, information on treatment medicines was available for 1,155 (70%) cases. Of these, there were 591 (51%) P. falciparum, 309 (27%) P. vivax, 33 (3%) P. malariae, 32 (3%) P. ovale, 13 (1%) mixed cases, and 177 (15%) where species type was unknown or not reported. The CDC Guidelines for Treatment of Malaria in the United States (10), herein referred to as the CDC Guidelines for Treatment, was used to determine whether the medicines listed for treatment were appropriate.
Of the 1,155 patients with uncomplicated malaria with available information on treatment, most (832 [72%]) were treated appropriately according to the CDC Guidelines for Treatment, and 323 (28%) patients received inappropriate treatment. This represents a significant decrease (6 percentage points) in patients with uncomplicated disease being treated appropriately compared with 2010. Among the patients who were treated appropriately, 190 (23%) indicated taking other antimalarial drugs in addition to those recommended by CDC guidelines. Because the CDC surveillance report form does not record the sequence of treatment events, it is difficult to understand and characterize the intended purpose of additional antimalarial treatment drugs. Therefore, for the purpose of this report, these 190 patients were considered to be treated appropriately. Among the 323 inappropriately treated patients, 18 (6%) had received the recommended chemoprophylaxis but subsequently had inappropriately received the same drug for treatment. Antimalarial drugs used for treatment should differ from the drugs received for chemoprophylaxis because of the potential for toxicity and reduced efficacy.
Adequacy of treatment varied by species. For the 591 P. falciparum cases, 517 (87%) patients were treated appropriately according to CDC Guidelines for Treatment, of which 120 (23%) received additional antimalarial drugs. The 75 P. falciparum cases that were treated with an inappropriate treatment regimen included three pregnant patients. Among the 33 P. malariae cases, 30 (91%) patients were treated appropriately according to CDC Guidelines for Treatment, of whom two (7%) received other antimalarial drugs in addition to those recommended by CDC. Only three (9%) patients were treated with an inappropriate treatment regimen.
Among the 309 patients with P. vivax cases for whom treatment information was reported, 264 (85%) patients were treated with an appropriate antimalarial drug to address their acute infection, of which 28 (11%) received other antimalarial drugs in addition to those recommended by CDC. Of the 264 P. vivax cases who received an appropriate treatment for their acute infection, the majority (n=136, 76%) were also treated with primaquine for relapse prevention. Among the 32 patients with P. ovale for whom treatment information was reported, 27 (84%) patients were treated with an appropriate antimalarial drug to address their acute infection, of whom two (7%) received other antimalarial drugs in addition to those recommended by CDC. Of the 27 P. ovale cases who received an appropriate treatment for their acute infection, nine (33%) also were treated with primaquine for relapse prevention. Among the 13 mixed cases for whom treatment information was reported, eight (62%) patients were treated appropriately according to CDC Guidelines for Treatment. Two of those received other antimalarial drugs in addition to the CDC-recommended regimens. Of the six mixed cases that were not treated appropriately, all included at least one relapsing species and five (83%) received primaquine.
According to the CDC Guidelines for Treatment, when species is unknown, a treatment regimen for a P. falciparum infection should be used to presumptively treat infection. Among the 177 cases where species was unknown, 130 (73%) patients were treated appropriately according to CDC Guidelines for Treatment, of whom 35 (27%) received other antimalarial drugs in addition to those recommended by CDC. Forty-seven (27%) patients received an inappropriate treatment regimen. Incomplete reporting of species and treatment medications might affect whether the case is classified as having been treated appropriately or not.
Severe Malaria
Among the 1,925 reported cases, 275 (14%) were classified as severe malaria, including five who died. This represents a significant increase from 2010, during which 11% were classified as severe. Most (84%) severe cases occurred in persons aged ≥18 years, and 16% occurred in children aged <18 years, nine (21%) of whom were aged <3 years. Children aged <5 years were more likely to have severe disease than those who were older. However, no association was found between severe disease and being aged >64 years or resident status. Among the 259 cases in patients with known resident status, 1,189 (75%) were U.S. residents. The predominant species among the severe cases was P. falciparum (n=203 [74%]), which is not significantly different than 2010 (n=149 [81%]).
Where information on prophylaxis was known (n=223), 57 (26%) persons reported taking a recommended chemoprophylaxis; however, only 13 reported adherence to the drug regimen, including four who used mefloquine, two who took atovaquone/proguanil, and one each who took doxycycline, chloroquine, or primaquine. There was no significant association between severe disease and use of prophylaxis. Although some patients had multiple clinical complications associated with an infection, the largest proportion of patients experienced severe anemia (19%) or renal failure (16%), unlike in 2010, when the largest proportion of patients experienced renal failure followed by cerebral malaria (10). More hospitalized cases of P. falciparum were considered severe in 2011 (n=183 [28%]) compared with 2010 (n=136 [20%]). Patients with severe cases were more likely to receive inappropriate treatment than those with uncomplicated cases. Among the 275 severe cases, 146 (53%) patients were treated with quinidine and 76 (28%) were treated with an oral antimalarial drug. Fifty-one (19%) patients were treated with intravenous (IV) artesunate provided by CDC through an investigational new drug protocol. Patients diagnosed with uncomplicated malaria can be effectively treated with oral antimalarial drugs. However, patients who are considered to have severe disease should be treated aggressively with parenteral antimalarial therapy (14).
Although there was no difference in the number of days from date of return to date of hospitalization between severe and uncomplicated P. falciparum cases (12.4 days for severe, 11.7 days for uncomplicated), severe cases had significantly longer intervals between date of onset and date of hospitalization than uncomplicated cases (5.7 days for severe, 2.2 days for uncomplicated). The dates on which patients first saw a medical provider were not collected, so time from first provider encounter to hospitalization could not be examined.
Among patients for whom reason for travel was known, most (59%) of the severe cases were in VFR travelers (comparable to 2010), of whom 65% specified acquisition from west Africa; 80% of severe cases were identified as P. falciparum infections. In addition, the number of severe cases acquired in Haiti decreased by 50% from 2010 to 2011. In Haiti, virtually all malaria is caused by P. falciparum.
No significant association was found between travel for business and severe malaria and, similar to 2010, a significant association was found between travel for missionary work and severe malaria in 2011. Among cases in missionaries or their dependents, 22% (22 of 102) developed severe disease, compared with 14% (261 of 1,823) of those traveling for other purposes.
Malaria During Pregnancy
A total of 37 cases of malaria were reported among pregnant women in 2011, representing 6% of cases among all women (n = 654). The number of pregnant women with malaria did not change significantly from the 41 cases reported in 2010. Additionally, no significant differences were noted among pregnant women with malaria compared with nonpregnant women in terms of species type, reason for travel, or region of infection acquisition. Of the 37 cases among pregnant women, 11 (30%) cases were severe. Among the 33 cases for whom Plasmodium species type was known, 27 (79%) were diagnosed with P. falciparum infection, including nine who presented with severe malaria. Six (18%) were diagnosed with P. vivax, including two with severe malaria, and one case was identified as a P. malariae infection. Seventeen (46%) cases occurred among U.S. civilians, of whom 12 (71%) reported travel to Africa. Among the 15 U.S. civilian pregnant women with known reason for travel, 93% reported VFR. Of the 17 cases of malaria reported among U.S. civilian pregnant women, only one reported taking malaria chemoprophylaxis and she reported adherence to chloroquine. No information was available on birth outcomes.
Selected Malaria Case Reports
Laboratory-acquired Malaria
Case. In April, a man aged 37 years who worked in a university research laboratory with Anopheles mosquitoes was treated in the emergency department for a P. falciparum infection. He reported no travel within the preceding 2 years but had lived in Kenya from December 2006 to May 2009. PCR testing confirmed that his infection matched the strain of P. falciparum being worked on in the laboratory and was chloroquine sensitive. It was unknown if any safety precautions were either required or in place during the time the man worked in the laboratory. He was started on a course of treatment using chloroquine, and all symptoms resolved within 7 days. The university conducted a safety investigation; no other employees were infected.
Transfusion Transmitted Infection
Case. In April, a man aged 76 years who was a veteran was admitted to a military hospital for presumed urosepsis. He had a 1-day history of fevers and, although he had been admitted several times during the previous 6 months for other conditions, had not complained of any symptoms consistent with acute malaria. A hematologist identified malaria parasites incidentally while performing a manual differential of a complete blood count, and the species was subsequently confirmed by PCR to be P. malariae. The patient was treated with an oral antimalarial regimen and recovered fully. The patient's travel history included a 1- year tour of duty in Vietnam and a short stay in Brazil, both in the early 1970s. The patient had no recent travel to malaria-endemic regions. The patient had been treated for anemia of chronic disease for several years and since December 2010 had been transfused with eight units of red blood cells. The blood bank was contacted and a trace-back investigation was initiated using banked serum samples from the eight donors. One of the donor serum samples had elevated titers against P. malariae and P. falciparum, indicating a previous infectious exposure at an indeterminate time. The implicated donor was contacted and his travel history and malaria exposure was ascertained. The donor lived in Liberia for the first five years of life (1990-1995) with no other travel history. The donor and his mother stated that he had never had malaria. As a result of this investigation, the donor agreed to antimalarial treatment. Donor blood specimens were collected and tested positive for P. malariae by PCR. The other blood products from this donor had been sent to theater of operations, likely Iraq or Afghanistan, and were not traceable.
Congenital Malaria
Two congenital cases, caused by transmission of parasites from mother to child during pregnancy or perinatally during labor, were reported in 2011. Clinical and laboratory data are limited.
Case 1. On November 6, a 1-day old male developed symptoms consistent with malaria infection. A peripheral blood film revealed P. falciparum infection with less than 0.01% parasitemia. His mother had immigrated to the United States from Cameroon 2 weeks earlier and reported having a previous infection with P. falciparum in 2009. Blood smears indicated that the mother also had P. falciparum infection, with a parasitemia of 5.1%. Treatment information was unavailable. Both mother and child suffered no complications and recovered fully.
Case 2. In March, a female aged 3 months was seen in an emergency department with fever and symptoms of an upper respiratory infection. Blood smears identified a P. vivax infection with 0.2% parasitemia. She was treated with atovaquone-proguanil and recovered. Although she was born in the United States and had never traveled outside of the country since her birth, her parents and three siblings emigrated from a refugee camp near the Thai/Burma border 6 months before her birth. The mother was symptomatic for malaria early in her pregnancy and, at that time, a thick film smear showed P. falciparum with moderate ring forms. She was successfully treated with artesunate tablets and mefloquine while she was in the process of leaving Thailand.
Cryptic Case
One case reported in 2011 was categorized as cryptic malaria because epidemiologic investigations did not identify a plausible mode of acquisition.
Case. In January, a female aged 12 years who moved to the United States in March 2007 from Nigeria, presented to a hospital with symptoms consistent with malaria, including fever. Blood smears and PCR testing indicated infection with P. falciparum. She was hospitalized for 4 days, treated with quinine, and discharged home. Her parents reported that she made one trip to Nigeria in 2008 to attend a funeral, but denied any travel outside of the United States since. They also denied that she had any contact with anyone sick and she had not received blood transfusions. Because of unfavorable environmental conditions, local malaria transmission in the northeastern region of the United States during the winter season was an unlikely source of her infection. The origin of the infection remains undetermined.
Fatal Cases
Case 1. On July 25, a man aged 45 years who had immigrated to the United States from Haiti 13 years earlier had onset of fever. He had returned from Haiti 6 days earlier after visiting friends and family in the Port-au-Prince and St. Louis areas; he did not take malaria chemoprophylaxis. On July 28, he sought care from his primary-care physician for fever, generalized weakness, fatigue, and abdominal pain. His doctor treated his febrile illness empirically with oral ciprofloxacin as an outpatient. The next day, he presented to an emergency department with worsening fatigue, ongoing fever, rigors, and abdominal pain. Blood smears revealed P. falciparum infection with a parasitemia of 5%, and he was administered oral chloroquine. He developed ARDS on his third day of hospitalization and was transferred to another hospital, where he was started on intravenous quinidine treatment for presumed treatment failure. However, malaria smear revealed <1% parasitemia. His condition continued to worsen. He became hypotensive, experienced multisystem organ failure, and died on August 2.
Case 2. A woman aged 83 years who was a United States resident of Indian ethnicity had a history of coronary artery disease, diabetes mellitus, and gastrointestinal bleeding and had been traveling in India for 6-7 months before her death. While in India, she reportedly suffered a hip fracture and was hospitalized. Her hospitalization in India was complicated by episodes of gastrointestinal bleeding, which was managed with blood transfusions. The time interval between hospital discharge and travel to the United States is unknown. On June 24, approximately 2 days after returning to the United States, she was found unresponsive at home. CPR was started and EMS was called; she was successfully resuscitated and admitted to a hospital. On arrival, it was determined that she had severe anemia, renal failure, and acidosis. Malaria parasites were detected by microscopy and subsequently identified as P. vivax. She received a dose of artesunate just after midnight on June 25. Later that day, the woman's family decided to remove her from life support, and she died the same day.
Case 3. In May 2011, a girl aged 4 years and her mother returned to the United States after having spent approximately 3 weeks visiting family in Uganda; neither had taken malaria chemoprophylaxis. While in Uganda, the girl became ill with fever and cough and presented to a clinic for treatment. Diarrhea and vomiting were reported. She had no rash or bleeding and did not report any chronic conditions. She was diagnosed with an unspecified bacterial infection was given acetaminophen suppositories and was treated with unspecified antibiotics. Care for the girl was sought six more times over a 2-week period for continued signs and symptoms. Malaria was reportedly tested for but not diagnosed. High fever persisted and she was returned to the United States. On the return flight to the United States, the girl was very thirsty and drank a large amount of liquid without subsequently urinating either while on the plane or while in the terminal after landing. While on a layover at an airport in the United States, she became unresponsive and was pronounced dead at a hospital an hour later. Malaria was diagnosed by autopsy (14). Histopathology revealed characteristic intraerythrocyte parasites suggestive of Plasmodium species. Immunohistochemistry and PCR assays of autopsy tissues and serum confirmed infection with Plasmodium falciparum. The pregnant mother began experiencing headaches, fever, chills, and generalized body aches three weeks after the death of her daughter. She was hospitalized and diagnosed with P. malariae. She was treated with atovaquone-proguanil and fully recovered.
Case 4. On January 12, a woman aged 33 years who was a missionary from Liberia and who had married a missionary from the United States was seen in an urgent-care clinic in the United States complaining of sore throat, fatigue, weakness, nausea, and vomiting. She was confused, not oriented to place or time. A complete blood count revealed leukocytosis and severe anemia (hemoglobin of 4.1 g/dL). She was transferred to a hospital by ambulance. She and her husband had returned to the United States from Liberia approximately 1 month earlier to visit her husband's family. At the hospital, it was determined that she had malaria with a 30% parasitemia (species was not determined) and Salmonella bacteremia. She was treated with intravenous quinidine, doxycycline, vancomycin, and exchange transfusion. She experienced multisystem organ failure. Her family decided to remove her from life support, and she died on January 17.
Case 5. On October 4, a man aged 58 years who was an Indian national with a history of coronary artery disease and diabetes and was in the United States on vacation was seen by a doctor for fever and nonproductive cough. Azithromycin was prescribed for a suspected upper respiratory infection. The fever continued and he began feeling short of breath at rest. On October 5, he went to a hospital where he was found to be anemic and thrombocytopenic with lactic acidosis, severe hypotension, and respiratory distress. Malaria parasites were observed on the manual differential of his complete blood count. He was intubated, admitted to intensive care, and started on intravenous quinidine and norepinephrine. He died on October 6, just 5 hours after being admitted to the hospital. A thin and thick blood smear confirmed the presence of malaria parasites that were believed but not confirmed to be P. falciparum. Information regarding level of parasitemia was not provided. PCR testing to determine the cause were cancelled after the patient died.
Discussion
The number of malaria cases reported in the United States in 2011 was the largest since 1971 (N = 3,180) (15), representing a 14% increase from 2010 (11) and a 48% increase from 2008 (16,17). Both travel and inadequate prevention measures might have contributed to this finding. These increases appear to be similar to those being reported in other parts of the world. For example, in the United Kingdom, 1,677 malaria cases were reported in 2011, a slight decrease from 2010 (5%) but a 22% increase from 2008 (18). The majority of the U.S. cases were acquired in sub-Saharan Africa, which is also similar to the data reported by the United Kingdom. Despite progress in reducing the number of malaria cases in regions where malaria is endemic (19), international travel appears to be growing steadily, and use of appropriate prevention measures by travelers is still inadequate. World Tourism Organization estimates that there were 983 million international travelers in 2011, with notable increases in overall travel (4.6%) and travel to Asia and the Pacific (6%) (19). Although travel to Africa increased only 1%, as a result of the Arab Spring and political transitions in North Africa, travel to Sub-Saharan Africa increased by 6.9% (20). As international travel increases, prevention messages and health communication strategies become even more important for protecting the traveling community from communicable diseases. Prevention messages directed toward Africa-bound travelers, particularly those whose destination is west Africa, should be emphasized in early spring, accompanied with a reminder in late fall through early winter. Malaria prevention messages directed toward Asia-bound travelers, specifically those bound for India, should be intensified in late spring. Travelers should be informed of the risk for malaria and strongly encouraged to use protective measures, including chemoprophylaxis. Imported malaria can reintroduce malaria into regions, including the United States, where the disease is not endemic and environmental conditions are present that can support the lifecycle of the parasite, including the presence of an appropriate Anopheles vector.
Of the 1,920 imported cases, 353 (19%) reports did not provide information regarding U.S. resident status, 250 (13%) did not have information regarding travel history, and 422 (22%) did not have information on species. This represents a reversal in a recent 2-year period of increases in reporting of complete information on the malaria case report form that began after 2008, when 46% of cases had no information about resident status and 41% had no information on species. In 2010, residential status and species was missing in only 11% and 18% of reports, respectively. For most of the cases with missing residential status, travel history, and species, reporting to CDC was done electronically to NNDSS and not by the NMSS case report form. NNDSS is unable to receive malaria-specific data elements from state and local health departments. States and local health departments are strongly encouraged to report cases using the NMSS case report form until malaria-specific data can be received electronically by NNDSS. All elements on the NMSS case report form should be completed, because incomplete reporting compromises efforts to examine trends in malaria cases in the United States and prevent infections among travelers. Local and state health departments, health-care providers, and other health personnel should be vigilant in reporting complete information for malaria cases. Specifically, if certain variables are not reported (e.g., species, residence, and country of acquisition), efforts should be made to obtain complete information for comprehensive analysis.
In the Caribbean region, endemic transmission of malaria ended in the mid-1960s, except in the island of Hispaniola, which includes the countries of the Dominican Republic and Haiti (20). An increase in the numbers of malaria cases acquired in Haiti had already been noted before the January 2010 earthquake (8). This increase continued throughout 2010 and was likely the result of both increased transmission in Haiti and increased volume of travel between the United States and Haiti by relief aid workers and Haitians returning to visit friends and relatives (11,21,22). The number of cases reported decreased from 172 cases in 2010 to 72 cases in 2011, which represents a significant decrease from 2010 but an increase in the number of cases being reported before the 2010 earthquake (21). Of the 72 cases that were acquired in Haiti, only four (6%) patients reported taking prophylaxis. One of the five reported taking all of the prophylaxis, and one of the patients that did not report taking prophylaxis died. Failure to take chemoprophylaxis is the most common risk factor for acquisition of malaria among travelers to regions where the disease is endemic. Messages must be conveyed to VFR travelers that they are at substantial risk for malaria despite beliefs that partial immunity offers protection from disease (23). Recent reports of emerging molecular markers of chloroquine drug resistance in Haiti (24,25) indicate a need for increased vigilance for evidence of clinical chloroquine chemoprophylaxis or treatment failure. However, chloroquine remains an effective choice for chemoprophylaxis and treatment of malaria acquired in Haiti. Health-care providers should contact CDC to assist with the evaluation of possible chloroquine failures identified among U.S. travelers or Haitian immigrants to the United States.
Airline crews based in the United States comprise a very small proportion of reported malaria cases; however, the number of reported cases might increase as direct daily flights between Africa (particularly to west Africa) and the United States increase (26). Nine cases were identified among airline crew members in 2011. Following a cluster of four cases among employees of one airline in 2010, a survey of knowledge, attitudes, and practices among airline staff found that the overall perception of malaria as an occupational risk was low (27). Organizations and companies with employees who must travel to malaria-endemic regions should incorporate employee education and training on the use of and compliance with measures to prevent malaria infection. Additionally, companies should review and remove any potential barriers that hinder employees from accessing necessary prevention medicines or information.
Although laboratory-acquired mosquitoborne infections are rare, cases have been reported in the United States (28,29). One case of malaria in 2011 occurred after laboratory exposure to mosquitoes carrying malaria parasites. Malaria can be acquired in a laboratory through accidental contact with infected blood from humans or animals or through accidental contact with an infective mosquito. Containment measures should be followed when working with infected mosquitoes, including continuous use of light traps (30).
In 2011, a total of 91 cases were reported among military personnel, a substantial increase from 2010 (n = 45). The majority of 2011 cases among military personnel were acquired in Afghanistan after an additional 30,000 troops were deployed in December 2010 (13). Although use of prophylaxis is higher in the military population in Afghanistan compared with the civilian population, adherence remains low (i.e., 38% among those who reported taking any chemoprophylaxis). Before 2010, cases among patients who were traveling for military duty to regions where malaria is endemic were only reported to CDC by local and state health departments and private health clinicians. However, after CDC partnered with AFHSC, additional cases occurring among the military are being identified that might have not been identified previously by local or state health departments or private health-care providers, thus improving opportunities to monitor and survey trends or changes (e.g., in geographical transmission and prophylaxis or treatment failures among the deployed military population).
Of the 435 uncomplicated cases of P. vivax or P. ovale in men and in nonpregnant women, only 177 (41%) received primaquine (which is contraindicated during pregnancy). Primaquine is the only antimalarial active against the dormant parasite liver forms and prevents relapses (31). In 2011, the Food and Drug Administration reported that primaquine was back ordered because of manufacturing issues reported by the pharmaceutical company, Sanofi-Aventis (32). Providers were urged to keep patients on weekly chloroquine prophylaxis to prevent relapses. Only 39 (15%) of P. vivax and P. ovale patients who did not receive primaquine received chloroquine. Because the CDC surveillance report form does not record the dates of treatment, understanding and determining the intended purpose of chloroquine treatment in these cases is difficult. Primaquine became available again in late 2012 (33). To prevent relapses of malaria, providers should contact patients that did not receive primaquine.
Five fatal cases were reported in 2011, less than half the number of fatal cases reported in 2010 but equal to that in 2009 (8). Two were P. falciparum infections, one was P. vivax, and two had no species identified. One of the cases with no identified species was suspected as P. falciparum, but further testing was cancelled after the patient died. Several patients had delayed seeking treatment after onset of symptoms, and two patients were discharged with empiric antibiotics without an appropriate workup for fever in a traveler returning from an area where malaria is endemic. The differential diagnosis of fever in a person who has returned from travel to an area where malaria is endemic should always include malaria. Signs and symptoms of malaria often are nonspecific but typically include fever. Other symptoms include headache, chills, increased sweating, back pain, myalgia, diarrhea, nausea, vomiting, and cough. Prompt diagnosis requires that malaria be included in the differential diagnosis of illness in a febrile person with a history of travel to an area where malaria is endemic. Health-care providers should ask all febrile patients for a travel history. Any delay in the diagnosis and treatment of malaria can result in complications, regardless of the effectiveness of the treatment regimen.
The choice of a specific antimalarial treatment regimen should be based on several key factors, including the probable geographic origin of the parasite, the Plasmodium species, parasite density, and the patient's clinical status (34). In a 2010 nationwide survey of laboratories in the United States, most laboratories surveyed offered malaria diagnostic testing services but very few were in complete compliance with all of the Clinical and Laboratory Standards Institute guidelines for analysis and reporting of results. Additionally, most laboratories reported very few cases annually (35). The case definition used for malaria surveillance in the United States was revised in 2013 to include identification of malaria parasites, determination of species, and quantification of the parasitemia (http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/13-ID-08.pdf). Microscopy is still considered the best method for the immediate diagnosis of malaria; however, PCR testing is particularly valuable for species confirmation and should be used in all instances when species cannot be determined by microscopy or to evaluate for mixed infections. CDC's Parasitology Diagnostic Service team provides free diagnostic services to laboratories and health professionals diagnosing cases of malaria, including microscopy, PCR testing, and drug resistance testing performed by experts who test thousands of specimens each year. Of the 1,925 cases, species confirmation was provided by CDC for <10% of cases. Increasing the proportion of cases with laboratory-confirmed diagnosis will improve the understanding of malaria epidemiology as presented in annual malaria surveillance summaries.
Patients with suspected or confirmed malaria who are severely ill should be treated aggressively with parenteral antimalarial therapy. Quinidine gluconate continues to be recommended for parenteral malaria therapy. However, this medication is no longer available in many hospital formularies. Because parenteral quinidine gluconate is potentially cardiotoxic, it should be administered in an intensive care setting with continuous cardiac and frequent blood pressure monitoring. As an alternative to quinidine gluconate, intravenous artesunate is also highly effective in the treatment of severe malaria and is available as an investigational new drug (IND) through CDC. Artesunate is stocked at nine sites around the United States and can be rapidly shipped at no cost to clinicians. Certain guidelines and eligibility requirements must be met to enroll a patient in the treatment protocol. Physicians who administer the drug to patients must notify CDC of any adverse event after administration and comply with the IND protocol (36). To enroll a patient with severe malaria in this treatment protocol, health-care providers should telephone the CDC Malaria Hotline at 770-488-7788 or toll-free at 855-856-4713, Monday–Friday, 9 a.m.–5 p.m., Eastern time. At other times, callers should telephone 770-488-7100 and ask to speak with a CDC Malaria Branch clinician. Travelers and health-care providers are encouraged to use CDC resources on malaria prevention and treatment, and contact the CDC Malaria Branch for assistance with diagnostic or case management needs.
Detailed recommendations for preventing malaria are available to the general public 24 hours a day online at http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/malaria.aspx#990. Additional information on malaria prevention recommendations is also available through the online CDC malaria map application at http://www.cdc.gov/malaria/map. The application is an interactive map that provides information on malaria throughout the world. Users can search or browse countries, cities, and place names and get information about malaria in that particular location and see recommended malaria prophylaxis for that area. Also, CDC biannually publishes recommendations in Health Information for International Travel (commonly referred to as The Yellow Book), which is available and updated on the CDC Travelers' Health website at http://www.cdc.gov/Features/TravelersHealth.html; the publication is also available for purchase from Oxford University Press, Inc., at http://www.oup.com/us or telephone 1-800-451-7556 (Table 7).
Health-care providers should be familiar with prevention, recognition, and treatment of malaria and are encouraged to consult appropriate sources for malaria prevention and treatment recommendations (Table 7). Health-care providers should be aware of diagnostic and treatment resources available in their facilities, including availability at night or on weekends. A recent evaluation of malaria diagnosis capabilities among U.S. laboratories demonstrated that although malaria diagnostic testing services were available to the majority of U.S. laboratories surveyed, very few were in compliance with all of the current guidelines (35). To maintain and improve malaria and other parasitic disease diagnosis capabilities in the United States, CDC's Parasitology Diagnostic Service team conducts training courses several times per year (www.dpd.cdc.gov/dpdx/HTML/Aboutdpdx.htm). Physicians seeking assistance with the diagnosis (including telediagnosis) or treatment of patients with suspected or confirmed malaria should call CDC's Malaria Hotline at telephone 770-488-7788 or toll-free 855-856-4713 during regular business hours or CDC's Emergency Operations Center at telephone 770-488-7100 during evenings, weekends, and holidays (ask to page the person on call for the Malaria Branch), or access CDC's Internet site at http://www.cdc.gov/malaria/diagnosis_treatment/index.html. These resources are intended for use by health-care providers only.
Acknowledgments
The authors acknowledge the state, territorial, and local health departments; health-care providers; laboratories; and the AFHSC for providing this information to CDC.
References
- World Health Organization. World malaria report 2012. Geneva, Switzerland: WHO Press; 2012.
- Pan American Health Organization. Report for Registration of Malaria Eradication from United States of America. Washington, DC: Pan American Health Organization; 1969.
- JM Andres, GE Quinby, AD Langmuir. Malaria eradication in the U.S. Am J Pub Health 1950;40:1405–11.
- CDC. Local transmission of Plasmodium vivax malaria—Palm Beach County, Florida, 2003. MMWR 2003;52:908–11.
- CDC. National notifiable disease surveillance system. Atlanta, GA: US Department of Health and Human Services, CDC; 2012. Available at: http://wwwn.cdc.gov/nndss/default.aspx.
- World Health Organization. Terminology of malaria and of malaria eradication: report of a drafting committee. Geneva, Switzerland: World Health Organization; 1963.
- World Health Organization. Management of severe malaria: a practical handbook. Third ed. Geneva, Switzerland: WHO Press; 2012.
- CDC. Malaria surveillance—United States, 2009. MMWR 2011;60(No. SS-3).
- CDC. Malaria rapid diagnostic test. MMWR 2007;56:686.
- BinaxNOW(r) Malaria [package insert]. Scarborough, Maine: Inverness Medical Professional Diagnostics; 2007.
- CDC. Malaria surveillance—United States, 2010. MMWR 2012;61(No. SS-2).
- World Tourism Organization. 415 million tourists expected worldwide in the May-August peak season. Madrid UNWTO Publications; 2012. Available at: http://media.unwto.org/en/press-release/2012-07-09/415-million-tourists-expected-worldwide-may-august-peak-season.
- Armed Forces Health Surveillance Center. Update: Malaria, U.S. Armed Forces, 2011. Medical Surveillance Monthly Report 2012;19:2–6.
- Taylor CA, Blau DM, DiAngelo CR, Shieh W-J, Zaki SR, Arguin PM. Malaria diagnosed by autopsy in a young traveler returning from Uganda: limitations of surveillance. Journal of Travel Medicine 2013;20:47–9.
- CDC. Guidelines for treatment of malaria in the United States. September 23, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. Available at: http://www.cdc.gov/malaria/resources/pdf/treatmenttable.pdf.
- CDC. Malaria surveillance, annual summary 1980. MMWR 1982;31.
- CDC. Malaria Surveillance—United States, 2008. MMWR 2010;59(No. SS-7).
- Redman C, Smith V. Travel health: malaria cases in Scotland and the UK: 2007-2011. HPS Weekly Report 2012;46.
- World Tourism Organization. UNWTO Tourism Highlights, 2012 Edition. Madrid: UNWTO Publications; 2012. Available at http://www.e-unwto.org/content/x6k11g/fulltext.pdf.
- Pan American Health Organization. Status of malaria eradication in the Americas, 18th report. PAHO CSP 18/7. Washington, DC: Pan American Health Organization; 1970. Available at http://new.paho.org/hq/index.php?lang=en.
- Agarwal A, McMorrow M, Arguin PM. The increase of imported malaria acquired in Haiti among US travelers in 2010. Am J Trop Med Hyg 2012;86:9–10.
- Townes D, Existe A, Boncy J, et al. Malaria survey in post-earthquake Haiti—2010. Am J Trop Med Hyg 2012;86:29–31.
- CDC. Immigrants returning home to visit friends and relatives (VFRs). In: CDC Health information for international travel 2012. New York: Oxford University Press; 2012. p. 547–51.
- Londono BL, Eisele TP, Keating J, et al. Chloroquine-resistant haplotype Plasmodium falciparum parasites, Haiti. Emerg Infect Dis 2009;15:735–40.
- Londono-Renteria B, Eisele TP, Keating J, Bennett A, Krogstad DJ. Genetic diversity in the merozoite surface protein 1 and 2 genes of Plasmodium falciparum from the Artibonite Valley of Haiti. Acta Tropica 2012;121:6–12.
- Travel Agent Central. Delta expands service to Africa with new Liberia flights. New York: Travel Agent Central; 2010. Available at http://www.travelagentcentral.com/west-africa/delta-expands-service-africa-new-liberia-flights-21705.
- Selent M, de Rochars VMB, Stanek D, et al. Malaria prevention Knowledge, Attitudes, and Practices (KAP) among international flying pilots and flight attendants of a US commercial airline. Journal of Travel Medicine 2012;19:366–72.
- CDC. Malaria surveillance—United States, 2004. MMWR 2006;55(No. SS-4).
- Herwaldt BL, Juranek DD. Laboratory-acquired malaria, leishmaniasis, trypanosomiasis, and toxoplasmosis. Am J Trop Med Hyg 1993;48:313–23.
- Herwaldt BL. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev 2001;14:659–88.
- Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA 2007;297:2264–77.
- CDC. Primaquine shortage, 2011. Available at http://www.cdc.gov/malaria/new_info/2011/primaquine.html.
- CDC. Primaquine now available, 2012. Available at http://www.cdc.gov/malaria/new_info/2012/primaquine.html.
- Baird JK. Effectiveness of Antimalarial Drugs. New Engl J Med 2005;352:1565–77.
- Abanyie FA, Arguin PM, Gutman J. State of malaria diagnostic testing at clinical laboratories in the United States, 2010: a nationwide survey. Malaria Journal 2011;10:340.
- CDC. New medication for severe malaria available under an investigational new drug protocol. MMWR 2007;56:769–73.
|
Year
|
U.S. military personnel
|
U.S. civilians
|
Foreign residents
|
Status not recorded
|
Total
|
|
1970
|
4,096
|
90
|
44
|
17
|
4,247
|
|
1971
|
2,975
|
79
|
69
|
57
|
3,180
|
|
1972
|
454
|
106
|
54
|
0
|
614
|
|
1973
|
41
|
103
|
78
|
0
|
222
|
|
1974
|
21
|
158
|
144
|
0
|
323
|
|
1975
|
17
|
199
|
232
|
0
|
448
|
|
1976
|
5
|
178
|
227
|
5
|
415
|
|
1977
|
11
|
233
|
237
|
0
|
481
|
|
1978
|
31
|
270
|
315
|
0
|
616
|
|
1979
|
11
|
229
|
634
|
3
|
877
|
|
1980
|
26
|
303
|
1,534
|
1
|
1,864
|
|
1981
|
21
|
273
|
809
|
0
|
1,103
|
|
1982
|
8
|
348
|
574
|
0
|
930
|
|
1983
|
10
|
325
|
468
|
0
|
803
|
|
1984
|
24
|
360
|
632
|
0
|
1,016
|
|
1985
|
31
|
446
|
568
|
0
|
1,045
|
|
1986
|
35
|
410
|
646
|
0
|
1,091
|
|
1987
|
23
|
421
|
488
|
0
|
932
|
|
1988
|
33
|
550
|
440
|
0
|
1,023
|
|
1989
|
35
|
591
|
476
|
0
|
1,102
|
|
1990
|
36
|
558
|
504
|
0
|
1,098
|
|
1991
|
22
|
585
|
439
|
0
|
1,046
|
|
1992
|
29
|
394
|
481
|
6
|
910
|
|
1993
|
278
|
519
|
453
|
25
|
1,275
|
|
1994
|
38
|
524
|
370
|
82
|
1,014
|
|
1995
|
12
|
599
|
461
|
95
|
1,167
|
|
1996
|
32
|
618
|
636
|
106
|
1,392
|
|
1997
|
28
|
698
|
592
|
226
|
1,544
|
|
1998
|
22
|
636
|
361
|
208
|
1,227
|
|
1999
|
55
|
833
|
381
|
271
|
1,540
|
|
2000
|
46
|
827
|
354
|
175
|
1,402
|
|
2001
|
18
|
891
|
316
|
158
|
1,383
|
|
2002
|
33
|
849
|
272
|
183
|
1,337
|
|
2003
|
36
|
767
|
306
|
169
|
1,278
|
|
2004
|
32
|
775
|
282
|
235
|
1,324
|
|
2005
|
36
|
870
|
297
|
325
|
1,528
|
|
2006
|
50
|
736
|
217
|
561
|
1,564
|
|
2007
|
33
|
701
|
263
|
508
|
1,505
|
|
2008
|
19
|
510
|
176
|
593
|
1,298
|
|
2009
|
18
|
661
|
201
|
604
|
1,484
|
|
2010
|
46
|
1,085
|
368
|
192
|
1,691
|
|
2011
|
91
|
1,098
|
386
|
350
|
1,925
|
|
Plasmodium species
|
2008
|
2009
|
2010
|
2011
|
|
No.
|
(%)
|
No.
|
(%)
|
No.
|
(%)
|
No.
|
(%)
|
|
P. falciparum
|
527
|
(40.6)
|
687
|
(46.3)
|
982
|
(58.1)
|
948
|
(49.3)
|
|
P. vivax
|
190
|
(14.6)
|
166
|
(11.2)
|
325
|
(19.2)
|
420
|
(21.8)
|
|
P. malariae
|
19
|
(1.5)
|
32
|
(2.1)
|
35
|
(2.1)
|
50
|
(2.6)
|
|
P. ovale
|
18
|
(1.4)
|
29
|
(2.0)
|
33
|
(1.9)
|
51
|
(2.6)
|
|
P. knowlesi
|
1
|
(0.1)
|
0
|
(0)
|
0
|
(0)
|
0
|
(0)
|
|
Mixed
|
8
|
(0.6)
|
13
|
(0.9)
|
13
|
(0.8)
|
21
|
(1.1)
|
|
Undetermined
|
535
|
(41.2)
|
557
|
(37.5)
|
303
|
(17.9)
|
435
|
(22.6)
|
|
Total
|
1,298
|
(100)
|
1,484
|
(100)
|
1,691
|
(100)
|
1,925
|
(100)
|
|
Country of Acquisition
|
P. vivax
|
P. falciparum
|
P. malariae
|
P. ovale
|
Mixed
|
Unknown
|
Total
|
|
Africa
|
76
|
786
|
42
|
34
|
10
|
196
|
1,144
|
|
Angola
|
0
|
8
|
2
|
1
|
0
|
1
|
12
|
|
Benin
|
0
|
4
|
0
|
0
|
0
|
0
|
4
|
|
Burkina Faso
|
1
|
8
|
0
|
0
|
0
|
1
|
10
|
|
Cameroon
|
2
|
52
|
2
|
2
|
0
|
4
|
62
|
|
Central African Republic
|
0
|
5
|
0
|
0
|
0
|
0
|
5
|
|
Congo, Republic of
|
1
|
11
|
0
|
0
|
0
|
3
|
15
|
|
Cóte d'Ivoire
|
1
|
23
|
0
|
2
|
0
|
2
|
28
|
|
Democratic Republic of Congo
|
0
|
3
|
0
|
0
|
0
|
0
|
3
|
|
Equatorial Guinea
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
Eritrea
|
11
|
0
|
0
|
0
|
3
|
4
|
18
|
|
Ethipoia
|
34
|
6
|
1
|
2
|
1
|
11
|
55
|
|
Gabon
|
0
|
1
|
1
|
0
|
0
|
0
|
2
|
|
Gambia
|
1
|
19
|
0
|
0
|
0
|
1
|
21
|
|
Ghana
|
3
|
112
|
5
|
8
|
2
|
26
|
156
|
|
Guinea
|
1
|
34
|
1
|
0
|
1
|
3
|
40
|
|
Guinea-Bissau
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
Kenya
|
0
|
24
|
4
|
1
|
0
|
8
|
37
|
|
Liberia
|
1
|
65
|
4
|
2
|
0
|
18
|
90
|
|
Madagascar
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
Malawi
|
1
|
5
|
0
|
0
|
0
|
2
|
8
|
|
Mali
|
0
|
7
|
0
|
0
|
0
|
1
|
8
|
|
Mozambique
|
1
|
3
|
0
|
0
|
0
|
2
|
6
|
|
Niger
|
0
|
2
|
0
|
0
|
0
|
1
|
3
|
|
Nigeria
|
3
|
160
|
1
|
4
|
2
|
43
|
213
|
|
Rwanda
|
1
|
1
|
0
|
1
|
0
|
0
|
3
|
|
Senegal
|
0
|
16
|
0
|
0
|
0
|
1
|
17
|
|
Sierra Leone
|
1
|
81
|
5
|
2
|
0
|
27
|
116
|
|
Somalia
|
1
|
0
|
0
|
0
|
0
|
0
|
1
|
|
South Africa
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
Sudan
|
1
|
26
|
2
|
1
|
0
|
2
|
32
|
|
Tanzania
|
0
|
5
|
0
|
2
|
0
|
0
|
7
|
|
Togo
|
0
|
9
|
1
|
1
|
0
|
3
|
14
|
|
Uganda
|
5
|
34
|
9
|
2
|
1
|
10
|
61
|
|
Zambia
|
0
|
2
|
0
|
0
|
0
|
2
|
4
|
|
Central Africa, unspecified
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
East Africa, unspecified
|
2
|
8
|
0
|
0
|
0
|
2
|
12
|
|
Southern Africa, unspecified
|
1
|
0
|
0
|
0
|
0
|
1
|
2
|
|
West Africa, unspecified
|
2
|
17
|
1
|
0
|
0
|
5
|
25
|
|
Africa, unspecified
|
1
|
30
|
3
|
3
|
0
|
12
|
49
|
|
Country of Acquisition
|
P. vivax
|
P. falciparum
|
P. malariae
|
P. ovale
|
Mixed
|
Unknown
|
Total
|
|
Asia
|
258
|
23
|
3
|
7
|
9
|
63
|
363
|
|
Afghanistan
|
43
|
1
|
0
|
2
|
1
|
13
|
60
|
|
Bangladesh
|
0
|
1
|
0
|
0
|
0
|
2
|
3
|
|
Burma (Myanmar)
|
1
|
3
|
0
|
0
|
0
|
1
|
5
|
|
Cambodia
|
1
|
1
|
0
|
0
|
0
|
2
|
4
|
|
India
|
161
|
13
|
3
|
4
|
7
|
35
|
223
|
|
Indonesia
|
3
|
1
|
0
|
0
|
0
|
0
|
4
|
|
Laos
|
1
|
0
|
0
|
0
|
0
|
0
|
1
|
|
Nepal
|
0
|
0
|
0
|
0
|
0
|
1
|
1
|
|
Pakistan
|
31
|
0
|
0
|
1
|
1
|
6
|
39
|
|
Philippines
|
1
|
0
|
0
|
0
|
0
|
1
|
2
|
|
Sri Lanka (Ceylon)
|
3
|
0
|
0
|
0
|
0
|
0
|
3
|
|
Thailand
|
9
|
2
|
0
|
0
|
0
|
1
|
12
|
|
Southeast Asia, unspecified
|
2
|
1
|
0
|
0
|
0
|
1
|
4
|
|
Asia, unspecified
|
2
|
0
|
0
|
0
|
0
|
0
|
2
|
|
Central American and the Caribbean
|
21
|
70
|
2
|
0
|
0
|
12
|
105
|
|
Belize
|
1
|
0
|
0
|
0
|
0
|
1
|
2
|
|
Dominican Republic
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
Guatemala
|
1
|
0
|
0
|
0
|
0
|
2
|
3
|
|
Haiti
|
0
|
65
|
1
|
0
|
0
|
6
|
72
|
|
Honduras
|
15
|
3
|
0
|
0
|
0
|
3
|
21
|
|
Mexico
|
2
|
0
|
0
|
0
|
0
|
0
|
2
|
|
Central America, unspecified
|
2
|
0
|
1
|
0
|
0
|
0
|
3
|
|
Caribbean, unspecified
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
South America
|
22
|
10
|
0
|
0
|
0
|
3
|
35
|
|
Brazil
|
6
|
0
|
0
|
0
|
0
|
1
|
7
|
|
Colombia
|
1
|
1
|
0
|
0
|
0
|
0
|
2
|
|
Ecuador
|
1
|
0
|
0
|
0
|
0
|
0
|
1
|
|
French Guiana
|
1
|
0
|
0
|
0
|
0
|
0
|
1
|
|
Guyana
|
10
|
8
|
0
|
0
|
0
|
1
|
19
|
|
Peru
|
3
|
0
|
0
|
0
|
0
|
1
|
4
|
|
Venezuela
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
Oceania
|
3
|
2
|
0
|
1
|
0
|
1
|
7
|
|
Papua New Guinea
|
3
|
1
|
0
|
1
|
0
|
1
|
6
|
|
Timor-Leste (East Timor)
|
0
|
1
|
0
|
0
|
0
|
0
|
1
|
|
Middle East
|
0
|
0
|
0
|
0
|
0
|
1
|
1
|
|
Middle East, unspecified
|
0
|
0
|
0
|
0
|
0
|
1
|
1
|
|
Unknown
|
39
|
54
|
2
|
9
|
2
|
159
|
265
|
|
Total
|
419
|
945
|
49
|
51
|
21
|
435
|
1,920
|
FIGURE 1. Number of malaria cases*, by state or territory in which case was diagnosed — United States, 2011
Alternate Text: The figure above presents a map of the United States that shows the number of malaria cases that occurred in each state and U.S. territory during 2011.
|
Area or region
|
United States
|
Foreign
|
Total
|
|
No.
|
(%)
|
No.
|
(%)
|
No.
|
(%)
|
|
Africa
|
818
|
(69.0)
|
239
|
(62.1)
|
1057
|
(67.4)
|
|
Asia
|
216
|
(18.2)
|
122
|
(31.7)
|
338
|
(21.5)
|
|
Central America/ Caribbean
|
87
|
(7.3)
|
12
|
(3.1)
|
99
|
(6.3)
|
|
South America
|
28
|
(2.4)
|
5
|
(1.3)
|
33
|
(2.1)
|
|
Oceania
|
5
|
(0.4)
|
1
|
(0.3)
|
6
|
(0.4)
|
|
Middle East
|
1
|
(0.1)
|
0
|
(0)
|
1
|
(0.1)
|
|
Unknown
|
31
|
(2.6)
|
6
|
(1.6)
|
37
|
(2.4)
|
|
Total
|
1,186
|
(100)
|
385
|
(100)
|
1,571
|
(100)
|
|
Interval (days)
|
P. falciparum
|
P. vivax
|
P. malariae
|
P. ovale
|
Mixed
|
Total
|
|
No
|
(%)
|
No
|
(%)
|
No
|
(%)
|
No
|
(%)
|
No
|
(%)
|
No
|
(%)
|
|
<0†
|
112
|
(15.2)
|
30
|
(11.1)
|
3
|
(9.1)
|
3
|
(9.1)
|
2
|
(25.0)
|
150
|
(13.9)
|
|
0-29
|
590
|
(80.0)
|
111
|
(41.1)
|
16
|
(48.5)
|
11
|
(33.3)
|
4
|
(50.0)
|
732
|
(67.7)
|
|
30-89
|
23
|
(3.1)
|
48
|
(17.8)
|
10
|
(30.3)
|
7
|
(21.2)
|
1
|
(12.5)
|
89
|
(8.2)
|
|
90-179
|
5
|
(0.7)
|
43
|
(15.9)
|
1
|
(3.0)
|
6
|
(18.2)
|
0
|
(0.0)
|
55
|
(5.1)
|
|
180-364
|
7
|
(1.0)
|
34
|
(12.6)
|
3
|
(9.1)
|
5
|
(15.2)
|
0
|
(0.0)
|
49
|
(4.5)
|
|
>365
|
1
|
(0.1)
|
4
|
(1.5)
|
0
|
(0.0)
|
1
|
(3.0)
|
1
|
(12.5)
|
7
|
(0.6)
|
|
Total
|
738
|
(100.0)
|
270
|
(100.0)
|
33
|
(100.0)
|
33
|
(100.0)
|
8
|
(100.0)
|
1,082
|
(100.0)
|
FIGURE 2. Number of malaria cases*, by species and month of onset — United States, 2011
Alternate Text: The figure above is a line graph that presents the number of malaria cases in 2011 by month and the type of infecting species.
|
Category
|
No.
|
(%)
|
|
Visiting friends and relatives
|
607
|
(55.4)
|
|
Tourist
|
45
|
(4.1)
|
|
Missionary or dependent
|
96
|
(8.8)
|
|
Business representative
|
78
|
(7.1)
|
|
Student or teacher
|
32
|
(2.9)
|
|
Air crew or sailor
|
10
|
(0.9)
|
|
Other
|
3
|
(0.3)
|
|
Unknown
|
224
|
(20.5)
|
|
Type of
information
|
Source
|
Availability
|
Telephone number, internet address,
or electronic mail address
|
|
Prophylaxis
|
CDC's Traveler's Health Internet site (includes online access to Health Information for International Travel)
|
24 hours/day
|
http://wwwnc.cdc.gov/travel
|
|
Health Information for International Travel (The Yellow Book)
|
Order from Oxford University Press, Inc. Order Fulfillment 198 Madison Avenue, New York, NY 10016-4314
|
800-451-7556 or http://www.oup.com/us/
|
|
CDC Malaria Map Application
|
24 hours/day
|
http://www.cdc.gov/malaria/map
|
|
Diagnosis
|
CDC's Division of Parasitic Diseases and Malaria diagnostic internet site (DPDx)
|
24 hours/day
|
http://www.dpd.cdc.gov/dpdx
|
|
CDC's Division of Parasitic Diseases and Malaria diagnostic CD-ROM (DPDx)
|
Order by electronic mail from CDC Division of Parasitic Diseases and Malaria
|
dpdx@cdc.gov
|
|
Treatment
|
CDC Malaria Branch
|
9:00 am–5:00 pm Eastern time, Monday–Friday
|
770-488-7788 or toll-free 855-856-4713*
|
|
CDC Malaria Branch
|
5:00 pm–9:00 am Eastern time on weekdays and all day weekends and holidays
|
770-488-7100* (This number is for the CDC's
Emergency Operations Center. Ask staff member
to page the person on call for the Malaria Branch). http://www.cdc.gov/malaria/diagnosis_treatment/treatment.html
|