FIGURE. Number* and percentage of respiratory specimens testing positive for influenza, by type, surveillance week, and year --- U.S. World Health Organization and National Respiratory and Enteric Virus Surveillance System collaborating laboratories, United States, May 22--September 3, 2011†
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Update: Influenza Activity --- United States and Worldwide, May 22--September 3, 2011
During May 22--September 3, 2011, the United States experienced low levels of influenza activity; 2009 influenza A (H1N1), influenza A (H3N2), and influenza B viruses were detected worldwide and identified sporadically in the United States. Typical seasonal patterns of influenza activity occurred in the Southern Hemisphere. This report summarizes influenza activity in the United States and worldwide since the last update (1).
United States
The U.S. influenza surveillance system is a collaborative effort between CDC and its federal, state, and local partners. CDC uses eight systems* to collect influenza information (2), six of which provide data year-round: 1) U.S. World Health Organization (WHO) collaborating laboratories; 2) the National Respiratory and Enteric Virus Surveillance System (NREVSS); 3) reports of novel influenza A virus cases from the National Notifiable Disease Surveillance System (NNDSS); 4) the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet); 5) the 122 Cities Mortality Reporting System; and 6) the Influenza-Associated Pediatric Mortality Reporting System.
During May 22-- September 3,† U.S. WHO and NREVSS collaborating laboratories tested 20,868 respiratory specimens for influenza viruses; 122 (0.6%) tested positive for influenza (Figure). Of these, 87 (71%) were influenza A viruses, and 35 (29%) were influenza B viruses. Of the influenza A viruses, 39 (45%) were subtyped: 24 (62%) were influenza A (H3N2) viruses and 15 (38%) were 2009 influenza A (H1N1) viruses. Four human infections with a novel influenza A virus (swine-origin influenza A [H3N2]) were reported in August (3) and September. These viruses are genetically and antigenically different from currently circulating influenza A (H3N2) viruses. Influenza viruses were reported from 26 states in all 10 U.S. Department of Health and Human Services (HHS) Regions. The largest proportion of positive samples came from the southeastern United States (HHS Region 4: Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, and Tennessee) (52%), followed by western states (HHS Region 9: Arizona, California, Hawaii, and Nevada) (17%).
During May 22--September 3, data from ILINet indicated that the weekly percentage of outpatient visits to ILINet providers for influenza-like illness (ILI)§ remained below the national baseline¶ of 2.5% and ranged from 0.5% to 1.2%. The percentage of deaths attributed to pneumonia and influenza (P&I), as reported by the 122 Cities Mortality Reporting System, remained below the epidemic threshold** except for 3 weeks in June. One influenza-associated pediatric death was reported in August and was associated with an influenza B virus.
Worldwide
During May 22--September 3, typical seasonal patterns of influenza activity occurred in the Southern Hemisphere. In Australia, influenza activity began increasing in mid-May; 2009 influenza A (H1N1) virus predominated and cocirculated with influenza B viruses, with small numbers of influenza A (H3N2) virus reported. However, in New Zealand, influenza B viruses predominated, with lower levels of influenza A (H3N2) and 2009 influenza A (H1N1) viruses cocirculating. In South America, influenza activity was low and influenza A viruses were reported more frequently, but the predominant subtype varied by country. In countries in southern Africa, 2009 influenza A (H1N1) viruses were the most common, followed by influenza B viruses, but in general influenza virus activity was low. The predominant subtype identified in Asia was influenza A (H3N2) virus, with a smaller number of influenza B viruses identified, although outbreaks of 2009 influenza A (H1N1) virus have been reported. In Europe and North America, influenza activity was low, and small numbers of 2009 influenza A (H1N1), influenza A (H3N2), and influenza B viruses were identified.
Antigenic Characterization of Influenza Virus Isolates
The WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza, located at CDC, receives and analyzes influenza virus isolates from laboratories worldwide. Sixty-eight 2009 influenza A (H1N1) viruses collected from May 22 to September 3 were analyzed (two from the United States, 44 from South America, 14 from Asia, and eight from Africa); all 68 (100%) were antigenically similar to A/California/7/2009, the influenza A (H1N1) component of the 2011--12 season influenza vaccine for the Northern Hemisphere. Of the 54 influenza A (H3N2) viruses characterized (two from the United States, 43 from South America, and nine from Asia), all 54 (100%) were antigenically similar to A/Perth/16/2009, the influenza A (H3N2) component of the 2011--12 influenza vaccine for the Northern Hemisphere. Finally, of 34 influenza B isolates collected during this period and analyzed by CDC, 31 (91%) belong to the B/Victoria lineage (two from the United States, 17 from South America, seven from Asia, and five from Africa), and all of those were antigenically similar to B/Brisbane/60/2008, the recommended influenza B component for the 2011--12 Northern Hemisphere influenza vaccine. The remaining three influenza B viruses (from Asia) belong to the B/Yamagata lineage and therefore are not related to the vaccine strain.
Antiviral Resistance Profiles of Influenza Virus Isolates
The WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza at CDC tested isolates collected during May 22--September 3 for resistance to influenza antiviral medications. Of 154 isolates tested for resistance to the neuraminidase inhibitor oseltamivir, 144 were received from foreign countries; 61 were 2009 influenza A (H1N1), 53 were influenza A (H3N2), and 30 were influenza B viruses. Ten were collected in the United States; five were 2009 influenza A (H1N1), three were influenza A (H3N2), and two were influenza B viruses. Of 151 isolates tested for resistance to the neuraminidase inhibitor zanamivir, 144 were received from foreign countries; 61 were 2009 influenza A (H1N1), 53 were influenza A (H3N2), and 30 were influenza B viruses. Seven were collected from the United States; two were 2009 influenza A (H1N1), three were influenza A (H3N2), and two were influenza B viruses. None of the tested viruses were found to be resistant to either oseltamivir or zanamivir. High levels of resistance to the adamantanes (i.e., amantadine and rimantadine) persisted among 2009 influenza A (H1N1) viruses and influenza A (H3N2) viruses circulating globally (4). Worldwide, oseltamivir-resistant 2009 influenza A (H1N1) viruses have been detected occasionally. For example, in the Newcastle region of Australia, in a limited geographic area, 25 cases of oseltamivir-resistant 2009 influenza A (H1N1) viruses were identified from May to August (5). CDC will continue to conduct surveillance for antiviral resistance among influenza viruses throughout the upcoming season.
Reported by
World Health Organization Collaborating Center for Surveillance, Epidemiology, and Control of Influenza. Lenee Blanton, MPH, Scott Epperson, MPH, Krista Kniss, MPH, Desiree Mustaquim, MPH, Amber Bishop, MPH, Lynnette Brammer, MPH, Margaret Okomo-Adhiambo, PhD, Larisa Gubareva, MD, Teresa Wallis, MS, Alexander Klimov, PhD, Joseph Bresee, MD, Nancy Cox, PhD, Lyn Finelli, DrPH, Influenza Div, National Center for Immunization and Respiratory Diseases, CDC. Corresponding contributor: Lenee Blanton, lblanton@cdc.gov, 404-639-3747.
Editorial Note
During May 22--September 3, surveillance data indicated that 2009 influenza A (H1N1), influenza A (H3N2), and influenza B viruses cocirculated worldwide. Although neither the influenza virus strain that will predominate nor the severity of influenza-related disease activity for the 2011--12 influenza season in the United States can be predicted, antigenic characterization of viral isolates submitted during the summer demonstrated that the vast majority of isolates were antigenically similar to the influenza vaccine strains in the Northern Hemisphere 2011--12 vaccine.
Influenza vaccination is the best method for preventing influenza and its associated complications. For optimal protection against influenza viruses, annual influenza vaccination is recommended regardless of whether the vaccine virus strains have changed since the previous season. In 2010, the Advisory Committee on Immunization Practices extended influenza vaccination recommendations to include all persons aged ≥6 months (6,7). Vaccine manufacturers project ample supplies of influenza vaccine in the United States for the 2011--12 influenza season; approximately 68 million doses had been distributed as of September 2, and influenza vaccination should proceed for all persons without contraindications to vaccination as soon as vaccine is available in their community. Multiple influenza vaccines are approved for use and are being distributed during the 2011--12 season, including trivalent inactivated vaccine (TIV) for persons aged ≥6 months, live, attenuated influenza vaccine (LAIV) for nonpregnant otherwise healthy persons aged 2--49 years; a high-dose inactivated vaccine for persons aged ≥65 years; and a new intradermally administered vaccine, Fluzone Intradermal, which was licensed by the Food and Drug Administration on May 10, 2011, for adults aged 18--64 years (7). Children aged 6 months--8 years who did not receive at least 1 dose of the 2010--11 seasonal influenza vaccine should receive 2 doses (administered at least 4 weeks apart) of the 2011--12 seasonal influenza vaccine. Children in this age group, who did receive at least 1 dose of the 2010--11 vaccine, and persons aged ≥9 years, should receive 1 dose of the 2011--12 seasonal vaccine (7).
For the 2011--12 influenza season, ACIP recommends that persons who have experienced only hives following exposure to eggs should still receive the influenza vaccine, with the following additional safety measures: vaccine should be administered by a health-care provider who is familiar with the subject of egg allergy, TIV should be used rather than LAIV, and the recipient should be observed for at least 30 minutes by the health-care provider after vaccination to monitor for possible reactions (7). Severe allergic reactions (e.g., anaphylaxis) to egg protein or other vaccine components continue to be contraindications to receipt of influenza vaccination. Also, severe allergic reaction to a previous dose of influenza vaccine, regardless of the component thought to be responsible for the reaction, continues to be a contraindication to the future receipt of vaccine.
Although annual vaccination is the best method for preventing and reducing the impact of influenza, influenza antiviral medications are an important adjunct. The benefits of influenza antiviral treatment are likely to be greatest if treatment is started as soon as possible after illness onset, and evidence for benefit is strongest in studies in which treatment was started within 48 hours of illness onset (8). Antiviral treatment is recommended as early as possible for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at greater risk for influenza-related complications (8).†† However, substantial observational data and one study in pregnant women (9) have indicated that antiviral treatment still can be beneficial in patients with severe, complicated, or progressive illness and in hospitalized patients even when administered >48 hours after illness onset (8,10). In such cases, decisions on starting antiviral treatment should not wait for laboratory confirmation of influenza. Antiviral treatment also may be considered for outpatients with confirmed or suspected influenza who do not have known risk factors for severe illness if treatment can be initiated within 48 hours of illness onset. Recommended antiviral medications include oseltamivir and zanamivir.
As a result of the ongoing investigation into the source of infection in the two cases of human infection with swine-origin influenza A (H3N2) virus in Indiana and Pennsylvania that were reported to CDC in August (3), two additional cases of swine-origin influenza A (H3N2) virus infection were identified in children in Pennsylvania and reported to CDC in September. Three of the four children had direct exposure to swine at an agricultural fair, but no exposure to swine was identified for the other child. One child is recovering at home, and the other three children have recovered fully.
Transmission of swine-origin influenza A viruses to humans is rare and usually occurs among persons in direct contact with swine or among persons who have visited places where swine are present (e.g., agricultural fairs, farms, and petting zoos). Clinicians should consider swine-origin influenza A virus infection as well as seasonal influenza virus infections in the differential diagnosis of patients with febrile respiratory illness who have been near swine (3). Clinicians who suspect influenza virus infection in humans with recent exposure to swine should obtain a nasopharyngeal swab from the patient, place the swab in a viral transport medium, contact their state or local health department to facilitate transport and timely diagnosis at a state public health laboratory, and consider empiric neuraminidase inhibitor antiviral treatment (3,6). Public health laboratories are requested to submit 1) summer specimens, 2) any specimens that cannot be subtyped by standard methods, or 3) specimens that are otherwise unusual, to CDC for further antigenic characterization, antiviral resistance monitoring, and identification of novel influenza A viruses. Early identification and prompt investigation of novel influenza A cases is critical to evaluating the extent of outbreaks and possible human-to-human transmission.
Influenza surveillance reports for the United States are posted online weekly and are available at http://www.cdc.gov/flu/weekly. Additional information regarding influenza viruses, influenza surveillance, influenza vaccine, influenza antiviral medications, and novel influenza A infections in humans is available at http://www.cdc.gov/flu.
Acknowledgments
State and territorial health departments and state public health laboratories; U.S. World Health Organization collaborating laboratories; National Respiratory and Enteric Virus Surveillance System collaborating laboratories; the U.S. Outpatient Influenza-like Illness Surveillance Network; the Influenza-Associated Pediatric Mortality Surveillance System; the 122 Cities Mortality Reporting System; and World Health Organization FluNet.
References
- CDC. Update: influenza activity--United States, 2010--11 season, and composition of the 2011--12 influenza vaccine. MMWR 2011;60:705--12.
- Brammer L, Blanton L, Epperson S, et al. Surveillance for influenza during the 2009 influenza A (H1N1) pandemic---United States, April 2009--March 2010. Clin Infect Dis 2011;52(Suppl 1):S27--35.
- CDC. Swine-origin influenza A (H3N2) virus infection in two children---Indiana and Pennsylvania, July--August 2011. MMWR 2011;60:1213--5.
- World Health Organization. Summary of influenza antiviral susceptibility surveillance findings, September 2010--March 2011. 2011. Geneva, Switzerland: World Health Organization; 2011. Available at http://www.who.int/csr/disease/influenza/influenzanetwork/flunet/antiviral_susceptibility/en. Accessed September 9, 2011.
- World Health Organization. Influenza virus activity in the world: 9 September 2011. Geneva, Switzerland: World Health Organization; 2011. Available at http://www.who.int/influenza/gisrs_laboratory/updates/summaryreport/en/index.html. Accessed September 13, 2011.
- CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59(No. RR-8).
- CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR 2011;60:1128--32.
- CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza---recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. RR-1).
- Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA 2010;303:1517--25.
- Yu H, Feng Z, Uyeki T, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis 2011;52:457--65.
* The CDC influenza surveillance system collects five categories of information from eight data systems: 1) viral surveillance (World Health Organization collaborating laboratories, the National Respiratory and Enteric Virus Surveillance System, and novel influenza A virus case reporting); 2) outpatient illness surveillance (U.S. Outpatient Influenza-like Illness Surveillance Network); 3) mortality (122 Cities Mortality Reporting System and influenza-associated pediatric mortality reports); 4) hospitalizations (FluSurv-NET, which includes the Emerging Infections Program and surveillance in four additional states); and 5) summary of the geographic spread of influenza (state and territorial epidemiologist reports).
† Data as of September 9, 2011.
§ Defined as a temperature of ≥100°F (≥37.8°C), oral or equivalent, and cough and/or sore throat, in the absence of a known cause other than influenza.
¶ The national and regional baselines are the mean percentage of visits for ILI during noninfluenza weeks for the previous three seasons plus two standard deviations. A noninfluenza week is a week during which <10% of specimens tested positive for influenza. National and regional percentages of patient visits for ILI are weighted on the basis of state population. Use of the national baseline for regional data is not appropriate.
** The seasonal baseline proportion of P&I deaths is projected using a robust regression procedure in which a periodic regression model is applied to the observed percentage of deaths from P&I that were reported by the 122 Cities Mortality Reporting System during the preceding 5 years. The epidemic threshold is set at 1.645 standard deviations above the seasonal baseline.
†† Persons at greater risk include children aged <5 years (especially those aged <2 years); adults aged ≥65 years; persons with chronic pulmonary (including asthma), cardiovascular (except hypertension alone), renal, hepatic, hematologic (including sickle cell disease), metabolic (including diabetes mellitus), or neurologic and neurodevelopmental conditions (including disorders of the brain, spinal cord, peripheral nerve, and muscle, such as cerebral palsy, epilepsy [seizure disorders], stroke, intellectual disability [mental retardation], moderate to severe developmental delay, muscular dystrophy, or spinal cord injury); persons with immunosuppression, including that caused by medications or by human immunodeficiency virus infection; women who are pregnant or postpartum (within 2 weeks after delivery); persons aged ≤18 years who are receiving long-term aspirin therapy; American Indians/Alaska Natives; persons who are morbidly obese (i.e., body mass index ≥40); and residents of nursing homes and other chronic-care facilities.
What is already known on this topic?
CDC collects, compiles, and analyzes data on influenza activity year-round in the United States. The influenza season generally begins in the fall and continues through the winter and spring months; however, the timing and severity of circulating influenza viruses can vary by geographic location and season.
What is added by this report?
The United States experienced low levels of influenza activity from May 22 to September 3, 2011, and influenza A (H3N2), 2009 influenza A (H1N1), and influenza B viruses were identified sporadically. The vast majority of viral isolates submitted during the summer demonstrated that they are antigenically similar to the influenza vaccine strains in the Northern Hemisphere 2011--12 vaccine.
What are the implications for public health practice?
To prevent influenza and its associated complications, influenza vaccination is recommended in all persons aged ≥6 months. Year-round influenza surveillance provides critical information for planning interventions to prevent and control influenza, developing vaccine recommendations and antiviral treatment guidance, and presenting information to the public regarding the progress and severity of the influenza season.
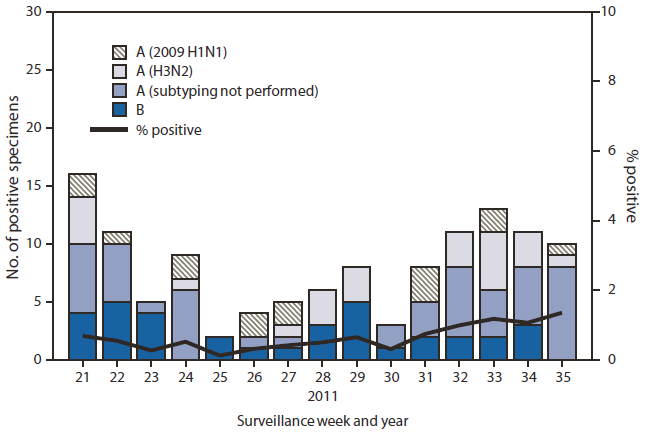
* N = 20,868.
† As of September 9, 2011.
Alternate Text: The figure above shows the number and percentage of respiratory specimens testing positive for influenza, by type, surveillance week, and year in the United States, from May 22-September 3, 2011, according to the U.S. World Health Organization and National Respiratory and Enteric Virus Surveillance System collaborating laboratories. During May 22- September 3, U.S. World Health Organization and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories tested 20,868 respiratory specimens for influenza viruses; 122 (0.6%) tested positive for influenza
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


