Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Use of a Reduced (4-Dose) Vaccine Schedule for Postexposure Prophylaxis to Prevent Human Rabies: Recommendations of the Advisory Committee on Immunization Practices
Please note: An erratum has been published for this article. To view the erratum, please click here.
Summary
This report summarizes new recommendation and updates previous recommendations of the Advisory Committee on Immunization Practices (ACIP) for postexposure prophylaxis (PEP) to prevent human rabies (CDC. Human rabies prevention---United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR 2008;57[No. RR-3]). Previously, ACIP recommended a 5-dose rabies vaccination regimen with human diploid cell vaccine (HDCV) or purified chick embryo cell vaccine (PCECV). These new recommendations reduce the number of vaccine doses to four. The reduction in doses recommended for PEP was based in part on evidence from rabies virus pathogenesis data, experimental animal work, clinical studies, and epidemiologic surveillance. These studies indicated that 4 vaccine doses in combination with rabies immune globulin (RIG) elicited adequate immune responses and that a fifth dose of vaccine did not contribute to more favorable outcomes. For persons previously unvaccinated with rabies vaccine, the reduced regimen of 4 1-mL doses of HDCV or PCECV should be administered intramuscularly. The first dose of the 4-dose course should be administered as soon as possible after exposure (day 0). Additional doses then should be administered on days 3, 7, and 14 after the first vaccination. ACIP recommendations for the use of RIG remain unchanged. For persons who previously received a complete vaccination series (pre- or postexposure prophylaxis) with a cell-culture vaccine or who previously had a documented adequate rabies virus-neutralizing antibody titer following vaccination with noncell-culture vaccine, the recommendation for a 2-dose PEP vaccination series has not changed. Similarly, the number of doses recommended for persons with altered immunocompetence has not changed; for such persons, PEP should continue to comprise a 5-dose vaccination regimen with 1 dose of RIG. Recommendations for pre-exposure prophylaxis also remain unchanged, with 3 doses of vaccine administered on days 0, 7, and 21 or 28. Prompt rabies PEP combining wound care, infiltration of RIG into and around the wound, and multiple doses of rabies cell-culture vaccine continue to be highly effective in preventing human rabies.
Introduction
Rabies is a zoonotic disease caused by RNA viruses in the family Rhabdoviridae, genus Lyssavirus (1). Virus is transmitted in the saliva of rabid mammals via a bite. After entry to the central nervous system, these viruses cause an acute, progressive encephalomyelitis. The incubation period usually ranges from 1 to 3 months after exposure, but can range from days to years. Rabies can be prevented by avoidance of viral exposure and initiation of prompt medical intervention when exposure does occur. In the United States, animal rabies is common. In a recent study, approximately 23,000 persons per year were estimated to have been exposed to potentially rabid animals and received rabies postexposure prophylaxis (PEP) (2). With the elimination of canine rabies virus variants and enzootic transmission among dogs, human rabies is now rare in the United States, with an average of one or two cases occurring annually since 1960 (3).
Prompt wound care and the administration of rabies immune globulin (RIG) and vaccine are highly effective in preventing human rabies following exposure. A variety of empirical schedules and vaccine doses have been recommended over time, based in part on immunogenicity and clinical experience in areas of the world with enzootic canine or wildlife rabies (4). As more potent vaccines were developed, the number of vaccine doses recommended for PEP has decreased, and studies aimed at further revision and reduction of PEP schedules and doses in humans have been encouraged. By the latter half of the 20th century, a 4- to 6-dose, intramuscular regimen using human diploid cell vaccine (HDCV) or purified chick embryo cell vaccine (PCECV) was being recommended (5--8). In the United States, a 5-dose PEP vaccine regimen was adopted during the 1980s (9--12). In 2007, when human rabies vaccine was in limited supply, an ad hoc National Rabies Working Group was formed to reassess the recommendations for rabies prevention and control in humans and other animals. In 2008, a smaller Advisory Committee on Immunization Practices (ACIP) Rabies Workgroup was formed to review rabies vaccine regimen options. This report provides updated ACIP recommendations regarding the use of a 4-dose vaccination regimen, replacing the previously recommended 5-dose regimen, for rabies PEP in previously unvaccinated persons.
Methods
The ACIP Rabies Workgroup* was formed in October 2008 to review 1) previous recommendations; 2) published and unpublished data from both national and global sources regarding rabies PEP; and 3) the immunogenicity, effectiveness, and safety of a 4-dose PEP rabies vaccination regimen. The ACIP Rabies Workgroup used an evidence-based process for consideration of a reduced vaccination regimen in human rabies PEP. This approach consisted of a review of information available from basic and applied studies of rabies prevention. Because rabies is almost always fatal among immunologically naïve persons once clinical symptoms of rabies occur, randomized, placebo-controlled efficacy studies of vaccine in humans cannot be conducted. The ACIP Rabies Workgroup reviewed six areas: 1) rabies virus pathogenesis, 2) experimental animal models, 3) human immunogenicity studies, 4) prophylaxis effectiveness in humans, 5) documented failures of prophylaxis in humans, and 6) vaccine safety. Studies for review were identified by searching the PubMed database and other relevant references and by consulting subject-matter experts. When definitive research evidence was lacking, the recommendations incorporated the expert opinion of the ACIP Rabies Workgroup members. The ACIP Rabies Workgroup also sought advice and comment from representatives of the vaccine industry, the National Association of State Public Health Veterinarians, the Council of State and Territorial Epidemiologists, state and local public health officials, additional national stakeholder groups, and other national and international experts. The proposed revised recommendations and a draft statement from the ACIP Rabies Workgroup were presented to the full ACIP during February 2009. After review and comment by ACIP, a revised draft, recommending a reduced regimen of 4 1-mL doses of rabies vaccine for PEP in previously unvaccinated persons, was prepared for consideration. These recommendations were discussed and accepted by ACIP at the June 2009 meeting (13).
Rationale for Reduced Doses of Human Rabies Vaccine
A detailed review of the evidence in support of a reduced, 4-dose schedule for human PEP has been published (14). The totality of the evidence, obtained from the available peer-reviewed literature, unpublished data sources, epidemiologic reviews, and expert opinion strongly supports a reduced vaccination schedule (Table 1). Since the 19th century, prophylactic interventions against rabies have recognized the highly neurotropic characteristics of lyssaviruses and have aimed at neutralizing the virus at the site of infection before it can enter the human central nervous system (Figure 1) (4,15,16). To accomplish this, immunologic interventions must be prompt and must be directed toward local virus neutralization, such as local infiltration with RIG and vaccination. Modern recommended rabies PEP regimens emphasize early wound care and passive immunization (i.e., infiltration of RIG in and around the wound) combined with active immunization (i.e., serial doses of rabies vaccine). Accumulated scientific evidence indicates that, following rabies virus exposure, successful neutralization and clearance of rabies virus mediated via appropriate PEP generally ensures patient survival (8).
The induction of a rabies virus-specific antibody response is one important immunologic component of response to vaccination (4). Development of detectable rabies virus-specific neutralizing antibodies is a surrogate for an adequate immune response to vaccination. Clinical trials of human rabies vaccination indicate that all healthy persons develop detectable rabies virus-neutralizing antibody titer rapidly after initiation of PEP. For example, in a literature review conducted by the ACIP Rabies Workgroup of at least 12 published rabies vaccination studies during 1976--2008 representing approximately1,000 human subjects, all subjects developed rabies virus-neutralizing antibodies by day 14 (14).
Observational studies indicate that PEP is universally effective in preventing human rabies when administered promptly and appropriately. Of the >55,000 persons who die annually of rabies worldwide, the majority either did not receive any PEP, received some form of PEP (usually without RIG) after substantial delays, or were administered PEP according to schedules that deviated substantially from current ACIP or World Health Organization recommendations (17). For example, a review of a series of 21 fatal human cases in which patients received some form of PEP indicated that 20 patients developed signs of illness, and most died before day 28 (Figure 2). In such cases, in which widespread infection of the central nervous system occurs before the due date (i.e., day 28) of the fifth vaccine dose, the utility of that dose must be nil. In the United States, of the 27 human rabies cases reported during 2000--2008, none of the patients had a history of receiving any PEP before illness, and this is the most common situation for human rabies deaths in both developed and developing countries (3,8). In India, an analysis from two animal bite centers during 2001--2002 demonstrated that in 192 human rabies cases, all deaths could be attributed to failure to seek timely and appropriate PEP, and none of them could be attributed to a failure to receive the fifth (day 28) vaccine dose (18). Even when PEP is administered imperfectly or not according to established scheduled dose recommendations, it might be generally effective. Several studies have reported cases involving persons who were exposed to potentially rabid animals and who received less than 5, 4, or even 3 doses of rabies vaccine but who nevertheless did not acquire rabies (Table 2). For example, in one series from New York, 147 (13%) of 1,132 patients had no report of receiving the complete 5-dose vaccine regimen. Of these patients, 26 (18%) received only 4 doses of vaccine, and two of these patients were exposed to animals with laboratory-confirmed rabies. However, no documented cases of human rabies occurred (CDC, unpublished data, 2003). The ACIP Rabies Working Group estimates that >1,000 persons in the United States receive rabies prophylaxis annually of only 3 or 4 doses, with no resulting documented cases of human rabies, even though >30% of these persons likely have exposure to confirmed rabid animals (14). In addition, no case of human rabies in the United States has been reported in which failure of PEP was attributable to receiving less than the 5-dose vaccine course. Worldwide, although human PEP failures have been reported very rarely, even in cases in which intervention appeared both prompt and appropriate (8), no cases have been attributed to the lack of receipt of the fifth human rabies vaccine dose on day 28 (4,17).
In vivo laboratory animal studies using multiple animal models from rodents to nonhuman primates have underscored the importance of timely PEP using RIG and vaccine, regardless of the absolute number of vaccine doses used or the schedule (14,19). For example, in a study in which 1, 2, 3, 4, or 5 doses of rabies vaccine were used in a Syrian hamster model in combination with human rabies immune globulin (HRIG), no statistically significant differences in elicited protection and consequent survivorship were observed among groups receiving different doses (20). In the same study, using a murine model, no differences were detected in immunogenicity and efficacy of PEP with 2, 3, or 4 vaccine doses. In another study using a nonhuman primate model, 1 dose of cell-culture vaccine, in combination with RIG administered 6 hours postexposure, provided substantial protection (21). In another study, a 3-dose regime was evaluated in a canine model and determined to be effective in preventing rabies (22).
Compared with older, nerve tissue-based products, adverse reactions associated with modern human rabies vaccination are uncommon (4). A review by the Workgroup of published and unpublished human rabies vaccine clinical trials and Vaccine Adverse Event Reporting System data identified no adverse events that were correlated to a failure to receive the fifth vaccine dose. As some adverse reactions might be independent clinical events with each vaccine administration, the omission of the vaccine dose on day 28 might have some positive health benefits. Otherwise, the overall safety of human rabies PEP is expected to be unchanged from the evidence provided in the 2008 ACIP report (12).
Preliminary economic assessments support the cost savings associated with a reduced schedule of vaccination (23,24). The ACIP Rabies Workgroup has estimated that, assuming 100% compliance with a recommended vaccine regimen, a change in recommendation from a 5-dose schedule to a 4-dose schedule would save approximately $16.6 million in costs to the U.S. health-care system. Persons who receive rabies vaccination might see some savings related to deletion of the fifth recommended dose of vaccine, measured in both the cost of the vaccine and the costs associated with the additional medical visit.
Revised Rabies Postexposure Prophylaxis Recommendations
This report presents revised recommendations for human rabies PEP (Table 3). Rabies PEP includes wound care and administration of both RIG and vaccine.
Postexposure Prophylaxis for Unvaccinated Persons
For unvaccinated persons, the combination of RIG and vaccine is recommended for both bite and nonbite exposures, regardless of the time interval between exposure and initiation of PEP. If PEP has been initiated and appropriate laboratory diagnostic testing (i.e., the direct fluorescent antibody test) indicates that the animal that caused the exposure was not rabid, PEP may be discontinued.
Vaccine Use
A regimen of 4 1-mL vaccine doses of HDCV or PCECV should be administered intramuscularly to previously unvaccinated persons (Table 3). The first dose of the 4-dose regimen should be administered as soon as possible after exposure. The date of the first dose is considered to be day 0 of the PEP series. Additional doses then should be administered on days 3, 7, and 14 after the first vaccination. Recommendations for the site of the intramuscular vaccination remain unchanged (e.g., for adults, the deltoid area; for children, the anterolateral aspect of the thigh also is acceptable). The gluteal area should not be used because administration of vaccine in this area might result in a diminished immunologic response. Children should receive the same vaccine dose (i.e., vaccine volume) as recommended for adults.
HRIG Use
The recommendations for use of immune globulin in rabies prophylaxis remain unchanged by the revised recommendation of a reduced rabies vaccine schedule. HRIG is administered once to previously unvaccinated persons to provide rabies virus-neutralizing antibody coverage until the patient responds to vaccination by actively producing virus-neutralizing antibodies. HRIG is administered once on day 0 at the time PEP is initiated, in conjunction with human rabies vaccines available for use in the United States. If HRIG was not administered when vaccination was begun on day 0, it can be administered up to and including day 7 of the PEP series (12,25). If anatomically feasible, the full dose of HRIG is infiltrated around and into any wounds. Any remaining volume is injected intramuscularly at a site distant from vaccine administration. HRIG is not administered in the same syringe or at the same anatomic site as the first vaccine dose. However, subsequent doses (i.e., on days 3, 7, and 14) of vaccine in the 4-dose vaccine series can be administered in the same anatomic location in which HRIG was administered.
Postexposure Prophylaxis for Previously Vaccinated Persons
Recommendations for PEP have not changed for persons who were vaccinated previously. Previously vaccinated persons are those who have received one of the ACIP-recommended pre- or postexposure prophylaxis regimens (with cell-culture vaccines) or those who received another vaccine regimen (or vaccines other than cell-culture vaccine) and had a documented adequate rabies virus-neutralizing antibody response. Previously vaccinated persons, as defined above, should receive 2 vaccine doses (1.0 mL each in the deltoid), the first dose immediately and the second dose 3 days later. Administration of HRIG is unnecessary, and HRIG should not be administered to previously vaccinated persons to avoid possible inhibition of the relative strength or rapidity of an expected anamnestic response (26). Local wound care remains an important part of rabies PEP for any previously vaccinated persons.
Vaccination and Serologic Testing
Postvaccination Serologic Testing
All healthy persons tested in accordance with ACIP guidelines after completion of at least a 4-dose regimen of rabies PEP should demonstrate an adequate antibody response against rabies virus (14). Therefore, no routine testing of healthy patients completing PEP is necessary to document seroconversion (12). When titers are obtained, serum specimens collected 1--2 weeks after prophylaxis (after last dose of vaccine) should completely neutralize challenge virus at least at a 1:5 serum dilution by the rapid fluorescent focus inhibition test (RFFIT). The rabies virus-neutralizing antibody titers will decline gradually since the last vaccination. Minimal differences (i.e., within one dilution of sera) in the reported values of rabies virus-neutralizing antibody results might occur between laboratories that provide antibody determination using the recommended RFFIT. Commercial rabies virus antibody titer determination kits that are not approved by the Food and Drug Administration are not appropriate for use as a substitute for the RFFIT. Discrepant results might occur after the use of such tests, and actual virus-neutralizing activity in clinical specimens cannot be measured.
Management of Adverse Reactions, Precautions, and Contraindications
Management of Adverse Reactions
Recommendations for management and reporting of vaccine adverse events have not changed. These recommendations have been described in detail previously (12).
Immunosuppression
Recommendations for rabies pre- and postexposure prophylaxis for persons with immunosuppression have not changed. General recommendations for active and passive immunization in persons with altered immunocompetence have been summarized previously (27,28). This updated report discusses specific recommendations for patients with altered immunocompetence who require rabies pre- and postexposure prophylaxis. All rabies vaccines licensed in the United States are inactivated cell-culture vaccines that can be administered safely to persons with altered immunocompetence. Because corticosteroids, other immunosuppressive agents, antimalarials, and immunosuppressive illnesses might reduce immune responses to rabies vaccines substantially, for persons with immunosuppression, rabies PEP should be administered using a 5-dose vaccine regimen (i.e., 1 dose of vaccine on days 0, 3, 7, 14, and 28), with the understanding that the immune response still might be inadequate. Immunosuppressive agents should not be administered during rabies PEP unless essential for the treatment of other conditions. If possible, immunosuppressed patients should postpone rabies preexposure prophylaxis until the immunocompromising condition is resolved. When postponement is not possible, immunosuppressed persons who are at risk for rabies should have their virus-neutralizing antibody responses checked after completing the preexposure series. Postvaccination rabies virus-neutralizing antibody values might be less than adequate among immunosuppressed persons with HIV or other infections (29,30). When rabies pre- or postexposure prophylaxis is administered to an immunosuppressed person, one or more serum samples should be tested for rabies virus-neutralizing antibody by the RFFIT to ensure that an acceptable antibody response has developed after completing the series. If no acceptable antibody response is detected after the final dose in the pre- or postexposure prophylaxis series, the patient should be managed in consultation with their physician and appropriate public health officials.
Variation from Human Rabies Vaccine Package Inserts
These new ACIP recommendations differ from current rabies vaccine label instructions, which still list the 5-dose series for PEP. Historically, ACIP review and subsequent public health recommendations for the use of various biologics has occurred after vaccine licensure and generally are in agreement with product labels. However, differences between ACIP recommendations and product labels are not unprecedented. For example, during the early 1980s, ACIP review and recommendations concerning the intradermal use of rabies vaccines occurred well in advance of actual label claims and licensing (9). On the basis of discussions with industry representatives, alterations of current product labels for HDCV and PCEC are not anticipated by the producers of human rabies vaccines licensed for use in the United States.
References
- Lyles DS, Rupprecht CE. Rhabdoviridae. In: Knipe D, Howley P, eds. Fields Virology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:1363--408.
- Christian KA, Blanton JD, Auslander M, Rupprecht CE. Epidemiology of rabies post-exposure prophylaxis---United States of America 2006--2008. Vaccine 2009;27:7156--61.
- Blanton JD, Robertson K, Palmer D, Rupprecht CE. Rabies surveillance in the United States during 2008. J Am Vet Med Assoc 2009;235:676--89.
- Plotkin SA, Koprowski H, Rupprecht CE. Rabies vaccines. In Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Philadelphia, PA: Saunders; 2008:687--714.
- World Health Organization. WHO Expert Committee on Rabies. 6th report. WHO Technical Report Series, No. 523. Geneva, Switzerland: World Health Organization; 1973.
- World Health Organization. WHO Expert Committee on Rabies. 7th report. WHO Technical Report Series, No. 709. Geneva, Switzerland: World Health Organization; 1984.
- World Health Organization. WHO Expert Committee on Rabies. 8th report. WHO Technical Report Series, No. 824. Geneva, Switzerland: World Health Organization; 1992.
- World Health Organization. WHO Expert Consultation on Rabies. 1st report. WHO Technical Report Series, No. 931. Geneva, Switzerland: World Health Organization; 2005.
- CDC. Rabies prevention---United States, 1984. y;33:393--408.
- CDC. Rabies prevention---United States, 1991: recommendations of the Immunization Practices Advisory Committee (ACIP). y40(No. RR-3).
- CDC. Human rabies prevention---United States, 1999: recommendations of the Advisory Committee on Immunization Practices (ACIP). y48(No. RR-1).
- CDC. Human rabies prevention---United States, 2008: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2008;57(No. RR-3).
- CDC. Human rabies biologics: consideration of reduced vaccine schedule in post-exposure prophylaxis. Advisory Committee on Immunization practices (ACIP): summary report of meeting held February 25--26, 2009 in Atlanta, GA. Available at http://www.cdc.gov/vaccines/recs/provisional/downloads/rabies-July2009-508.pdf. Accessed March 3, 2010.
- Rupprecht CE, Briggs D, Brown C, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine 2009;27:7141--8.
- Charlton KM, Nadin-Davis S, Casey GA, Wandeler AI. The long incubation period in rabies: delayed progression of infection in muscle at the site of exposure. Acta Neuropathol 1997;94:73--7.
- Dietzschold B, Schnell M, Koprowski H. Pathogenesis of rabies. Curr Top Microbiol Immunol 2005;292:45--56.
- Wilde H. Failures of post-exposure rabies prophylaxis. Vaccine 2007;25:7605--9.
- Ichhpujani RL, Mala C, Veena M, et al. Epidemiology of animal bites and rabies cases in India: a multicentric study. J Commun Dis 2008;40:27--36.
- Baer GM. Animal models in the pathogenesis and treatment of rabies. Rev Infect Dis 1988;10(Suppl 4):S739--50.
- Franka R, Wu X, Jackson RF, et al. Rabies virus pathogenesis in relationship to intervention with inactivated and attenuated rabies vaccines. Vaccine 2009;27:7149--55.
- Sikes RK, Cleary WF, Koprowski H, Wiktor TJ, Kaplan MM. Effective protection of monkeys against death from street virus by post-exposure administration of tissue-culture rabies vaccine. Bull World Health Organ 1971;45:1--11.
- Manickama R, y, y. Post-exposure prophylaxis (PEP) of rabies-infected Indian street dogs. y 2008;26:6564--8.
- Meltzer MI, Rupprecht CE. A review of the economics of the prevention and control of rabies: I. Global impact and rabies in humans. PharmacoEconomics 1998:14:365--83.
- Dhankhar P, Vaidya SA, Fishbien DB, Meltzer MI. Cost effectiveness of rabies post exposure prophylaxis in the United States. Vaccine 2008;26:4251--5.
- Khawplod P, Wilde H, Chomchey P, et al. What is an acceptable delay in rabies immune globulin administration when vaccine alone had been given previously? Vaccine 1996;14:389--91.
- Schumacher CL, Ertl HC, Koprowski H, Dietzschold B. Inhibition of immune responses against rabies virus by monoclonal antibodies directed against rabies virus antigens. Vaccine 1992;10:754--60.
- CDC. Recommendations of the Advisory Committee on Immunization Practices (ACIP): use of vaccines and immune globulins for persons with altered immunocompetence. y42(No. RR-4).
- CDC. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). y55(No. RR-15).
- Tantawichien T, Jaijaroensup W, Khawplod P, Sitprija V. Failure of multiple-site intradermal postexposure rabies vaccination in patients with human immunodeficiency virus with low CD4+ T lymphocyte counts. Clin Infect Dis 2001:33:E122--4.
- Pancharoen C, Thisyakorn U, Tantawichien T, Jaijaroensup W, Khawplod P, Wilde H. Failure of pre- and postexposure rabies vaccinations in a child infected with HIV. Scand J Infect Dis 2001;33:390--1.
Advisory Committee on Immunization Practices
Membership as of June 24, 2009
Chair: Dale Morse, MD, New York State Department of Health, Albany, New York.
Executive Secretary: Larry Pickering, MD, National Center for Immunization and Respiratory Diseases, CDC, Atlanta, Georgia.
Members: Carol Baker, MD, Baylor College of Medicine, Houston, Texas; Robert Beck, JD, Consumer Representative, Palmyra, Virginia; Lance Chilton, MD, University of New Mexico, Albuquerque, New Mexico; Paul Cieslak, MD, Oregon Public Health Division, Portland, Oregon; Kristen Ehresmann, MPH, Minnesota Department of Health, St. Paul, Minnesota; Janet Englund, MD, University of Washington and Children's Hospital and Regional Medical Center, Seattle, Washington; Franklyn Judson, MD, University of Colorado Health Sciences Center, Denver, Colorado; Susan Lett, MD, Massachusetts Department of Public Health, Boston, Massachusetts; Michael Marcy, MD, UCLA Center for Vaccine Research, Torrance, California; Cody Meissner, MD, Tufts Medical Center, Boston, Massachusetts; Kathleen Neuzil, MD, University of Washington; Seattle, Washington; Mark Sawyer, MD, University of California--San Diego, California; Ciro Valent Sumaya, MD, Texas A&M Health Science Center, College Station, Texas; Jonathan Temte, MD, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin.
Ex Officio Members: James E. Cheek, MD, Indian Health Service, Albuquerque, New Mexico; Wayne Hachey, DO, Department of Defense, Falls Church, Virginia; Geoffrey S. Evans, MD, Health Resources and Services Administration, Rockville, Maryland; Bruce Gellin, MD, National Vaccine Program Office, Washington, District of Columbia; Linda Murphy, Centers for Medicare and Medicaid Services, Baltimore, Maryland; George T. Curlin, MD, National Institutes of Health, Bethesda, Maryland; Norman Baylor, MD, Food and Drug Administration, Bethesda, Maryland; Linda Kinsinger, MD, Department of Veterans Affairs, Durham, North Carolina.
Liaison Representatives: American Academy of Family Physicians, Doug Campos-Outcalt, MD, Phoenix, Arizona; American Academy of Pediatrics, Joseph Bocchini, MD, Shreveport, Louisiana, David Kimberlin, MD, Birmingham, Alabama; American College Health Association, James C. Turner, MD, Charlottesville, Virginia; American College of Obstetricians and Gynecologists, Stanley Gall, MD, Louisville, Kentucky; American College of Physicians, Gregory Poland, MD, Rochester, Minnesota; American Geriatrics Society, Kenneth Schmader, MD, Durham, North Carolina; America's Health Insurance Plans, Tamara Lewis, MD, Salt Lake City, Utah; American Medical Association, Litjen Tan, PhD, Chicago, Illinois; American Osteopathic Association, Stanley Grogg, DO, Tulsa, Oklahoma; American Pharmacists Association, Stephan L. Foster, PharmD, Memphis, Tennessee; Association for Prevention Teaching and Research, W. Paul McKinney, MD, Louisville, Kentucky; Biotechnology Industry Organization, Clement Lewin, PhD, Cambridge, Massachusetts; Canadian National Advisory Committee on Immunization, Joanne Langley, MD, Halifax, Nova Scotia, Canada; Department of Health, United Kingdom, David M. Salisbury, MD, London, United Kingdom; Healthcare Infection Control Practices Advisory Committee, Alexis Elward, MD, St Louis, Missouri; Infectious Diseases Society of America, Samuel L. Katz, MD, Durham, North Carolina; National Association of County and City Health Officials, Jeff Duchin, MD, Seattle, Washington; National Association of Pediatric Nurse Practitioners, Patricia Stinchfield, MPH; National Foundation for Infectious Diseases, William Schaffner, MD, Nashville, Tennessee; National Immunization Council and Child Health Program, Mexico, Vesta Richardson, MD, Mexico City, Mexico; National Medical Association, Patricia Whitley-Williams, MD, New Brunswick, New Jersey; National Vaccine Advisory Committee, Guthrie Birkhead, MD, Albany, New York; Pharmaceutical Research and Manufacturers of America, Damian A. Braga, Swiftwater, Pennsylvania; Peter Paradiso, PhD, Collegeville, Pennsylvania; Society for Adolescent Medicine, Amy Middleman, MD, Houston, Texas; Society for Healthcare Epidemiology of America, Harry Keyserling, MD, Atlanta, Georgia.
ACIP Rabies Workgroup
Membership as of June 24, 2009
Chair: Paul Cieslak, MD, Oregon Department of Public Health, Corvallis, Oregon.
Members: Deborah Briggs, PhD, Kansas State University, Manhattan, Kansas; Catherine Brown, DVM, Massachusetts Department of Public Health, Jamaica Plain, Massachusetts; Samuel L. Katz, MD, Duke University Medical Center, Durham, North Carolina; Harry D. Kerr, MD, American College of Emergency Physicians, Dallas, Texas; Susan M. Lett, MD, Massachusetts Department of Public Health, Jamaica Plain, Massachusetts; Robin Levis, PhD, Food and Drug Administration, Washington, District of Columbia; William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tennessee. Charles E. Rupprecht, VMD, PhD, Richard Franka, DVM, PhD, Martin I. Meltzer, PhD, CDC, Atlanta, Georgia.
FIGURE 1. Schematic of dynamics of rabies virus pathogenesis* in the presence and absence of postexposure prophylaxis (PEP)--mediated immune responses†
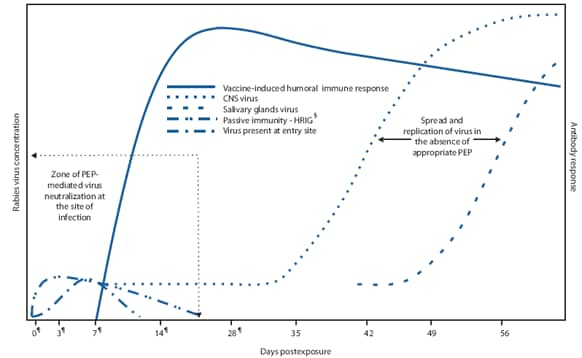
* Rabies can progress through five stages: incubation period (5 days to >2 years: U.S. median ~35 days), prodrome state (0--10 days), acute neurologic period (2--7 days), coma (5--14 days), and death.
† Once in tissues at the entry site, rabies virus can be neutralized by passively administered rabies immune globulin (RIG). Active immunization (vaccine) stimulates the host immune system, and, as a result, virus-neutralizing antibodies (VNA) are produced approximately 7--10 days after initiation of vaccination. By approximately day 14--28 (after administration of 4 vaccine doses), VNAs peak. In the absence of early and adequate PEP, virus enters host neurons, spreads to the central nervous system (CNS), and causes disease, with inevitably fatal consequence.
§ Human rabies immune globulin.
¶ Day vaccine administered.
Alternate Text: Figure 1 depicts an idealized outline of dynamics of rabies virus pathogenesis in the presence and absence of PEP-mediated immune responses. Clinical stages of rabies can progress from incubation period (5 days to >2 years: U.S. median ~35 days) to prodrome state (0-10 days) to acute neurologic period (2-7 days) to coma (5-14 days) to death. Once in tissues at the entry site, rabies virus can be neutralized by passively administered rabies immune globulin (RIG). Active immunization (vaccine) stimulates the host immune system, and, as a result, virus-neutralizing antibodies (VNA) are produced approximately 7-10 days after initiation of vaccination. By approximately day 14-28 (after administration of 4 vaccine doses), VNAs peak. In the absence of early and adequate PEP, virus enters host neurons, spreads to the central nervous system, and causes disease, with inevitably fatal consequence.
FIGURE 2. Number of documented rabies postexposure prophylaxis (PEP) failures --- Burma, India, the Philippines, South Africa, Sri Lanka, and Thailand, 1984--2007*
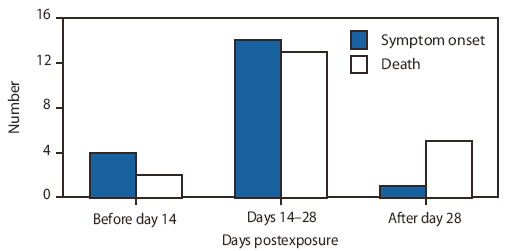
SOURCES: Wilde H. Failures of post-exposure rabies prophylaxis. Vaccine 2007;25:7605--9; Wilde H, Sirikawin S, Sabcharoen A, et al. Failure of postexposure treatment of rabies in children. Clin Infect Dis 1996;22:228--32; Matha IS, Salunke SR. Immunogenicity of purified vero cell rabies vaccine used in the treatment of fox-bite victims in India. Clin Infect Dis 2005;40:611--3.
* Of 21 reported PEP failures described, 20 patients had symptoms and 15 died before day 28.
Alternate Text: Figure 2 depicts the number of cases of documented rabies PEP failures in Burma, India, the Philippines, South Africa, Sri Lanka, and Thailand during 1984-2007. Of 21 reported PEP failures described, 20 patients had symptoms and 15 died before day 28. The majority of cases of symptom onset and death occurred during days 14-28.
|
TABLE 2. Number and percentage of patients with suspected rabies exposures who received <5 doses of vaccine --- India, 2003; New York, 1998--2000; and Puerto Rico, 2008* |
||||
|---|---|---|---|---|
|
Location (year) |
No. of persons exposed |
Persons who received <5 doses of vaccine |
No. of documented rabies deaths |
|
|
No. |
(%) |
|||
|
New York (1998--2000)† |
1,132 |
147 |
(13) |
0 |
|
India (2003)§ |
439 |
261 |
(59) |
0 |
|
Puerto Rico (2008)¶ |
191 |
30 |
(16) |
0 |
|
* No cases of human rabies were recorded that were attributable to receipt of only 4 doses of vaccine. † SOURCE: CDC, unpublished data, 2003. § SOURCE: Association for the Prevention and Control of Rabies (APCRI) in India. Assessing the burden of rabies in India: WHO sponsored national multi-centric rabies survey 2003. Final report May 2004. Available at http://rabies.org.in. Accessed March 8, 2010. Sudarshan MK, Madhusudana SN, Mahendra BJ, et al. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Intl J Infect Dis 2007;11:29--35. ¶ SOURCE: CDC, unpublished data, 2008. |
||||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


