Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Diagnosis and Management of Q Fever — United States, 2013: Recommendations from CDC and the Q Fever Working Group
Please note: An erratum has been published for this article. To view the erratum, please click here.
The material in this report originated in the National Center for Emerging and Zoonotic Infectious Diseases, Beth P. Bell, MD, Director; and the Division of Vector-Borne Diseases, Lyle R. Petersen, MD, Director.
Corresponding preparer: Alicia Anderson, DVM, Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC, 1600 Clifton Road, MS A-30, Atlanta, GA 30333. Telephone: 404-639-4499; Fax: 404-639-2778; E-mail: aha5@cdc.gov.
Summary
Q fever, a zoonotic disease caused by the bacterium Coxiella burnetii, can cause acute or chronic illness in humans. Transmission occurs primarily through inhalation of aerosols from contaminated soil or animal waste. No licensed vaccine is available in the United States. Because many human infections result in nonspecific or benign constitutional symptoms, establishing a diagnosis of Q fever often is challenging for clinicians. This report provides the first national recommendations issued by CDC for Q fever recognition, clinical and laboratory diagnosis, treatment, management, and reporting for health-care personnel and public health professionals. The guidelines address treatment of acute and chronic phases of Q fever illness in children, adults, and pregnant women, as well as management of occupational exposures. These recommendations will be reviewed approximately every 5 years and updated to include new published evidence.
Introduction
Q fever, first described in 1937, is a worldwide zoonosis that has long been considered an underreported and underdiagnosed illness because symptoms frequently are nonspecific, making diagnosis challenging (1–3). The causative organism, Coxiella burnetii, is an intracellular bacterium that tends to infect mononuclear phagocytes but can infect other cell types as well. Infection in humans usually occurs by inhalation of bacteria from air that is contaminated by excreta of infected animals. Other modes of transmission to humans, including tick bites, ingestion of unpasteurized milk or dairy products, and human-to-human transmission, are rare (1). Laboratory diagnosis relies mainly on serology, and doxycycline is the most effective treatment for acute illness. No vaccine is available commercially in the United States.
Q fever was designated a nationally notifiable disease in the United States in 1999. Since then, reports of Q fever have increased, with 167 cases reported in 2008, an increase greater than ninefold compared with 2000, in which 17 cases were reported (4). The national seroprevalence of Q fever is estimated to be 3.1% based on data from the National Health and Nutrition Examination Survey (2003–2004), and human infections have been reported from every state in the United States (5). Q fever infections in humans and animals have been reported from every world region except Antarctica (6).
Q fever has acute and chronic stages that correspond to two distinct antigenic phases of antibody response. During an acute infection, an antibody response to C. burnetii phase II antigen is predominant and is higher than the response to the phase I antigen, whereas a chronic infection is associated with a rising phase I immunoglobulin G (IgG) titer. Although acute Q fever symptoms in humans vary, the condition typically is characterized by a nonspecific febrile illness, hepatitis, or pneumonia. Asymptomatic infections followed by seroconversion have been reported in up to 60% of cases identified during outbreak investigations (6–8). Onset of symptoms usually occurs within 2–3 weeks of exposure, and symptomatic patients might be ill for weeks or months if untreated.
Chronic Q fever can manifest within a few months or several years after acute infection and can follow symptomatic or asymptomatic infections. Chronic disease is rare (<5% of patients with acute infections) and typically is characterized by endocarditis in patients with preexisting risk factors such as valvular or vascular defects (9). Unlike acute Q fever, which has a low mortality rate (<2%), chronic Q fever endocarditis is always fatal if untreated (10). Routine blood cultures are negative in patients with chronic Q fever endocarditis. Diagnosis of chronic Q fever endocarditis can be extremely difficult because vegetative lesions are visualized by echocardiography in approximately 12% of patients (6).
Q fever is an occupational disease in persons whose work involves contact with animals, such as slaughterhouse workers, veterinarians, and farmers, although infection is not limited to these groups. Urban outbreaks and cases with no known exposure or close proximity to livestock have been reported, as have nonoccupational exposures such as through a hobby farm (a small farm that is not a primary source of income) (11). Data collected from Q fever case report forms submitted to CDC during 2000–2010 indicate that 320 of 405 (79%) cases in patients who reported occupational status are recognized in patients who are not in previously defined high-risk occupations, and 243 of 405 (60%) cases are in patients who do not report livestock contact (CDC, unpublished data, 2010). These findings underscore the need for health-care professionals to consider Q fever in the differential diagnosis in patients with a compatible illness, even in the absence of occupational risk or history of direct contact with animal reservoirs. Approximately 200 cases of acute Q fever were reported in U.S. military personnel who had been deployed to Iraq since 2003. Investigations of these cases linked illness to tick bites, sleeping in barns, and living near helicopter zones with environmental exposure resulting from helicopter-generated aerosols (12,13).
The largest known reported Q fever outbreak involved approximately 4,000 human cases and occurred during 2007–2010 in the Netherlands. This outbreak was linked to dairy goat farms near densely populated areas and presumably involved human exposure via a windborne route (14).
Prompt diagnosis and appropriate treatment shortens the illness and reduces the risk for severe complications (15,16). In patients with chronic Q fever illness, early treatment might be lifesaving. Physician awareness of the epidemiologic and clinical characteristics of Q fever is required to make a prompt and correct diagnosis. Information in this report is designed to assist U.S. clinicians with the following:
- Recognize common epidemiologic features and clinical manifestations of Q fever.
- Consider Q fever as the cause of a patient's illness if appropriate.
- Obtain relevant history (e.g., medical history and exposure) and diagnostic tests for Q fever.
- Identify the limitations and utility of laboratory diagnostic testing.
- Make treatment decisions based on epidemiologic and clinical evidence.
- Recognize that doxycycline is the treatment of choice for patients of any age with severe illness.
- Recognize potential severe manifestations of acute and chronic Q fever and understand appropriate strategies to monitor and manage these patients.
- Manage infected children and pregnant women appropriately.
- Provide effective risk communication for persons at high risk for Q fever exposure.
- Report suspect and confirmed cases to appropriate public health officials.
A compilation of the key point summaries for Q fever clinical features, diagnosis, treatment, occupational exposures, and surveillance and reporting are provided (Appendix A).
Methods
This report provides the first national guidelines for the diagnosis and management of Q fever in the United States. The recommendations were prepared by the Q Fever Working Group, which includes CDC scientists, infectious disease specialists, laboratorians, epidemiologists, and clinical practitioners with expertise in the diagnosis and management of Q fever. These recommendations were developed through expert consultation and consensus and represent the best judgment of Q fever subject-matter experts, many of whom are international experts because of the low number of Q fever clinical subject-matter experts in the United States. In 2009, CDC created the first draft using previously published guidelines, review articles, and multiple search strategies of medical and professional computerized databases. During 2010–2012, each member of the Q Fever Working Group reviewed, revised, and refined the recommendations. In 2012, the CDC National Institute of Occupational Safety and Health reviewed the recommendations. When possible, recommendations were based on existing recommendations or guidelines (referenced within the text), with emphasis on U.S. populations. Published guidelines and the peer-reviewed literature were reviewed to ensure the relevance, completeness, and accuracy of the recommendations. If no adequate guidelines existed, the guidelines and recommendations were based on the experience and expertise of the Q Fever Working Group members.
Epidemiology
Overview
Cattle, sheep, and goats are the primary reservoirs for C. burnetii. However, infection has been confirmed in multiple vertebrate species, including wildlife, marine mammals, domestic mammals, birds, and reptiles (17). C. burnetii has been isolated from approximately 40 species of ticks, and possible tickborne transmission of C. burnetii to humans has been reported (18–20). Any infected animal has the potential to transmit the pathogen via bacterial shedding in their body secretions. Human outbreaks and cases have been epidemiologically linked to exposure to multiple species including pigeons, dogs, and rabbits (21–23). Human cases and outbreaks linked to exposure to infected parturient cats also have been reported (24–26).
The majority of animal infections are asymptomatic. Clinical illness in ruminants is primarily characterized by reproductive disorders such as abortion, stillbirth, endometritis, mastitis, and infertility (27). The highest numbers of organisms are shed in birth products, although viable organisms also might be shed in the urine, milk, and feces of infected animals (28,29). A positive antibody titer in an infected animal does not correlate with active shedding of organisms because some seronegative animals might actively shed bacteria (30,31). Conversely, animals might seroconvert after exposure to C. burnetii but not shed the bacteria (31–34). Thus, serologic testing is not a reliable method to determine whether specific animals are a potential source of transmission of C. burnetii to humans or other animals. Polymerase chain reaction (PCR) testing of body fluids (e.g., feces, milk, and vaginal mucus) is a more reliable method to detect shedding, which might be intermittent.
The most common mode of transmission in humans is inhalation of infectious aerosols directly from birth fluids of infected animals or via inhalation of dust contaminated with dried birth fluids or excreta. C. burnetii is extremely resistant to physical stresses, including heat and desiccation and can survive in the environment for months to years. The bacteria can become airborne, traveling on wind currents for miles, resulting in outbreaks (35,36). In one outbreak, Q fever cases were documented in persons who lived 10 miles from the farm that was the source of the outbreak. In a recent outbreak in the Netherlands, living within 2 km of an infected farm was a significant risk factor for infection (36–38). Less common routes of transmission include ingestion of raw milk and dairy products or contact with contaminated clothing (39,40).
Person-to-person transmission of Q fever is possible but rarely reported. Persistent infection of the genital tract has been documented both in animals and humans, and sexual transmission and transplacental transmission of disease have been reported (41–44). Rare cases of transmission caused by blood transfusion or bone marrow transplantation from infected human donors have been reported (45,46). C. burnetii has been isolated from human breast milk, and lactogenic transmission is possible, although no cases have been documented via this route of transmission (47). Sporadic cases of nosocomial transmission associated with autopsies and obstetrical procedures of infected women have been reported (48,49).
The reported incidence and seroprevalence of acute Q fever is higher among persons aged ≥40 years than among younger persons, and disease severity increases with age (5,50). Persons aged 60–64 years have the highest age-related risk of Q fever in the United States (4). In addition, males have a higher risk for symptomatic Q fever illness than females (6), which might be partly explained by sex-associated occupational exposures or the protective effects of 17β-estradiol in females, which has been validated in animal studies (51,52).
Although infections occur year round, acute Q fever cases in the United States peak in the spring. Seasonal incidence of acute Q fever likely correlates with livestock birthing times or farm management practices such as manure spreading (4,53,54). In addition to host factors, other elements that might influence disease susceptibility and clinical manifestations include route of infection and size of inoculum (6,55,56).
Epidemiologic Factors Associated with Q Fever
When compiling a medical history, health-care providers should consider the following factors:
- occupations with increased contact with animals or animal products (particularly livestock), including veterinarians, butchers, slaughterhouse workers, farmers, and laboratory workers
- living in a rural area or living on or within 10 miles of a farm that houses livestock, particularly cattle, sheep, or goats
- recent travel to areas of higher risk for Q fever, such as rural, agricultural communities (domestic and international), areas with recent outbreaks such as the Netherlands, or regions such as the Middle East where the disease is highly endemic
- sexual contact with a person who has recently had Q fever or contact with contaminated clothing and linens leading to fomite transmission
- Q fever symptoms in a person who has a partner or family member who has received a diagnosis of Q fever
- chronic Q fever symptoms in anyone with a history of acute Q fever infection, particularly persons with valvular heart disease or a vascular graft or arterial aneurysm, immunosuppressed persons, and women who are pregnant
Although a detailed exposure history, including animal contact, might assist health-care providers in identifying potential Q fever in a patient, a lack of direct animal contact should not preclude a clinical suspicion of diagnosis because airborne transmission of C. burnetii can occur.
Assessment of Clinical Signs and Symptoms
Acute Q Fever
Adults
Symptomatic acute Q fever, which occurs in approximately half of infected persons, is characterized by a wide variety of clinical signs and symptoms. After an incubation period of 2–3 weeks, the most common clinical manifestation is a nonspecific febrile illness that might occur in conjunction with pneumonia or hepatitis (6). The most frequently reported symptoms include fever, fatigue, chills, and myalgia (Table 1). In a study of deployed U.S. military personnel, the three most common International Classification of Diseases, Clinical Modification (ICD-9-CM) codes assigned to patients who were later identified as having Q fever were as follows: fever, not otherwise specified (780.6); pneumonia, organism unspecified (486); and viral infection, not otherwise specified (079.99) (12).
Severe, debilitating headaches also are a frequent symptom, and lumbar punctures have been performed on patients for suspected meningitis who were later shown to have Q fever (J. Hartzell, MD, Walter Reed National Military Medical Center, 2012, personal communication). The headache might be retroorbital and associated with photophobia (6). In patients with acute Q fever illness, this has been misclassified as a new onset migraine headache or a potentially infected tooth because the headache pain radiates to the jaw (11).
Pneumonia is an important clinical manifestation of acute Q fever, and C. burnetii might be an underrecognized cause of community-acquired pneumonia. In North America in the 1980s, the prevalence of acute Q fever in 1,306 cases of community-acquired pneumonia in hospitalized patients was 30 cases (2.3%) (57). Features of Q fever pneumonia are similar to other etiologies of community-acquired pneumonia and cannot be distinguished clinically, radiologically, or by any other routine laboratory evaluation. Q fever pneumonia can range from mild to severe, and numerous patients have extrapulmonary manifestations (including severe headache, myalgia, and arthralgia). Cough is often present and is nonproductive in 50% of patients; upper respiratory signs are less likely to be reported in persons with Q fever pneumonia (58–62).
Fever lasts a median of 10 days in untreated patients (range: 5–57 days); the majority of cases defervesce within 72 hours of doxycycline administration (63,64). The duration of fever increases with age; one study demonstrated that 60% of patients aged >40 years had a fever duration of >14 days, compared with 29% of patients aged <40 years (64). In another study, 5%–21% of patients with acute Q fever had a maculopapular or purpuric rash (53). Onset of symptoms can be gradual or abrupt, with variable severity. Although mortality is <2% in patients with acute Q fever, in the Netherlands outbreak, which included approximately 4,000 reported cases, up to 50% of acute Q fever patients were hospitalized (6,38). Less frequently described clinical symptoms include pericarditis, myocarditis, aseptic meningitis, encephalitis, and cholecystitis (53,65).
Children
Children with Q fever are less likely to have symptoms than adults and might have a milder illness. Acute Q fever in symptomatic children typically is characterized by a febrile illness, often accompanied by headache, weakness, cough, and other nonspecific systemic symptoms. Illness frequently is self-limited, although a relapsing febrile illness lasting for several months has been documented in some children (66). Gastrointestinal symptoms such as diarrhea, vomiting, abdominal pain, and anorexia are reported in 50%–80% of pediatric cases (66–68). Skin rash is more common in children than adults, with a prevalence as high as 50% among children with diagnosed cases (66–69). Q fever pneumonia is usually moderate with mild cough; respiratory distress and chest pain might occur. Severe manifestations of acute disease are rare in children and include hepatitis, hemolytic uremic syndrome, myocarditis, pericarditis, encephalitis, meningitis, hemophagocytosis, lymphadenitis, acalculous cholecystitis, and rhabdomyolysis (70–74).
Pregnant Women
Q fever infections in women that occur shortly before conception or during pregnancy might result in miscarriage, stillbirth, premature birth, intrauterine growth retardation, or low birthweight (75). Adverse pregnancy outcomes are likely to be caused by vasculitis or vascular thrombosis resulting in placental insufficiency, although direct infection of the fetus has been documented (76). Of the reports that describe outcomes of infected pregnant women, none have documented an increased risk for congenital malformations because of infection (75,76).
Pregnant women might be less likely to have symptoms of Q fever compared with other adults (e.g., a febrile illness), although they remain at risk for adverse pregnancy outcomes (50). As a result, if a pregnant woman with no history of clinical illness has only a single increased antibody titer, it is difficult for the health-care provider to determine whether the increase is from a previous or current infection. Serosurveys of pregnant women evaluating a possible association between a single, elevated C. burnetii antibody titer (which cannot differentiate between previous or current infection) and adverse pregnancy outcomes have reported mixed findings (77–80). A woman with a previous infection (>30 days before conception) with no evidence of progression to chronic disease does not require treatment during pregnancy. However, a Q fever infection during pregnancy requires antibiotic treatment (Table 2), and health-care providers should consider several factors to determine the best treatment approach. Careful assessment of serologic results are useful because the phase II antibody response is increased in patients with an acute infection but decreases during convalescence as the phase I antibody response increases. Factors to consider before initiating treatment include whether the patient had contact with infected livestock, occupational animal contact, or an epidemiological link to another person with Q fever to guide treatment decisions.
The risk for adverse effects on the fetus and the risk that the mother will develop chronic Q fever are highest when an acute infection occurs during the first trimester (81,82). Untreated infection in the first trimester is more likely to result in miscarriage, whereas infection later in pregnancy is more likely to cause premature delivery (75). Women infected with acute Q fever during pregnancy, including those who were asymptomatic or experienced no adverse pregnancy outcomes, might be at risk for recrudescent infection during subsequent pregnancies (83). Therefore, pregnant women with a history of Q fever infection during a previous pregnancy should be monitored closely for recrudescent infection in all subsequent pregnancies.
Health-care providers should educate women of child-bearing age who receive a diagnosis of acute Q fever of potential risks to the fetus. These women should be advised to avoid pregnancy for at least 1 month after diagnosis and treatment and should receive a pregnancy test to determine whether long-term antibiotic treatment is needed.
Radiologic Evaluation
Pneumonia is one of the primary clinical manifestations of acute Q fever (6). Chest radiograph abnormalities are seen in the majority of patients with acute Q fever, although patients in the early stages of disease might have normal radiographic findings. Radiographic evaluation of acute Q fever patients during the Netherlands outbreak showed infiltrates in >96% of patients (84). Radiographic patterns for acute Q fever pneumonia are nonspecific; the most common abnormalities are segmental or lobar consolidation, which might be unilateral or bilateral, involve upper or lower lobes, or feature multiple or single opacities. Patchy infiltrations are an uncommon feature of Q fever pneumonia (58,85,86). Acute respiratory distress syndrome is a rare manifestation of Q fever but has occurred (87,88). It is not possible to differentiate Q fever pneumonia from other causes of community-acquired pneumonia solely on the basis of radiographic findings.
Laboratory Findings
Although up to 25% of patients with acute Q fever have an increased leukocyte count, most patients have normal white blood cell counts. Mild thrombocytopenia in early illness, which occurs in approximately one third of patients, might be followed by subsequent thrombocytosis. Increased erythrocyte sedimentation rate, hyponatremia, hematuria, increased creatine kinase, and increased C-reactive protein levels have been reported. The most common laboratory abnormalities are increased liver enzyme levels, which are observed in up to 85% of cases (89). Hyperbilirubinemia occurs in one in four patients (90–93). Hepatomegaly or splenomegaly (unrelated to thrombocytopenia) also might be present, although jaundice is rare (90). Q fever causes significant immune activation that might result in cross-reactivity with other laboratory tests for autoimmune or infectious processes or agents, including tests for antineutrophil cytoplasmic antibodies, human immunodeficiency virus (HIV), brucellosis, or rapid plasma reagin (94).
Summary of Acute Q Fever
- Prolonged fever (>10 days) with a normal leukocyte count, thrombocytopenia, and increased liver enzymes is suggestive of acute Q fever infection.
- Children with Q fever generally have a milder acute illness than adults.
- Children are more likely to have a rash than adults. Rash has been reported in up to 50% of children with acute Q fever.
- Women infected with Q fever during pregnancy are at increased risk for miscarriage and preterm delivery.
- Women of child-bearing age who receive a diagnosis of Q fever can benefit from pregnancy screening and counseling to guide health-care management decisions
Chronic Q Fever
Adults
Chronic Q fever is rare, occurring in <5% of persons with acute infection, and might occur within a few months, years, or even decades after the initial acute infection (6). Chronic disease can occur after symptomatic or asymptomatic infections. Potential signs and symptoms include endocarditis, chronic hepatitis, chronic vascular infections, osteomyelitis, osteoarthritis, and chronic pulmonary infections (6). Although patients likely have lifelong immunity to reinfection, disease recrudescence might occur and has been well documented (95).
The patients at highest risk for chronic Q fever are those with valvular heart disease, a vascular graft, or an arterial aneurysm. Acute infection in immunosuppressed persons and pregnant women also has been linked to later development of chronic disease (6,96). Since Q fever was categorized as a notifiable disease in the United States in 1999, CDC has received 49 reports of chronic Q fever, of which 24 manifested as endocarditis (CDC, unpublished data, 2012). Endocarditis is the major form of chronic Q fever, comprising 60%–78% of all cases worldwide. Endocarditis is a severe condition that is invariably fatal due to heart failure if untreated and has a 10-year mortality rate of 19% in treated patients (97,98). The second most common form of chronic Q fever is infection of aneurysms or vascular prostheses, followed by chronic Q fever infections after pregnancy (98). However, during the Q fever outbreak in the Netherlands, vascular infections were the most common form of chronic disease reported (15).
A clinical assessment of patients with acute Q fever should be performed to determine whether they are at high risk for subsequent chronic infection. Approximately 40% of persons with a known valvulopathy with an acute Q fever diagnosis subsequently develop infective endocarditis (99). Patients with endocarditis are predominantly men aged >40 years (97). Similar to other infective endocarditis etiologies, patients at highest risk for development of Q fever endocarditis after acute infection are those with a prosthetic valve, followed by patients with aortic bicuspid valves, mitral valve prolapse, and moderate mitral insufficiency (99,100).
The initial clinical signs and symptoms in patients with chronic Q fever often are nonspecific and highly variable. Features might include fatigue, fever, abdominal or chest pain, weight loss, night sweats, or hepatosplenomegaly (101). Arterial embolism, pulmonary embolism, or deep venous thrombosis also might occur (97,102). Valvular vegetations usually are small and are detected by echocardiogram in approximately 12% of cases. Cardiologists evaluating the echocardiogram should be made aware of the suspected diagnosis of chronic Q fever because the lesions, which often are subtle, might be missed (97). A transesophageal echocardiogram (TEE) is more sensitive than a transthoracic echocardiogram (TTE) in detecting valvular defects; TEEs should be performed in patients with suspected endocarditis who have TTEs that are negative or not definitive (103). However, a negative echocardiogram, whether TTE or TEE, does not rule out a diagnosis of chronic Q fever endocarditis.
In a retrospective study of patients with Q fever endocarditis, the most common abnormality found by echocardiography in patients with chronic Q fever endocarditis was a newly discovered or worsening valvular insufficiency (97). Other echocardiographic findings included vegetations, valvular thickening, calcification, cardiac abscess, stenosis, prosthetic leakage or avulsion, or pericardial effusion. The mitral and aortic valves were most commonly affected. Patients often did not have a previous diagnosis of acute Q fever. The median onset time for endocarditis was 2.5 months (range: 1–66 months) after acute illness among those who received a diagnosis of acute Q fever (97).
Persons with arterial aneurysms or vascular grafts also are at risk for chronic Q fever, and C. burnetii vascular infection carries a high mortality rate even in treated patients (96,104). Treated patients have an estimated 3-year mortality rate of 25%, a rate much higher than patients with treated Q fever endocarditis, who have an estimated 3-year mortality rate of 7% (97,104). The majority of C. burnetii vascular infections reported in the literature are aortic infections, which complicate an aortic aneurysm or previously placed endovascular prosthesis (104). The infected aneurysm frequently is diagnosed during surgery because the aortic wall ruptures; death is most commonly caused by vascular rupture. Infections develop more slowly in grafts than in aneurysms. Patients might have culture-negative aortitis (105). Spondylodiscitis and vertebral involvement might occur and should prompt testing for Q fever in patients with aortic defects (104). Imaging techniques that might prove useful for diagnosis of vascular infections include computed tomography, magnetic resonance imaging, or duplex ultrasound. Fluorodeoxyglucose positron emission tomography combined with computed tomography has high sensitivity and specificity for diagnosis of low-grade vascular infections and can visualize other potential infectious foci that cannot be visualized by other imaging methods (101,105).
Pregnant Women
Women infected with Q fever during pregnancy are at high risk for developing chronic Q fever, possibly because of a failure to mount an appropriate immune response to acute infection or the ability of C. burnetii to use placental trophoblasts as a replicative niche (106,107). The earlier during pregnancy a woman is infected, the greater her risk for development of chronic disease (75). After a diagnosis of new onset acute infection, treatment throughout pregnancy is recommended to decrease the risk for an adverse birth outcome as well as the risk for future development of chronic Q fever (75). Chronic infection might be evidenced by increased phase I IgG C. burnetii titers that do not decrease after pregnancy and can lead to adverse outcomes during subsequent pregnancies (81).
Children
Chronic Q fever is rarely reported in children. Pediatric chronic Q fever manifests most frequently as chronic relapsing or multifocal osteomyelitis, blood-culture–negative endocarditis, or chronic hepatitis (108). Children with Q fever osteomyelitis often experience a prolonged course with recurrent episodes affecting multiple bones before diagnosis (71,109). Like adults, children who are immunocompromised or have underlying heart valve disease might be at higher risk for chronic Q fever.
Summary of Chronic Q Fever
- Persons who are at high risk for development of chronic Q fever include persons with preexisting valvular heart disease, vascular grafts, or arterial aneurysms.
- Infection during pregnancy and immunosuppression (e.g., from chemotherapy) are both conditions that have been linked to chronic Q fever development.
- Endocarditis and infections of aneurysms or vascular prostheses are the most common forms of chronic Q fever and generally are fatal if untreated.
- Chronic Q fever is rarely reported in children.
- In contrast with adults, osteomyelitis is one of the most common findings in children with pediatric chronic Q fever.
Post-Q Fever Fatigue Syndrome
Post-Q fever fatigue syndrome has been reported in up to 20% of patients with acute Q fever and is the most common chronic outcome after acute infection (110–122). However, the data related to post-Q fever fatigue syndrome are limited, and its rate of occurrence in the United States is unknown. This syndrome is distinct from other sequelae of acute infection such as chronic Q fever manifesting as endocarditis or osteomyelitis. The majority of patients with post-Q fever fatigue syndrome are previously healthy persons with no underlying medical or psychological problems who develop a complex of symptoms dominated by a debilitating fatigue after symptomatic acute Q fever infection. Other accompanying symptoms might include nausea, headache, night sweats, myalgia, intermittent muscle fasciculations, enlarged lymph nodes, arthralgia, difficulty sleeping, alcohol intolerance, photophobia, irrational and out-of-proportion irritability, depression, decreased concentration, and impaired short-term memory. There is no apparent organ involvement (111).
Although any or all of these symptoms might occur in patients with an acute infection and last up to a year followed by full recovery, post-Q fever fatigue syndrome is characterized by fatigue and other expected Q fever symptoms that last beyond a year and for many patients last for several years or for life. No consensus has been reached on the pathogenesis of post-Q fever fatigue syndrome, although genetic predisposition and the severity of acute illness have been suggested to play a role in its development (117,123).
Diagnosis of post-Q fever fatigue syndrome relies on persistence of characteristic symptoms >1 year after a symptomatic acute Q fever infection, elevated antibody titers against C. burnetii antigen, and a lack of clinical and laboratory evidence of chronic Q fever with organ involvement. All other causes of similar symptoms should be excluded, and a thorough search for organ involvement or nidus of infection is imperative before making a diagnosis of post-Q fever fatigue syndrome because Q fever with organ involvement is responsive to antibiotic treatment. Management strategies for post-Q fever fatigue syndrome might reflect those used for chronic fatigue syndrome, such as graded exercise therapy and cognitive behavioral therapy. There are anecdotal reports of limited success using antibiotic therapy; however, no evidence-based recommendations exist for treatment of post-Q fever fatigue syndrome (124,125).
Diagnosis
Acute Q Fever
Because most persons with acute Q fever have nonspecific symptoms, health-care providers typically do not suspect Q fever during the acute stage of the disease. Although a laboratory diagnosis of acute Q fever can be made on the basis of serologic results, the requirement of a fourfold rise in phase II IgG antibody titer between acute and convalescent samples for definitive diagnosis makes this primarily a retrospective diagnosis (Table 3). For a definitive diagnosis in the early stages of acute Q fever illness, serologic testing in combination with PCR is recommended. PCR of whole blood or serum can be positive very early after symptom onset but becomes negative as the antibody titer increases and after administration of antibiotics (Table 4).
When interpreting serologic and PCR data, particularly if appropriately timed acute and convalescent titers were not obtained, empiric treatment should be based on the presence of a clinically compatible syndrome. Treatment should never be withheld pending receipt of diagnostic test results or discontinued because of a negative acute serologic or PCR result. Conversely, because antibodies might remain detectable for months to years after infection, treatment should not be provided based solely on elevated titers (such as those detected through routine screening or baseline occupational assessments) without clinical manifestation of acute illness (e.g., fever, pneumonia, hepatitis, or other acute symptoms).
Serologic Testing
For serologic testing, the indirect immunofluorescence assay (IFA) is commercially available and is the most commonly used method for serologic diagnosis of Q fever in the United States. Other methods described for Q fever serologic diagnosis include complement fixation, radioimmunoassay, enzyme-linked immunosorbent assay, and Western immunoblotting, although assay kits for these tests are not readily available in the United States.
The interpretation of serologic results for possible Q fever must include differential reactivity to Coxiella antigens. C. burnetii exists in two antigenic phases, phase I and phase II. Phase I is the virulent, highly infectious form that undergoes a transition to phase II, the avirulent form, during serial laboratory passages in embryonated eggs or cell cultures. In acute infection, the phase II antibody response to C. burnetii appears first and is higher than the phase I antibody response (6).
The most commonly used means of confirming the diagnosis of acute Q fever is demonstration of a fourfold rise in phase II IgG by IFA between serum samples from the acute and convalescent phases taken 3–6 weeks apart. Ideally, the first serum specimen should be taken during the first week of illness. Although this specimen can be tested immediately, results often are negative or too low for detection pending production of measurable antibodies. Therefore, serum samples from the acute phase are not helpful for guiding immediate treatment decisions. Various values are used by individual laboratories to categorize patients as seropositive or seronegative.
Alternatively, the serum specimen from the acute phase could be appropriately stored (refrigerated at 4°C [32.9°F] or frozen at ≤-20°C [-4°F]) until the convalescent serum specimen is drawn. Both specimens can be sent together to the laboratory for IFA testing in the same assay run to eliminate interassay variation. If the samples cannot be tested simultaneously, interassay and interlaboratory variations can lead to misinterpretation of perceived changes in titer. End-point titers also might be influenced by the use of different antigen preparations and assay protocols (126). Because results from different laboratories can differ significantly, using the same laboratory service whenever possible can provide the most accurate results (126).
Seroconversion typically occurs 7–15 days after symptoms appear, and 90% of patients seroconvert by the third week of illness. Immunoglobulin M (IgM) antibodies to phase II antigen develop in the second week of acute illness, with an increase in phase II IgG occurring almost simultaneously. In successfully treated or spontaneously resolving disease, IgG and IgM titers to phase I antigen might continue to increase in later specimens but typically do not exceed phase II titers.
Regardless of whether the infection is symptomatic or asymptomatic, after the infection, antibodies might remain detectable for many months, for years, or for life (127). In a nationally representative serosurvey of otherwise healthy persons, 3.1% of the general adult U.S. population had detectable antibodies to C. burnetii (5). Therefore, a single serum sample is less useful for the diagnosis of acute Q fever than paired acute and convalescent serum samples collected 3–6 weeks apart. Despite these limitations, a single serum titer is the most commonly applied diagnostic criterion among cases reported to CDC, likely because clinical suspicion for Q fever is uncommon for patients who initially seek care for symptoms. In the absence of positive PCR results and the ability to obtain an acute serum sample, a single positive convalescent serum sample (IgG phase II ≥1:128) in a patient who has been ill >1 week indicates a probable acute infection
IgM results provide ancillary information to the IgG titers; however, because of persistence (>1 year in some cases), the IgM test provides limited diagnostic value as a standalone test. IgM antibodies have a much lower specificity than IgG and might have a higher cross-reactivity. Cross-reactions between Coxiella, Legionella, and Bartonella species have been reported (128,129). However, the cross-reacting antibodies generally have low titers and should not result in misdiagnosis.
Because early doxycycline treatment (within the first 3 days of symptoms) is most effective, treatment of a patient suspected of having Q fever should be based on clinical findings and should not be delayed while awaiting laboratory confirmation (16). No evidence indicates that early administration of doxycycline blunts the antibody response or prevents seroconversion (130,131).
Nucleic Acid Detection
Rapid, sensitive, and quantitative PCR techniques have been developed for Q fever testing. Multiple gene targets have been used, and physicians should be aware that they can differ in sensitivity and specificity (132).
Either whole blood collected in anticoagulant-treated tubes or serum can be used for PCR testing. Whole blood might have a higher concentration of C. burnetii DNA than serum but is also likely to have more PCR inhibitors. For PCR results to be useful, the clinical sample must be obtained in the acute phase of infection (optimally during the first 2 weeks of symptom onset) and either before or shortly after (within 24–48 hours) antibiotic administration. When appropriate samples are drawn (i.e., during the acute phase and before or shortly after antibiotic administration), PCR results are positive in almost all patients with early acute Q fever before the antibody response develops (133).
Chronic Q Fever
The Duke criteria, a set of validated diagnostic criteria for infective endocarditis, were revised in 2000 to include redefined Q fever serologic parameters (134). That revision defined a phase I IgG antibody titer >1:800 or a single positive blood culture for C. burnetii as a major criterion for infective endocarditis. The Duke Endocarditis Service also advocated for use of TEEs as the initial diagnostic test of choice in patients categorized as having possible infective endocarditis, those with suspected complicated infective endocarditis, and those with suspected prosthetic valve infective endocarditis (Appendix B). A patient with a phase I IgG antibody titer >1:800 or a single positive blood culture for C. burnetii and one of the following minor criteria would be classified as having possible infective endocarditis, thereby warranting use of an initial TEE: predisposition, predisposing heart condition or injection drug use, fever, vascular phenomena, immunologic phenomena, or microbiologic evidence.
Serologic Testing
Chronic Q fever is diagnosed primarily by serologic testing. Establishing an identifiable nidus of chronic infection (e.g., endocarditis, vascular infection, or osteomyelitis) is required, as is laboratory confirmation. The distinct antigenic phases to which humans develop antibodies play an important role in the diagnosis. In contrast to acute Q fever infection, chronic infection is associated with continued increasing phase I IgG titers (typically ≥1:1024) that might be higher than phase II IgG. However, there are reports of chronic Q fever patients who retain extremely high phase II IgG antibody titers that equal or exceed their phase I IgG titers (135,136). If an acute Q fever case progresses to chronic disease, phase I IgG titer will continue to rise to levels ≥1:1024 and might exceed the phase II titer. It is possible for a patient with previously diagnosed acute Q fever who no longer has clinical symptoms to have increased phase I IgG titers for several months that subsequently decrease or stabilize without ever progressing to chronic disease (135,137).
Nucleic Acid Detection
Patients with suspected chronic Q fever should have whole blood or serum PCR performed because they can experience a recurrent bacteremia similar to early acute infection. Reported rates of PCR positivity in blood or serum of patients with Q fever endocarditis have ranged from 33% to 64% (97,135,136). PCR assays also can be performed on excised heart valve tissue from the site of active infection, even if frozen or embedded in paraffin. Infected heart valves, procured fresh or as formalin-fixed, paraffin-embedded specimens, are excellent for laboratory diagnosis because they typically contain abundant numbers of bacteria. PCR can be performed on cerebrospinal fluid, pleural fluid, bone marrow, bone biopsies, liver biopsies, milk, placenta, and fetal tissue.
Immunohistochemistry
Immunohistochemistry can be used to detect the presence of C. burnetii antigens in formalin-fixed, paraffin-embedded tissues and is particularly valuable for examining cardiac valve specimens excised from patients with culture-negative endocarditis for whom chronic Q fever is suspected (138). This assay is particularly useful because it can stain C. burnetii bacteria in tissues from patients even after they have received antibiotic therapy. The assay also can provide a crucial retrospective diagnosis in patients who relapse after valve replacement surgery for unrecognized or undiagnosed Q fever endocarditis. In the United States, this test can be referred to CDC through state public health laboratories.
Isolation
Cultivation of C. burnetii is not recommended for routine diagnosis because the process is difficult, time consuming, and dangerous; culture requires a biosafety level 3 (BSL-3) laboratory because bacteria are highly infective and can be hazardous for laboratory workers. Often, patients with chronic Q fever have already received antibiotics, which further complicates isolation attempts; a negative culture does not rule out a C. burnetii infection. Specimens can be referred to CDC through state public health laboratories for culture.
Collection and Storage of Specimens
Clinical specimens for evaluation of C. burnetii can be tested at some state public health laboratories or private referral laboratories. Health-care providers should contact their state health department for assistance with specimen submission and reporting infected patients. CDC accepts samples and performs testing at no charge if the samples have been submitted with the approval of or through a state health department. In 2011, the Food and Drug Administration approved a PCR test, for use by deployed military health-care providers, that includes a Department of Defense assay for the diagnosis of Q fever (139).
Serum. Using a red-top or serum separator tube, the acute-phase specimen should be collected as soon as possible after symptom onset (within the first 2 weeks) with a convalescent-phase specimen collected 3–6 weeks later. Sera should be refrigerated and shipped by express shipping on frozen gel packs separated from the specimen by packing material. Samples can be frozen in a non–frost-free freezer and shipped on dry ice to the laboratory.
Blood. Whole blood for PCR testing should be collected before antibiotic administration in EDTA-treated anticoagulant tubes and shipped refrigerated on frozen gel packs by overnight shipping. If samples are to be prepared for other laboratory tests, the buffy coat can be saved for DNA amplification and stored frozen in a non–frost-free freezer.
Tissue. Heart valve tissue is the most commonly evaluated specimen used for confirmation of chronic Q fever. Fresh tissue specimens, which are the most effective and have the widest range of diagnostic techniques, should be refrigerated if they are being transported within 24 hours, and they should be shipped on frozen gel packs. If transport does not occur within 24 hours, specimens should be frozen in a non–frost-free freezer and shipped on dry ice for either culture or PCR analysis. In preparation for transport, fresh tissue should not be immersed in saline but should be placed on a gauze pad moistened with sterile saline and placed in a sterile collection cup. PCR, immunohistochemistry staining, and culture isolation for C. burnetii can be attempted on fresh tissue. Should culture attempts be performed, biopsy specimens should be kept at -80°C (-112°F) before shipping and shipped on dry ice.
Formalin-fixed paraffin-embedded blocks for PCR and immunohistochemistry can be stored and shipped at room temperature and should never be frozen. During warmer months, the blocks should be shipped refrigerated with a frozen gel pack to prevent melting. Formalin-fixed wet tissue should be stored and shipped at room temperature. Length of time in formalin might adversely affect assay results. If sending glass slides with sections from paraffin-embedded blocks, 10–12 treated (e.g., with silane or poly-L-lysine) glass slides with sections of affected tissue cut at a thickness of 3 µm (no greater than 5 µm) should be submitted. These may be shipped at room temperature or refrigerated on cold packs and should never be frozen.
Summary of Q Fever Diagnosis
- PCR of whole blood or serum provides rapid results and can be used to diagnose acute Q fever in approximately the first 2 weeks after symptom onset but before antibiotic administration.
- A fourfold increase in phase II IgG antibody titer by IFA of paired acute and convalescent specimens is the diagnostic gold standard to confirm diagnosis of acute Q fever. A negative acute titer does not rule out Q fever because an IFA is negative during the first stages of acute illness. Most patients seroconvert by the third week of illness.
- A single convalescent sample can be tested using IFA in patients past the acute stage of illness; however, a demonstrated fourfold rise between acute and convalescent samples has much higher sensitivity and specificity than a single elevated, convalescent titer.
- Diagnosis of chronic Q fever requires demonstration of an increased phase I IgG antibody (≥1:1024) and an identifiable persistent infection (e.g., endocarditis).
- PCR, immunohistochemistry, or culture of affected tissue can provide definitive confirmation of infection by C. burnetii.
- Test specimens can be referred to CDC through state public health laboratories.
Treatment and Management
Acute Q Fever in Adults
The majority of acute Q fever cases resolve spontaneously within 2–3 weeks, even without treatment. Symptomatic patients with confirmed or suspected acute Q fever, including children with severe infections, should be treated with doxycycline (Table 2). Doxycycline is the most effective treatment for Q fever. Treatment is most effective if given within the first 3 days of symptoms, shortens the illness, and reduces the risk for severe complications (15,16). Other antibiotic regimens that can be used if doxycycline is contraindicated because of allergies include moxifloxacin, clarithromycin, trimethoprim/sulfamethoxazole, and rifampin (75,140,141). Treatment for acute Q fever is not routinely recommended for asymptomatic persons or for those whose symptoms have resolved, although it might be considered in those at high risk for developing chronic Q fever. In one study of acute Q fever patients who were monitored over time for progression to chronic disease, those who eventually had chronic Q fever were more likely to have not received appropriate doxycycline treatment during their acute illness because their symptoms were mild or they were asymptomatic (15).
Patients with acute Q fever should undergo a careful clinical assessment to determine whether they might be at risk for progression to chronic Q fever because patients at high risk require closer observation during the convalescent period. A thorough clinical assessment should include review of possible immunosuppressive conditions, pregnancy testing when appropriate, and assessment for vascular and heart valve defects because certain valvular lesions might not be detectable by auscultation (142). A medical history and clinical examination alone might not be sufficient to identify patients with existing heart valve defects (143,144); health-care providers should use their clinical judgment to determine the most appropriate tools for assessment of risk (Figure).
Chronic Q Fever in Adults
Management of chronic Q fever is evaluated through both serologic and clinical monitoring. Using the same laboratory and testing procedures for serologic monitoring is important because variations among laboratories might give an inaccurate appearance of significant titer decreases or increases.
Patients who are healthy and have no identified risk factor for chronic illness should receive a clinical and serologic evaluation approximately 6 months after diagnosis of acute infection to identify potential progression to chronic disease. Phase I and phase II IgG and IgM antibodies should be measured to endpoint by IFA and compared with previous titers. Patients with a phase I IgG antibody titer ≥1:1024 should be carefully assessed for clinical evidence of progression to chronic Q fever infection. If a patient has no serologic or clinical evidence of progression to chronic infection, serologic monitoring can either be discontinued or continued less frequently if deemed appropriate by the health-care provider. However, patients should be advised to seek medical care immediately should symptoms of chronic Q fever occur at any time throughout their lives.
Patients with cardiovascular risk factors for chronic disease (e.g., heart valve defect, vascular graft, or aneurysm) at the time of acute infection should be serologically monitored and receive a physical examination at intervals of 3, 6, 12, 18, and 24 months (Figure). Women infected during pregnancy should be serologically and clinically monitored at the same intervals (3, 6, 12, 18, and 24 months) after delivery. If there is no evidence of an increase in phase I IgG titers ≥1:1024 after 2 years and no evidence of clinical progression to chronic infection, serologic monitoring may be discontinued or continued less frequently if deemed appropriate by the health-care provider. However, patients should be advised to seek medical care immediately should symptoms occur at any time throughout their lives, because those with valvular defects or vascular abnormalities remain at high risk for chronic Q fever for life. In addition, patients who have been infected with acute Q fever and develop valvular disease later in life from any cause are at risk for a recrudescent infection that can result in chronic Q fever endocarditis.
It is not uncommon for patients with an acute Q fever infection to develop serologic profiles of chronic Q fever that eventually regress. Clinical evidence of chronic Q fever must accompany increased phase I IgG antibody titers to confirm a chronic diagnosis, and treatment should not be given based on increased titers alone. In all monitored patients, diagnosis of chronic Q fever is based on a rising or elevated phase I IgG titer (typically ≥1:1024) and an identifiable nidus of infection (e.g., endocarditis, vascular infection, and osteomyelitis). Any symptomatic patient with serologic evidence of chronic Q fever (phase I IgG antibody titer ≥1:1024) should be given a thorough clinical assessment to identify potential organ infection. The phase I IgG antibody titer might be higher than the phase II IgG titer; however, this is not a diagnostic criterion because patients with chronic Q fever might retain extremely high phase II IgG titers that equal or exceed phase I IgG titers (136).
Adults who receive a diagnosis of chronic Q fever should receive a treatment regimen of doxycycline and hydroxychloroquine (100 mg of doxycycline twice daily with 200 mg of hydroxychloroquine three times daily); duration of treatment might vary by the site of infection (Table 2) (6). A combination regimen is necessary to eradicate the organism because hydroxychloroquine raises the pH in the acidified phagosomal compartment and, in combination with doxycycline, has been shown to have in vitro bactericidal activity against C. burnetii. Because of potential retinal toxicity from long-term use of hydroxychloroquine, a baseline ophthalmic examination should be performed before treatment and every 6 months thereafter. Both doxycycline and hydroxychloroquine can cause photohypersensitivity, and hypersensitivity to sunlight is a potential complication with acute and chronic treatment regimens. Hydroxychloroquine is contraindicated in persons with glucose-6-phosphate dehydrogenase deficiency and persons with retinal or visual field deficits.
During treatment for chronic Q fever, patients should receive monthly serologic testing for C. burnetii phase I and II IgG and IgM antibodies and monthly clinical evaluations. If an appropriate treatment response is not achieved, monthly monitoring for hydroxychloroquine plasma levels (which should be maintained at 0.8–1.2 µg/mL) and doxycycline plasma levels (which should be maintained at ≥5 µg/mL) should also be performed during the treatment (145,146). Treatment should continue for at least 18 months for native valve infections and at least 24 months for prosthetic valve infections (97).
Although treatment of vascular infections, such as infected aneurysms or grafts, is less clearly defined because of the smaller patient group, duration of antibiotic therapy reported in recovered patients is similar (18–24 months) (104). Early surgical intervention improves patient survival and might be necessary to remove an infected graft if the patient does not respond to antibiotic therapy (104,105). Treatment and management of rarer manifestations of chronic disease (e.g., osteoarticular infections) depends on clinical and serologic response, and consultation with an infectious disease physician is recommended.
The definition of a cured case of chronic fever on the basis of serologic testing previously was defined as phase I IgG ≤1:200, although other researchers have recommended a phase I IgG cutoff of <1:800 to determine treatment duration (145,147). Because these specific titer dilutions are not available from commercial laboratories in the United States, they are more difficult to interpret in the United States. Rather than rely on indiscriminate application of predetermined cutoff titers, health-care providers should use serologic testing as a tool to ensure that the phase I IgG is decreasing during treatment in conjunction with recovery from clinical symptoms. A patient who has been treated appropriately for ≥18 months and has recovered from clinical symptoms but whose phase I IgG remains ≥1:1024 might not benefit from continued treatment. One study found that a favorable prognostic indicator for treated endocarditis patients who had no progression of clinical disease yet who were not considered cured on the basis of serologic testing was a fourfold decrease in phase I IgG and IgA and the complete disappearance of phase II IgM (97). Twice yearly serologic monitoring of treated patients should continue for a minimum of 5 years after treatment, and lifelong serologic monitoring might be warranted in patients with severe valvular defects (97).
Treatment of chronic Q fever is challenging. Because of the highly variable clinical nature both of acute and chronic Q fever, clinical judgment remains the most crucial factor in the treatment and management. Health-care providers should contact their state health department for assistance with specimen submission and reporting of infected patients. Health-care providers who need an epidemiologic consultation on Q fever can contact their state health department or CDC at 1-800-CDC-INFO (Appendix C).
Acute and Chronic Q Fever in Pregnant Women
Treatment of pregnant women who received an acute Q fever diagnosis during pregnancy with trimethoprim/sulfamethoxazole throughout pregnancy has been shown to significantly decrease the risk for adverse consequences for the fetus (75). Up to 81% of untreated infected pregnant women might have adverse pregnancy outcomes (75).
Although approximately 40% of pregnant women who receive long-term trimethoprim/sulfamethoxazole treatment might still experience adverse outcomes, complications are more likely to be limited to intrauterine growth retardation and premature delivery instead of stillbirth or miscarriage (75). Long-term trimethoprim/sulfamethoxazole treatment during pregnancy has decreased the risk for conversion to chronic Q fever in the mother and prevented adverse pregnancy events in subsequent pregnancies (75).
Doxycycline is classified as a category D drug because of demonstrated concerns about the effects of tetracyclines on the bone structure and dentitia of the developing fetus (see drug categories for pregnancy at http://chemm.nlm.nih.gov/pregnancycategories.htm). An effective alternative, trimethoprim/sulfamethoxazole, has been used as a treatment in pregnant women who received an acute Q fever diagnosis, although the drug is classified as a category C drug. The use of trimethoprim/sulfamethoxazole during pregnancy might increase the risk for congenital abnormalities (primarily including urinary tract and cardiovascular abnormalities) because of antifolate effects (148), and concomitant use of folic acid is recommended. Research to assess the potential fetal risk from trimethoprim/sulfamethoxazole during pregnancy has been inconclusive (149).
Because pregnant women with acute Q fever are considered to be at high risk for chronic Q fever infection or recrudescent infections activated during subsequent pregnancies, patients should be monitored after delivery for postpartum progression to chronic disease and during subsequent pregnancies. Although rare, the development of Q fever endocarditis in a pregnant woman presents a difficult clinical dilemma because the safety of the treatment of choice (doxycycline and hydroxychloroquine) has not been evaluated during pregnancy. Health-care providers who are treating chronic Q fever endocarditis during pregnancy should consult with an expert in infectious diseases.
Women who are treated for acute Q fever during pregnancy should be monitored similarly to other patients at high risk for progression to chronic disease (e.g., serologic monitoring at 3, 6, 12, 18, and 24 months after delivery). Women should be advised of potential risks to the fetus should they become pregnant during the monitoring or treatment period. In one study, seven women treated for chronic Q fever with doxycycline and hydroxychloroquine for at least 1 year had normal subsequent pregnancies with no recurrent miscarriages (81). Q fever serologic testing should be resumed for women previously treated during pregnancy who become pregnant again during this 2-year period; reinitiation of long-term trimethoprim/sulfamethoxazole is indicated when IgG titers demonstrate a fourfold rise indicating a recrudescent infection, even if other signs or a definite nidus of infection cannot be identified. In these women, the nidus of infection is assumed to be the reproductive system, and the only clinical sign might be an adverse pregnancy event in a subsequent pregnancy.
Acute and Chronic Q Fever in Children
Doxycycline is the drug of choice for treatment of acute Q fever in children and is recommended for patients aged ≥8 years and for severe infections in children of any age. The pediatric doxycycline dose for treatment of acute Q fever is 2.2 mg/kg twice per day for 2 weeks (maximum 100 mg per dose). The clinical benefit of using doxycycline to treat Q fever in children aged <8 years who meet the criteria for being considered high risk is greater than the potential risk for dental staining. Children aged <8 years who are considered high risk and should therefore receive the full 2-week treatment with doxycycline include children who are hospitalized or have severe illness, children with preexisting heart valvulopathy, children who are immunocompromised, or children with delayed Q fever diagnosis who have experienced illness for >2 weeks without resolution of symptoms.
Although short courses (≤5 days) of doxycycline for the treatment of rickettsial infections such as Rocky Mountain spotted fever have not resulted in significant dental staining in children, the possible long-term dental effects of 2 weeks of doxycycline in children aged <8 years have not been well studied (150). Because acute Q fever is frequently a mild or self-limiting illness with a low risk for death or a poor prognosis, health-care providers should use their clinical judgment to determine whether a 2-week course of doxycycline treatment should be used to treat Q fever infections in children aged <8 years who have a mild or uncomplicated illness. For these patients, health-care providers might consider a 5-day course of doxycycline, which does not cause dental staining. Children with continued mild symptoms after a short course of doxycycline can be treated with 14 days of trimethoprim/sulfamethoxazole.
Limited data are available on treatment of chronic Q fever in children; therefore, consultation with an expert in pediatric infectious diseases is recommended. The safety of long-term hydroxychloroquine treatment in children has not been determined, and evaluation for retinal toxicity might be limited because of difficulties in evaluation of color vision. Alternative long-term treatments that might be considered in children with chronic Q fever include use of a fluoroquinolone (e.g., moxifloxacin or levofloxacin) with rifampin or trimethoprim/sulfamethoxazole with doxycycline.
Summary of Q Fever Treatment and Management
- Because of the delay in seroconversion often necessary to confirm diagnosis, antibiotic treatment should never be withheld pending laboratory tests or discontinued on the basis of a negative acute specimen. In contrast, treatment of chronic Q fever should be initiated only after diagnostic confirmation.
- Treatment for acute or chronic Q fever should only be provided for patients with clinically compatible cases and not based on elevated serologic titers alone (see Pregnancy section for exception).
- Doxycycline is the drug of choice, and 2 weeks of treatment is recommended for adults, children aged ≥8 years, and for severe infections in patients of any age.
- Children aged <8 years with uncomplicated illness may be treated with trimethoprim/sulfamethoxazole or a shorter duration (5 days) of doxycycline.
- Women who are pregnant when acute Q fever is diagnosed should be treated with trimethoprim/sulfamethoxazole throughout the duration of pregnancy.
- Serologic monitoring is recommended after an acute Q fever infection to assess possible progression to chronic infection. The recommended schedule for monitoring is based on the patient's risk for chronic infection.
Occupational Exposure and Prevention
Overview
Certain occupations are associated with increased risk for exposure to C. burnetii, as might their associated institutions and businesses. Multiple Q fever outbreaks have been reported among workers in slaughterhouses, farms, animal research facilities, military units, and, rarely, hospitals and diagnostic laboratories (12,151–156). Employees in high-risk occupations should be educated about the risk for exposure and the clinical presentation of Q fever. Educational efforts should describe groups vulnerable to development of chronic Q fever, such as workers who have preexisting valvulopathy, a prosthetic heart valve, a vascular prosthesis, an aneurysm, are pregnant or might become pregnant, or are immunosuppressed, because these employees have a higher risk for a severe outcome or death if infected. Although protection for at-risk workers can be provided by Q fever vaccination, a licensed vaccine for humans is only commercially available in Australia (157). Therefore, most workers in high-risk occupations in the United States are not vaccinated.
Transmission of C. burnetii to health-care personnel has been rarely reported (158,159). One obstetrician was infected through contact with the birth fluids of an infected parturient woman (48). Hospital personnel have become infected after autopsies of patients with Q fever, although the infection control precautions used, if any, are unknown (49,160).
Adherence to standard precautions is recommended to prevent Q fever infection in health-care personnel during routine care (161). During autopsies of patients who have died of Q fever, health-care personnel should use either a BSL-3 facility or use the barrier precautions of BSL-2 and the negative airflow and respiratory precautions of BSL-3 as recommended by the CDC Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories (162). During procedures that put health-care personnel at risk for infection from splashing of infected material, such the delivery of an infant from an infected woman, standard precautions including the use of a face mask and eye protection or a face shield are recommended. Care should be used when handling soiled laundry (e.g., bedding, towels, and personal clothing) of Q fever patients. Soiled laundry should not be shaken or otherwise handled in a way that might aerosolize infectious particles.
During any procedure that might generate aerosols of infectious materials (e.g., a procedure involving use of a surgical power instrument such as an oscillating bone saw) in a patient with suspected or confirmed Q fever, health-care personnel should also take the following precautions:
1. Use a fit-tested N-95 (or comparable) respirator and eye protection (e.g., goggles or face shield).
2. Contain and dispose of contaminated waste (e.g., dressings or birth products) in accordance with facility-specific guidelines for infectious waste.
3. Place the patient in an airborne infection isolation room or a private room if one is not available during the procedure. The patient does not need to wear a face mask, because Q fever is not transmitted by sneezing or coughing.
4. Handle used patient-care equipment in a way that prevents contamination of skin and clothing. Ensure that used equipment has been cleaned and reprocessed appropriately.
5. Ensure that procedures are in place for cleaning and disinfecting environmental surfaces in the patient care environment (see #5 in the Research Facility Safety Standards section that follows for chemical disinfectant recommendations).
Precautions used in addition to standard precautions are only recommended during an aerosol-generating procedure. Procedures that do not generate aerosols, such as drawing blood or giving physical examinations, do not pose a risk for transmission of Q fever. Transmission through coughing or sneezing is not a documented route of infection, and there is no evidence that Q fever is transmitted by any type of casual contact (e.g., hugging, shaking hands, kissing, or sharing food).
Laboratory transmission of C. burnetii is primarily a concern when bacteria are propagated using specialized techniques (i.e., tissue culture), during lapses in standard precautions leading to specimen aerosolization, and through protocols involving passage through animals. Handling of usual biomedical specimens, including routine blood culture testing, from humans or animals collected in medical or veterinary settings is not considered an exposure risk for Q fever and can be processed by routine standard precautions and handling techniques.
Laboratory safety and containment recommendations for C. burnetii should be followed as described in the CDC Biosafety in Microbiological and Biomedical Laboratories manual (163). Samples known or suspected to contain viable C. burnetii (i.e., birth products or other biologic material from infected animals or humans) should be handled in a BSL-3 facility and rendered nonviable or destroyed. Appropriate personal protective equipment (PPE) can be effective at reducing the risk for exposure in handling these types of specimens. In the BSL-3 laboratory, attire worn while working with viable C. burnetii should be sterilized after use. Protective eyewear such as splatter-proof safety goggles or face shields, disposable gloves, and shoe covers also should be worn, and showering after working with C. burnetii under BSL-3 conditions is recommended. In laboratories that work with viable C. burnetii organisms in culture media or live animals, unvaccinated workers should wear respiratory protection such as an N95 respirator. An N95 respirator filters at least 95% of airborne particles when used correctly but might not completely eliminate infection risk. A powered air purifying respirator with P100 filtration also can be used in research laboratories that experiment with C. burnetii, particularly by employees unable to wear an N95 respirator. Whether this higher level of protection further reduces transmission of C. burnetii is unknown. If respirators are used, compliance with the Occupational Safety and Health Administration Respiratory Protection Standard is required (164). Compliance includes but is not limited to establishment of a written respiratory protection program, a medical evaluation, annual fit testing, and training in proper use and maintenance (164).
Biomedical facilities that house sheep and goats but do not directly experiment with C. burnetii are the mostly common settings for occupationally related outbreaks of Q fever in the United States (156,165–167), and these outbreaks have primarily been associated with exposure to pregnant ewes. Cases have occurred not only among persons who worked directly with animals but also among administrative staff, janitorial staff, and family members of workers infected through fomite transmission from contaminated clothing. The following general recommendations should reduce the risk for infection and focus on facilities that house animals at high risk for C. burnetii, such as pregnant, small ruminants. Specific safety standards vary by the size, purpose, and location of each facility.
Research Facility Safety Standards
1. A Q fever medical surveillance program should be established. Workers should receive a preemployment medical screening to document any risk factors for chronic illness if infected, and education for Q fever exposure risks and symptoms of illness should be provided. A baseline serum sample should be drawn within 30 days of beginning work and tested for evidence of previous exposure to C. burnetii infection. Sera should then be tested for IgG antibodies against phase I and phase II C. burnetii annually to determine whether seroconversion has occurred and whenever an employee develops symptoms consistent with Q fever. Facilities should store a baseline or previously drawn serum specimen for comparison testing of paired samples when evaluating workers. Seroconversion or a greater than fourfold increase in titer indicates infection with C. burnetii. Workers with documented seroconversion should be questioned to determine whether a clinically compatible illness occurred in the previous year and was treated with an appropriate regimen. Seroconversion of employees should trigger a review of exposure risk in the work environment and standard operating procedures. Workers who develop symptoms of Q fever should immediately be evaluated by a health-care provider and tested.
2. Appropriate changing, washing, and showering facilities should be available to workers. Employees should shower and change before leaving work, and work clothes should be laundered on site. Work footwear should not be worn outside of the facility. Employees should thoroughly wash their hands after any type of animal contact.
3. Access to the facility should be limited to necessary personnel who are informed of the Q fever risk.
4. Gloves, protective clothing, and respiratory protection (typically an N95 respirator) should be worn when the probability of exposure is high (e.g., when assisting with birth or removing retained placentas from infected livestock). Protective clothing might consist of laboratory coats, smock, aprons, or coveralls. They should be changed daily and should not be worn outside of the work environment. Staff should use respiratory protection during high-risk activities such as changing or cleaning air filters or during any dust-generating tasks. A face shield or splatter-proof goggles in conjunction with an N95 respirator should be used during procedures with a high risk for droplet contamination (e.g., assistance with birth).
5. Because of the extracellular sporelike form of C. burnetii, the bacteria are highly resistant to inactivation by chemical disinfectants, heat, pressure, or drying. Contaminated surfaces can be cleaned with MicroChem-Plus (a dual-quaternary ammonium/detergent compound), which completely inactivates the bacteria using a 30-minute contact time. Seventy-percent ethanol also completely deactivates the organism, although rapid evaporation makes this a less feasible treatment (168). Use of humidified ethylene oxide gas or vapor phase hydrogen peroxide effectively sterilizes C. burnetii in contaminated rooms that can be isolated and sealed (168). Treatment with a 1:100 dilution of household bleach or a 1% Virkon S treatment produces >90% reduction in infectivity (169,170). All visible organic matter should be removed before cleaning, and proper PPE should be worn.
6. If available, facility air filtration should be used and negative air pressure should be maintained in all animal holding areas with respect to areas not designated as part of the Q fever biohazard area. Consultation with a ventilation engineer experienced with infection control practices in animal facilities is recommended.
7. Determining whether an animal or a flock is free of Q fever is difficult. A positive antibody titer in infected animals does not correlate with active shedding of organisms because some seronegative animals also might actively shed bacteria. Although body secretions (vaginal mucus, feces, or milk) can be tested by PCR, bacterial shedding is intermittent. Therefore, even frequent serologic and PCR testing and intensive surveillance of animals cannot completely ensure that an animal is not infected and shedding the bacteria. However, an ongoing animal surveillance program that isolates and quarantines animals until they have two negative serologic tests might reduce the likelihood of infection and reveal infected source herds that should not be purchased by the facility.
8. No animals (regardless of whether infection is suspected) should be transported through areas not designated as part of the Q fever biohazard area.
9. Use male and nonpregnant female animals for research when possible.
10. Birth products and all other organic materials should be removed and disposed of immediately via incineration, composting, or burial in a designated sanitary landfill. Appropriate PPE should be used during removal and disposal, and training in safe handling and disposal of animal products (e.g., birth products, urine, feces, or milk) should be provided. Facilities should consult with state or local environmental agencies regarding regulations on manure management and carcass disposal because the regulations vary by state.
11. Workers should participate in an initial and periodic training program for the recommendations. Standard operating procedures should be developed for all safety and containment practices recommended.
No recent data support the use of postexposure prophylaxis outside of an experimental setting and the use of prophylactic antimicrobial agents are not routinely recommended for workers after a known or potential exposure and before symptom onset. A 1956 U.S. military study of five men who received tetracycline prophylaxis 8–12 days after a C. burnetii challenge dose exposure resulted in asymptomatic infection; five men equally exposed and administered tetracycline prophylaxis 1 day after exposure had delayed symptom onset (130). One report theorized that providing postexposure prophylaxis after a bioterrorism release of C. burnetii would be useful if the timing of exposure were known (171). However, the recommendation was based on the limited data provided by the 1956 study. Prophylaxis prevented symptomatic illness in this study but did not prevent infection. In persons at high risk, the risk for chronic Q fever development is still present regardless of whether symptomatic or asymptomatic infection occurs. Because of the limited treatment duration in the 1956 study, the lack of additional studies verifying its findings, and use of oxytetracycline instead of doxycycline, the benefit of prophylactic antimicrobial agents is questionable and therefore not recommended.
A daily fever monitoring log should be kept for a minimum of 3 weeks after exposure to C. burnetii. The incubation period for Q fever is dose dependent. The majority of infected persons have symptom onset 2–3 weeks after exposure, although onset can occur up to 6 weeks after exposure. If fever occurs during the monitoring period, immediate treatment with doxycycline should be administered and testing should be performed. Treatment within 24 hours of fever onset is extremely effective in shortening illness duration and symptom severity (16,130). Baseline serologic testing can be performed to evaluate previous infection status with a convalescent sample drawn 6 weeks later to determine whether asymptomatic seroconversion has occurred. Although asymptomatic infections do not routinely require treatment, even asymptomatic infection carries a risk for progression to chronic disease in groups at high risk; therefore, treatment for asymptomatic infection might be considered in these groups. Determination of infection status might provide useful data to guide future health management.
Summary of Occupational Exposure to Q Fever
- The majority of occupationally related Q fever outbreaks in the United States have occurred among biomedical research facility workers exposed to infected pregnant ewes.
- Workplaces with employees at high risk for C. burnetii exposure (e.g., laboratories that experiment with C. burnetii and animal research facilities) should institute a Q fever medical surveillance and health education monitoring program. Engineering controls, administrative controls, and use of PPE are recommended when appropriate.
- Use of standard precautions by health-care providers is sufficient to prevent Q fever transmission during routine care. Additional precautions should be used during aerosol-generating procedures.
- Use of postexposure prophylaxis is not recommended for workers after a known or potential exposure; any acute febrile illness that occurs within 6 weeks of exposure warrants immediate treatment and medical evaluation.
Surveillance and Reporting
Q fever is a nationally notifiable disease in the United States. Health-care providers should report suspected or confirmed cases through local or state reporting mechanisms in place for notifiable disease conditions. Many state laboratories have systems in place that automatically report specific diseases, although this varies by state. The most recent human case definition, revised in 2009, provides separate reporting categories for acute and chronic Q fever (Table 3). The CDC case definition is used for national reporting as a public health surveillance tool and is not intended for clinical diagnosis. A medical diagnosis is made to treat a patient and should consider all aspects of the illness. Surveillance case definitions are used for standardization, not patient care.
National surveillance for Q fever in the United States relies on accurate and timely reporting of cases by health-care providers. When health-care providers identify a potential case of acute or chronic Q fever, they should notify the local or state health department, which can assist with obtaining appropriate diagnostic testing. Epidemiologic and clinical patient information is reported through state health departments to CDC on a standard, confidential case report form (Appendix D). When reporting cases of Q fever, clinical signs and symptoms should be included as well as laboratory results. CDC compiles reports of Q fever from state health departments including diagnosis, date of onset, and basic demographic and geographic data, and reports the summary data.
Although Q fever is a zoonotic disease, infection in animals is not considered reportable to national agricultural authorities. However, many states consider the disease reportable when it is diagnosed in animals. Veterinary health authorities should follow state-specific reporting guidelines.
Summary of Q Fever Surveillance and Reporting
- Human Q fever infection is a notifiable disease in the United States.
- Health-care providers who identify a potential case of Q fever should notify the local/state health department, which can assist with diagnostic testing.
- Surveillance and reporting of Q fever are key components of public health education and disease prevention efforts.
Acknowledgments
Joanna Regan, Naomi Drexler, and Randy Nett, CDC. Nicola Marsden-Haug, Washington Department of Health. Dutch Consensus Group Q Fever: Chantal Bleeker, MD, PhD, Corine Delsing MD, PhD, Alphons Horrevorts MD, PhD, Linda Kampschreur, Marion Koopmans, PhD, Peter Lestrade, MD, PhD, Jan Marcelis MD, PhD, Marrigje Nabuurs-Franssen MD, PhD, Daan Notermans, MD, PhD, Jan Jelrik Oosterheert MD, PhD, Nicole Renders, MD PhD, Peter Schneeberger MD, PhD, Wim van der Hoek, Pepijn van der Voort, MD, PhD, Marjolijn Wegdam, MD, and Peter Wever, MD, PhD.
References
- Derrick EQ. Q fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Med J Aust 1937;2:281–99.
- Dyer RE. A filter-passing infectious agent isolated from ticks. IV. Human infection. Public Health Rep 1938;53:2277–83.
- Davis GE, Cox HR. A filter-passing infectious agent isolated from ticks. I. Isolation from Dermacentor andersoni, reactions in animals, and filtration experiments. Public Health Rep 1938;53:2259–61.
- CDC. Q fever: in-depth information. [Internet site]. Atlanta, GA; 2010. Available at http://www.cdc.gov/qfever/info/index.html. Accessed February 13, 2012.
- Anderson AD, Kruszon-Moran D, Loftis AD, et al. Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg 2009;81:691–4.
- Maurin M, Raoult DQ. Fever. Clin Microbiol Rev 1999;12:518–53.
- Bamberg WM, Pape WJ, Beebe JL, et al. Outbreak of Q fever associated with a horse-boarding ranch, Colorado, 2005. Vector Borne Zoonotic Dis 2007;7:394–402.
- Delgado Naranjo J, Alonso Fustel E, et al. Study and management of a Q fever outbreak among machine tool workers in the Basque country (Spain). Epidemiol Res Int 2011;2011.
- Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis 2001;33:312–6.
- Rolain JM, Boulos A, Mallet MN, Raoult D. Correlation between ratio of serum doxycycline concentration to MIC and rapid decline of antibody levels during treatment of Q fever endocarditis. Antimicrob Agents Chemother 2005;49:2673–6.
- Bjork A, M-HN, Nett R, Kersh G, et al. First reported multi-state human Q fever outbreak in the United States, 2011. Vector Borne Zoonotic Dis. In press 2013.
- Anderson AD, Baker TR, Littrell AC, Mott RL, Niebuhr DW, Smoak BL. Seroepidemiologic survey for Coxiella burnetii among hospitalized U.S. troops deployed to Iraq. Zoonoses Public Health 2011;58:276–83.
- Faix DJ, Harrison DJ, Riddle MS, et al. Outbreak of Q fever among U.S. military in western Iraq, June–July 2005. Clin Infect Dis 2008;46:e65–8.
- Schimmer B, Dijkstra F, Velllema P, et al. Sustained intensive transmission of Q fever in the south of the Netherlands, 2009. Euro Surveill 2009;14:3.
- Kampschreur LM, Dekker S, Hagenaars JC, et al. Identification of risk factors for chronic Q fever, the Netherlands. Emerg Infect Dis 2012;18:563–70.
- Powell OW, Kennedy KP, Mc IM, Silverstone H. Tetracycline in the treatment of "Q" fever. Australas Ann Med 1962;11:184–8.
- Babudieri B. Q fever: a zoonosis. Adv Vet Sci 1959;5:81.
- Eklund CM, Parker RR, Lackman DB. A case of Q fever probably contracted by exposure to ticks in nature. Public Health Rep 1947;62:1413–6.
- Beaman MH, Hung J. Pericarditis associated with tick-borne Q fever. Aust N Z J Med 1989;19:254–6.
- Thompson HDD, Dasch GQ. Fever. In: Goodman J, ed. Tick-borne diseases of humans. Washington, DC: ASM Press; 2005:328–42.
- Stein A, Raoult D. Pigeon pneumonia in Provence: a bird-borne Q fever outbreak. Clin Infect Dis 1999;29:617–20.
- Buhariwalla F, Cann B, Marrie TJ. A dog-related outbreak of Q fever. Clin Infect Dis 1996;23:753–5.
- Marrie TJ, Williams J, Schlech W, Yates L. Q fever pneumonia associated with exposure to wild rabbits. Lancet 1986;327:427–9.
- Marrie TJ, Durant H, Williams JC, Mintz E, Waag DM. Exposure to parturient cats: a risk factor for acquisition of Q fever in maritime Canada. J Infect Dis 1988;158:101–8.
- Langley JM, Marrie TJ, Covert A, Waag DM, Williams JC. Poker players' pneumonia. An urban outbreak of Q fever following exposure to a parturient cat. N Engl J Med 1988;319:354–6.
- Pinsky RL, Fishbein DB, Greene CR, Gensheimer KF. An outbreak of cat-associated Q fever in the United States. J Infect Dis 1991;164:202–4.
- Lang GH. Coxiellosis (Q fever) in animals. In: Marrie TJ, ed. Q fever. The disease (vol 1). Boca Raton, FL: CRC Press; 1990:23–48.
- Angelakis E, Raoult DQ. Fever. Vet Microbiol 2010;140:297–309.
- Rodolakis A, Berri M, Hechard C, et al. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J Dairy Sci 2007;90:5352–60.
- Enright JB, Franti CE, Behymer DE, Longhurst WM, Dutson VJ, Wright ME. Coxiella burnetii in a wildlife-livestock environment. Distribution of Q fever in wild mammals. Am J Epidemiol 1971;94:79–90.
- Berri M, Souriau A, Crosby M, Crochet D, Lechopier P, Rodolakis A. Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep. Vet Rec 2001;148:502–5.
- Rousset E, Berri M, Durand B, et al. Coxiella burnetii shedding routes and antibody response after outbreaks of Q fever-induced abortion in dairy goat herds. Appl Environ Microbiol 2009;75:428–33.
- Muskens J, van Engelen E, van Maanen C, Bartels C, Lam TJ. Prevalence of Coxiella burnetii infection in Dutch dairy herds based on testing bulk tank milk and individual samples by PCR and ELISA. Vet Rec 2011;168:79.
- Berri M, Crochet D, Santiago S, Rodolakis A. Spread of Coxiella burnetii infection in a flock of sheep after an episode of Q fever. Vet Rec 2005;157:737–40.
- Tissot-Dupont H, Torres S, Nezri M, Raoult D. Hyperendemic focus of Q fever related to sheep and wind. Am J Epidemiol 1999;150:67–74.
- Tissot-Dupont H, Amadei MA, Nezri M, Raoult D. Wind in November, Q fever in December. Emerg Infect Dis 2004;10:1264–9.
- Hawker JI, Ayres JG, Blair I, et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health 1998;1:180–7.
- van der Hoek W, Dijkstra F, Schimmer B, et al. Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill 2010;15.
- Fishbein DB, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg 1992;47:35–40.
- Oliphant J, Gordon D, Meis A, Parker R. Q fever in laundry workers, presumably transmitted from contaminated clothing. Am J Hyg 1949;49:76–82.
- Milazzo A, Hall R, Storm PA, Harris RJ, Winslow W, Marmion BP. Sexually transmitted Q fever. Clin Infect Dis 2001;33:399–402.
- Stein A, Raoult D. Q fever during pregnancy: a public health problem in southern France. Clin Infect Dis 1998;27:592–6.
- Kruszewska D, Lembowicz K, Tylewska-Wierzbanowska S. Possible sexual transmission of Q fever among humans. Clin Infect Dis 1996;22:1087–8.
- Kruszewska D, Tylewska-Wierzbanowska SK. Coxiella burnetii penetration into the reproductive system of male mice, promoting sexual transmission of infection. Infect Immun 1993;61:4188–95.
- CDC. Q fever—California. MMWR 1977;26:86–7.
- Kanfer E, Price C, MacDonald D, Coleman J, Barrett AJ. Q fever following bone marrow transplantation. Bone Marrow Tran 1988;3:165–6.
- Kumar A, Yadav MP, Kakkar S. Human milk as a source of Q-fever infection in breast-fed babies. Indian J Med Res 1981;73:510–2.
- Raoult D, Stein A. Q fever during pregnancy—a risk for women, fetuses, and obstetricians. N Engl J Med 1994;330:371.
- Harman JB. Q fever in Great Britain; clinical account of eight cases. Lancet 1949;254:1028–30.
- Tissot-Dupont H, Vaillant V, Rey S, Raoult D. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis 2007;44:232–7.
- Leone M, Honstettre A, Lepidi H, et al. Effect of sex on Coxiella burnetii infection: protective role of 17β-estradiol. J Infect Dis 2004;189:339–45.
- Marc Leone JT, Textoris J, Capo C, Mege JL. Sex hormones and bacterial infections. In: Dubey RK, ed. Sex hormones. InTech; 2012.
- Tissot-Dupont H, Raoult D, Brouqui P, et al. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am J Med 1992;93:427–34.
- Dijkstra F, van der Hoek W, Wijers N, et al. The 2007–2010 Q fever epidemic in the Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol Med Microbiol 2012;64:3–12.
- La Scola B, Lepidi H, Raoult D. Pathologic changes during acute Q fever: influence of the route of infection and inoculum size in infected guinea pigs. Infect Immun 1997;65:2443–7.
- Raoult D. Host factors in the severity of Q fever. Ann N Y Acad Sci 1990;590:33–8.
- Marrie TJ, Durant H, Yates L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis 1989;11:586–99.
- Caron F, Meurice JC, Ingrand P, et al. Acute Q fever pneumonia: a review of 80 hospitalized patients. Chest 1998;114:808–13.
- Marrie TJ. Coxiella burnetii pneumonia. Eur Respir J 2003;21:713–9.
- Marrie TJ. Q fever pneumonia. Curr Opin Infect Dis 2004;17:137–42.
- Huebner RJ, Jellison WL, Beck MD. Q fever; a review of current knowledge. Ann Intern Med 1949;30:495–509.
- Cunha BA, Nausheen S, Busch L. Severe Q fever community-acquired pneumonia (CAP) mimicking Legionnaires' disease: Clinical significance of cold agglutinins, anti-smooth muscle antibodies and thrombocytosis. Heart Lung 2009;38:354–62.
- Sobradillo V, Zalacain R, Capelastegui A, Uresandi F, Corral J. Antibiotic treatment in pneumonia due to Q fever. Thorax 1992;47:276–8.
- Derrick EH. The course of with Coxiella burnetii. Med J Aust 1973;1:1051–7.
- Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000;79:109–23.
- Richardus JH, Dumas A, Huisman J, Schapp G. Q fever in infancy: a review of 18 cases. Pediatr Infect Dis 1985;4:369–73.
- Maltezou HC, Constantopoulou I, Kallergi C, et al. Q fever in children in Greece. Am J Trop Med Hyg 2004;70:540–4.
- Terheggen U, Leggat PA. Clinical manifestations of Q fever in adults and children. Travel Med Infect Dis 2007;5:159–64.
- Ruiz-Contreras J, Montero RG, Amador JTR, Corradi EG, Vera AS. Q fever in children. Am J Dis Child 1993;147:300–2.
- Maltezou HC, Kallergi C, Kavazarakis E, Stabouli S, Kafetzis DA. Hemolytic-uremic syndrome associated with Coxiella burnetii infection. Pediatr Infect Dis J 2001;20:811–3.
- Maltezou HC, Raoult D. Q fever in children. Lancet Infect Dis 2002;2:686–91.
- Carrascosa M, Pascual F, Borobio MV, González Z, Napal J. Rhabdomyolysis associated with acute Q fever. Clin Infect Dis 1997;25:1243–4.
- Rolain JM, Lepidi H, Harlé JR, et al. Acute acalculous cholecystitis associated with Q fever: report of seven cases and review of the literature. Eur J Clin Microbiol Infect Dis 2003;22:222–7.
- Foucault C, Lepidi H, Poujet-Abadie JF, et al. Q fever and lymphadenopathy: report of four new cases and review. Eur J Clin Microbiol Infect Dis 2004;23:759–64.
- Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A. Managing Q fever during pregnancy: the benefits of long-term cotrimoxazole therapy. Clin Infect Dis 2007;45:548–55.
- Stein A, Raoult D. Q Fever during pregnancy: a public health problem in southern France. Clin Infect Dis 1998;27:592–6.
- van der Hoek W, Meekelenkamp JC, Leenders AC, Wijers N, Notermans DW, Hukkelhoven CW. Antibodies against Coxiella burnetii and pregnancy outcome during the 2007–2008 Q fever outbreaks in the Netherlands. BMC Infect Dis 2011;11:44.
- McCaughey C, McKenna J, McKenna C, et al. Human seroprevalence to Coxiella burnetii (Q fever) in Northern Ireland. Zoonoses Public Health 2008;55:189–94.
- Baud D, Peter O, Langel C, Regan L, Greub G. Seroprevalence of Coxiella burnetii and Brucella abortus among pregnant women. Clin Microbiol Infect 2009;15:499–501.
- Rey D, Obadia Y, Tissot-Dupont H, Raoult D. Seroprevalence of antibodies to Coxiella burnetii among pregnant women in South Eastern France. Eur J Obstet Gynecol Reprod Biol 2000;93:151–6.
- Raoult D, Fenollar F, Stein A. Q fever during pregnancy: diagnosis, treatment, and follow-up. Arch Intern Med 2002;162:701–4.
- Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A. Q fever during pregnancy. Ann N Y Acad Sci 2009;1166:79–89.
- Syrucek L, Sobeslavsky O, Gutvirth I. Isolation of Coxiella burnetii from human placentas. J Hyg Epidemiol Microbiol Immunol 1958;2:29–35.
- de Wit NCJ, de Jager CPC, Meekelenkamp JCE, et al. Markers of infection in inpatients and outpatients with acute Q-fever. Clin Chem Lab Med 2001;47:1407–9.
- Gikas A, Kofteridis D, Bouros D, Voloudaki A, Tselentis Y, Tsaparas N. Q fever pneumonia: appearance on chest radiographs. Radiology 1999;210:339–43.
- Jacobson G, Denlinger RB, Carter RA. Roentgen manifestations of Q fever. Radiology 1949;53:739–49.
- Oddo M, Jolidon RM, Peter O, Poli S, Cometta A. Q fever pneumonia complicated by acute respiratory distress syndrome. Intensive Care Med 2001;27:615.
- Hartzell JD, Peng SW, Wood-Morris RN, et al. Atypical Q fever in U.S. soldiers. Emerg Infect Dis 2007;13:1247–9.
- Fournier P-E, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol 1998;36:1823–34.
- Smith DL, Ayres JG, Blair I, et al. A large Q fever outbreak in the West Midlands: clinical aspects. Respir Med 1993;87:509–16.
- Marrie TJ. Coxiella burnetii (Q fever) pneumonia. Clin Infect Dis 1995;21(Suppl 3):S253–64.
- Chang K, Yan JJ, Lee HC, Liu KH, Lee NY, Ko WC. Acute hepatitis with or without jaundice: a predominant presentation of acute Q fever in southern Taiwan. J Microbiol Immunol Infect 2004;37:103–8.
- Spelman DW. Q fever: a study of 111 consecutive cases. Med J Aust 1982;1:547–8, 551, 553.
- Holmes RO Jr, Hartzell JD, Tofferi JK, Roebuck JD, Kelly WF. Dual high titer antineutrophil cytoplasmic autoantibodies in association with systemic Q fever. J Clin Rheumatol 2009;15:411–3.
- Williams JC. Infectivity, virulence, and pathogenicity of Coxiella burnetii for various hosts. In: Williams JC, Thompson HA, eds. Q fever: the biology of Coxiella burnetii. Boca Raton, FL: CRC Press, Inc; 1991:21–72.
- Fournier PE, Casalta JP, Piquet P, Tournigand P, Branchereau A, Raoult D. Coxiella burnetii infection of aneurysms or vascular grafts: report of seven cases and review. Clin Infect Dis 1998;26:116–21.
- Million M, Thuny F, Richet H, Raoult D. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis 2010;10:527–35.
- Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis 2005;5:219–26.
- Fenollar F, Fournier P-E, Carrieri MP, Habib G, Messana T, Raoult D. Risk factors and prevention of Q fever endocarditis. Clin Infect Dis 2001;33:312–6.
- Raoult D, Million M, Thuny F, Carrieri P. Chronic Q fever detection in the Netherlands. Clin Infect Dis 2011;53:1170–1.
- Wegdam-Blans MC, Kampschreur LM, Delsing CE, et al. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J Infect 2012;64:247–59.
- Brouqui P, Dupont HT, Drancourt M, et al. Chronic Q fever: ninety-two cases from France, including 27 cases without endocarditis. Arch Intern Med 1993;153:642–8.
- Shively BK, Gurule FT, Roldan CA, Leggett JH, Schiller NB. Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis. J Am Coll Cardiol 1991;18:391–7.
- Botelho-Nevers E, Fournier PE, Richet H, et al. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur J Clin Microbiol Infect Dis 2007;26:635–40.
- Wegdam-Blans MC, Ter Woorst JF, Klompenhouwer EG, Teijink JA. David procedure during a reoperation for ongoing chronic Q fever infection of an ascending aortic prosthesis. Eur J Cardiothorac Surg 2012;42:e19–20.
- Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A. Q Fever during pregnancy: a cause of poor fetal and maternal outcome. Ann N Y Acad Sci 2009;1166:79–89.
- Ben Amara A, Ghigo E, Le Priol Y, et al. Coxiella burnetii, the agent of Q fever, replicates within trophoblasts and induces a unique transcriptional response. PLoS ONE 2010;5:e15315.
- McQuiston JH. Coxiella burnetii (Q fever). In: Long SS, ed. Principles and practices of pediatric infectious diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone; 2009:885–7.
- Nourse C, Allworth A, Jones A, et al. Three cases of Q fever osteomyelitis in children and a review of the literature. Clin Infect Dis 2004;39:e61–6.
- Delsing CE, Kullberg BJ, Bleeker-Rovers CP. Q fever in the Netherlands from 2007 to 2010. Neth J Med 2010;68:382–7.
- Ayres JG, Flint N, Smith EG, et al. Post-infection fatigue syndrome following Q fever. QJM 1998;91:105–23.
- Harris RJ, Storm PA, Lloyd A, Arens M, Marmion BP. Long-term persistence of Coxiella burnetii in the host after primary Q fever. Epidemiol Infect 2000;124:543–9.
- Helbig KJ, Heatley SL, Harris RJ, Mullighan CG, Bardy PG, Marmion BP. Variation in immune response genes and chronic Q fever. Concepts: preliminary test with post-Q fever fatigue syndrome. Genes Immun 2003;4:82–5.
- Marmion BP, Shannon M, Maddocks I, Storm P, Penttila I. Protracted debility and fatigue after acute Q fever. Lancet 1996;347:977–8.
- Marmion BP, Storm PA, Ayres JG, et al. Long-term persistence of Coxiella burnetii after acute primary Q fever. QJM 2005;98:7–20.
- Marmion BP, Sukocheva O, Storm PA, et al. Q fever: persistence of antigenic non-viable cell residues of Coxiella burnetii in the host—implications for post Q fever infection fatigue syndrome and other chronic sequelae. QJM 2009;102:673–84.
- Sukocheva OA, Marmion BP, Storm PA, Lockhart M, Turra M, Graves S. Long-term persistence after acute Q fever of non-infective Coxiella burnetii cell components, including antigens. QJM 2010;103:847–63.
- Penttila IA, Harris RJ, Storm P, Haynes D, Worswick DA, Marmion BP. Cytokine dysregulation in the post-Q-fever fatigue syndrome. QJM 1998;91:549–60.
- Helbig K, Harris R, Ayres J, et al. Immune response genes in the post-Q-fever fatigue syndrome, Q fever endocarditis and uncomplicated acute primary Q fever. QJM 2005;98:565–74.
- Limonard GJ, Peters JB, Nabuurs-Franssen MH, et al. Detailed analysis of health status of Q fever patients 1 year after the first Dutch outbreak: a case-control study. QJM 2010;103:953–8.
- Morroy G, Peters JB, van Nieuwenhof M, et al. The health status of Q-fever patients after long-term follow-up. BMC Infect Dis 2011;11:97.
- Morroy G, Bor HH, Polder J, et al. Self-reported sick leave and long-term health symptoms of Q-fever patients. Eur J Public Health 2012;22:814–9.
- Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 2006;333:575.
- Arashima Y, Kato K, Komiya T, et al. Improvement of chronic nonspecific symptoms by long-term minocycline treatment in Japanese patients with Coxiella burnetii infection considered to have post-Q fever fatigue syndrome. Intern Med 2004;43:49–54.
- Ledina D, Bradaric N, Milas I, Ivic I, Brncic N, Kuzmicic N. Chronic fatigue syndrome after Q fever. Med Sci Monit 2007;13:CS88–92.
- Healy B, van Woerden H, Raoult D, et al. Chronic Q fever: different serological results in three countries–results of a follow-up study 6 years after a point source outbreak. Clin Infect Dis 2011;52:1013–9.
- Murphy AM, Field PR. The persistence of complement-fixing antibodies to Q-fever (Coxiella burnetii) after infection. Med J Aust 1970;1:1148–50.
- La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol 1996;34:2270–4.
- Musso D, Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin Diagn Lab Immunol 1997;4:208–12.
- Benenson AS, Tigertt WD. Studies on Q fever in man. Trans Assoc Am Physics 1956;69:98–104.
- Wielders CC, Kampschreur LM, Schneeberger PM, et al. Early diagnosis and treatment of patients with symptomatic acute Q fever do not prohibit IgG antibody responses to Coxiella burnetii. Clin Vaccine Immunol 2012;19:1661–6.
- Tilburg JJ, Melchers WJ, Pettersson AM, et al. Interlaboratory evaluation of different extraction and real-time PCR methods for detection of Coxiella burnetii DNA in serum. J Clin Microbiol 2010;48:3923–7.
- Schneeberger PM, Hermans MH, van Hannen EJ, Schellekens JJ, Leenders AC, Wever PC. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin Vaccine Immunol 2010;17:286–90.
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8.
- van der Hoek W, Versteeg B, Meekelenkamp JC, et al. Follow-up of 686 patients with acute Q fever and detection of chronic infection. Clin Infect Dis 2011;52:1431–6.
- Fenollar F, Fournier PE, Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J Clin Microbiol 2004;42:4919–24.
- Limonard GJ, Nabuurs-Franssen MH, Weers-Pothoff G, et al. One-year follow-up of patients of the ongoing Dutch Q fever outbreak: clinical, serological and echocardiographic findings. Infection 2010;38:471–7.
- Lepidi H, Houpikian P, Liang Z, Raoult D. Cardiac valves in patients with Q fever endocarditis: microbiological, molecular, and histologic studies. J Infect Dis 2003;187:1097–106.
- Hamilton LR, George DL, Scoville SL, Hospenthal DR, Griffith ME. PCR for rapid diagnosis of acute Q fever at a combat support hospital in Iraq. Mil Med 2011;176:103–5.
- Gikas A, Kofteridis D, Manios A, Pediaditis J, Tselentis Y. Newer macrolides as empiric treatment for acute Q fever infection. Antimicrob Agents Chemother 2001;45:3644–6.
- Dijkstra F, Riphagen-Dalhuisen J, Wijers N, et al. Antibiotic therapy for acute Q fever in the Netherlands in 2007 and 2008 and its relation to hospitalization. Epidemiol Infect 2011;139:1332–41.
- Fenollar F, Thuny F, Xeridat B, Lepidi H, Raoult D. Endocarditis after acute Q fever in patients with previously undiagnosed valvulopathies. Clin Infect Dis 2006;42:818–21.
- Healy B, Llewelyn M, Westmoreland D, Lloyd G, Brown N. The value of follow-up after acute Q fever infection. J Infect 2006;52:e109–12.
- Palmer SR, Young SE. Q-fever endocarditis in England and Wales, 1975–81. Lancet 1982;320:1448–9.
- Raoult D, Houpikian P, Tissot Dupont H, Riss JM, Arditi-Djiane J, Brouqui P. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med 1999;159:167–73.
- Rolain JM, Mallet MN, Raoult D. Correlation between serum doxycycline concentrations and serologic evolution in patients with Coxiella burnetii endocarditis. J Infect Dis 2003;188:1322–5.
- Parker NR, Barralet JH, Bell AM. Q fever. Lancet 2006;367:679–88.
- Czeizel AE, Rockenbauer M, Sørensen HT, Olsen J. The teratogenic risk of trimethoprim-sulfonamides: a population based case-control study. Reprod Toxicol 2001;15:637–46.
- Forna F, McConnell M, Kitabire F, et al. Systematic review of the safety of trimethoprim-sulfamethoxazole for prophylaxis in HIV-infected pregnant women: implications for resource-limited settings. AIDS Rev 2006;8:24–36.
- American Academy of Pediatrics. Rocky Mountain spotted fever. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red book 2009: Report of the Committee on Infectious Diseases. 28th ed. Elk Grove, IL: American Academy of Pediatrics; 2009:573–75.
- CDC. Q fever among slaughterhouse workers—California. MMWR 1986;35:223–6.
- Perry S, Dennie CJ, Coblentz CL, Cleland S. Minimizing the risk of Q fever in the hospital setting. Can J Infect Control 1994;9:5–8.
- Spicknall CG, Huebner RJ, et al. Report on an outbreak of Q fever at the National Institute of Health; clinical features. Ann Intern Med 1947;27:28–40.
- Steiner HA, Raveh D, Rudensky B, et al. Outbreak of Q fever among kitchen employees in an urban hospital. Eur J Clin Microbiol Infect Dis 2001;20:898–900.
- Thomas DR, Treweek L, Salmon RL, et al. The risk of acquiring Q fever on farms: a seroepidemiological study. Occup Environ Med 1995;52:644–7.
- Simor AE, Brunton JL, Salit IE, Vellend H, Ford-Jones L, Spence LP. Q fever: hazard from sheep used in research. Can Med Assoc J 1984; 130:1013–6.
- Gidding HF, Wallace C, Lawrence GL, McIntyre PB. Australia's national Q fever vaccination program. Vaccine 2009;27:2037–41.
- Deutsch DL, Peterson ET. Q fever: Transmission from one human being to others. J Am Med Assoc 1950;143:348–50.
- Siegert R, Simrock W, Stroder U. A hospital outbreak of Q fever. Ztschr f Tropenmed u Parasit 1950;2:1.
- Gerth HJ, Leidig U, Riemenschneider T. Q-fever epidemic in an institute of human pathology. Dtsch Med Wochenschr 1982;107:1391–5.
- Siegel JRE, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee. 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings; 2007 Available at http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf. Accessed February 13, 2013.
- CDC. Guidelines for safe work practices in human and animal medical diagnostic laboratories. Recommendations of a CDC-convened, Biosafety Blue Ribbon Panel. MMWR 2012;61(Suppl).
- Chosewood LCWD. Biosafety in microbiological and biomedical laboratories. 5th ed. Atlanta, GA: CDC; 2007.
- US Department of Labor. Respiratory protection. Washington, DC: 2012. Available at http://www.osha.gov/SLTC/respiratoryprotection/index.html. Accessed February 13, 2013.
- Hall CJ, Richmond SJ, Caul EO, Pearce NH, Silver IA. Laboratory outbreak of Q fever acquired from sheep. Lancet 1982;1:1004–6.
- Ruppanner R, Brooks D, Franti CE, Behymer DE, Morrish D, Spinelli J. Q fever hazards from sheep and goats used in research. Arch Environ Health 1982;37:103–10.
- Curet LB, Paust JC. Transmission of Q fever from experimental sheep to laboratory personnel. Am J Obstet Gynecol 1972;114:566–8.
- Scott GH, Williams JC. Susceptibility of Coxiella burnetii to chemical disinfectants. Ann N Y Acad Sci 1990;590:291–6.
- Banazis M. Sciences MUFoH. Development of tools for surveillance of Coxiella burnetii in domestic ruminants and Australian marsupials and their waste: Murdoch University; 2009.
- Priestley RaM. R. Decontamination issues with Coxiella burnetii: an evaluation of currently available disinfectants. American Society of Rickettsiology 21st Meeting; 2007 September 8–11, 2007; Colorado Springs, CO; 2007.
- Moodie CE, Thompson HA, Meltzer MI, Swerdlow DL. Prophylaxis after exposure to Coxiella burnetii. Emerg Infect Dis 2008;14:1558–66.
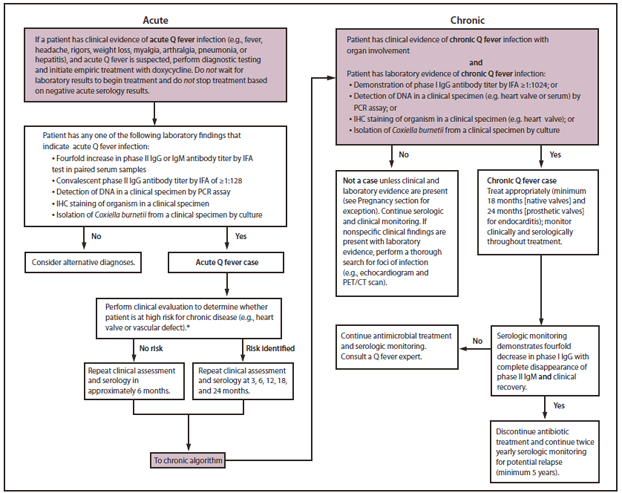
Abbreviations: CT = computed tomography; IFA = immunofluorescent assay; IgG = immunoglobulin G; IgM = immunoglobulin M; IHC = immunohistochemistry; PCR = polymerase chain reaction; PET = positron emission tomography.
* This algorithm is intended for use as a general guide and does not replace the physician's clinical judgment. It is intended to provide a management strategy for patients under the care of a physician and is not intended for those who might be tested for Q fever as part of an occupational monitoring program. Women infected during pregnancy should be treated and monitored as described in the text of the report.
Alternate Text: This figure shows a flow chart describing the management of acute Q fever and chronic Q fever.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.


