National Enteric Disease Surveillance: Shiga Toxin-producing E. coli (STEC) Annual Report, 2016
The Laboratory-based Enteric Disease Surveillance (LEDS) system contributes to the understanding of human Shiga toxin-producing Escherichia coli (STEC) in the United States by collecting reports of infections submitted by state and regional public health laboratories1. Reporting to LEDS is voluntary; the number of laboratories submitting reports varies somewhat from year to year, although almost all public health laboratories report every year. Occasionally, more than one isolate is reported from a single episode of infection in a person; this report includes only one isolate of a given STEC serogroup per person within a 30-day period.
Data in this report are current as of April 16, 2018.
Summary
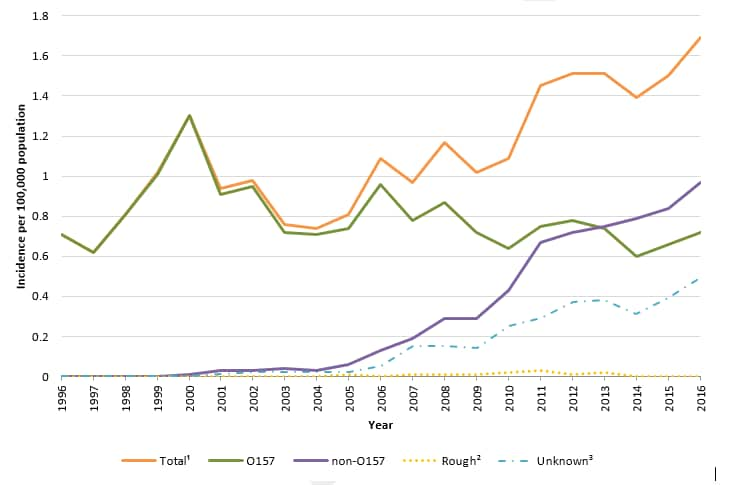
- In 2016, 52 state and regional public health laboratories reported 5,441 cases of culture-confirmed Shiga toxin-producing Escherichia coli (STEC) infections, including 2,323 O157 and 3,104 non-O157 cases.
- An additional 1,574 STEC reports were submitted with unknown serogroup, representing a 26% increase from 2015, likely due to increased culture-independent diagnostic testing.
- Compared with 2015, the incidence of both STEC O157 and non-O157 infections in 2016 was higher (9% and 15% increase respectively).
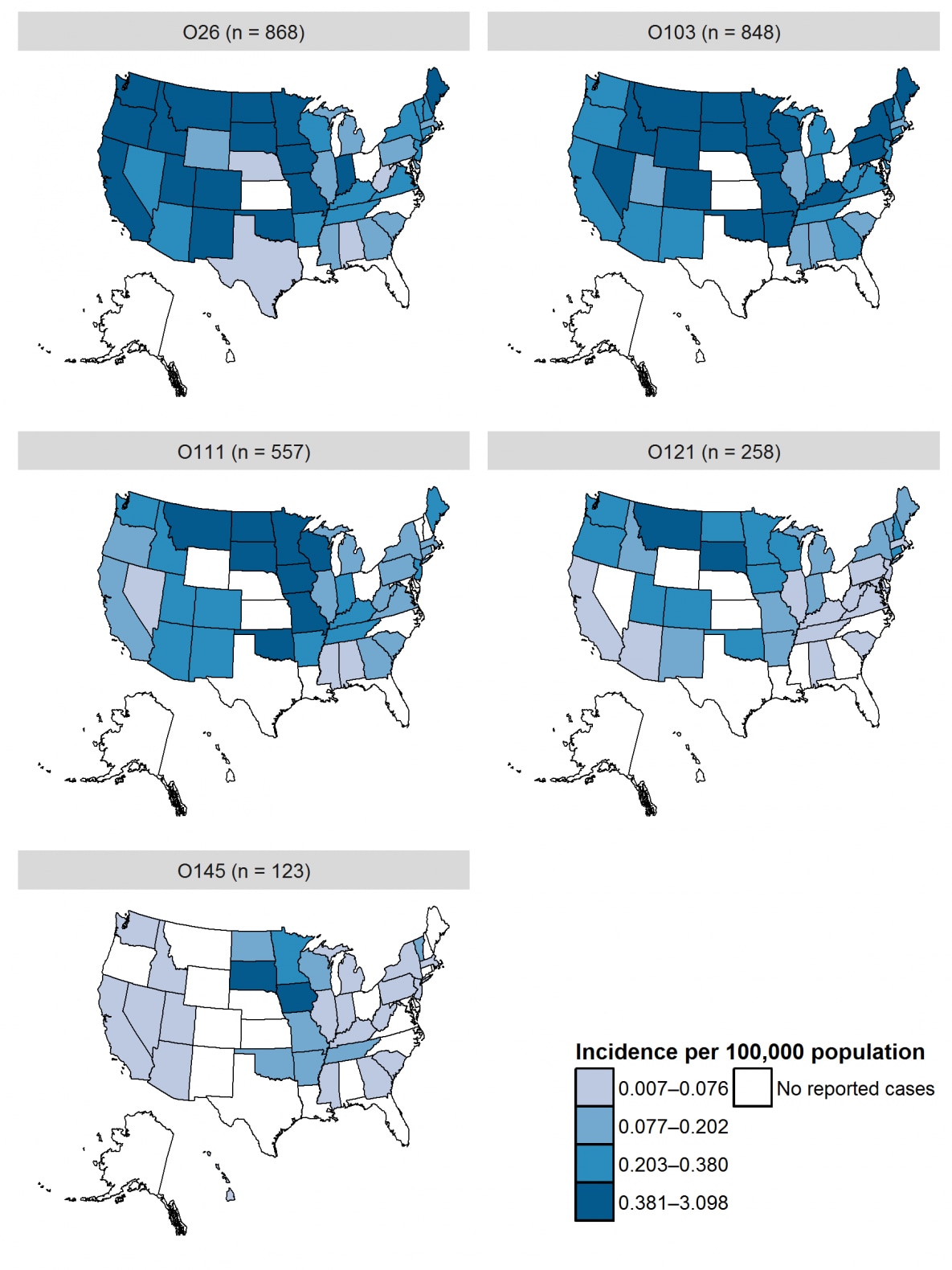
- At least half of states in the Midwest and West regions2 had incidence above the national average for culture-confirmed infection due to O157 and non-O157 serogroups (including Iowa, Minnesota, Missouri, North Dakota, South Dakota, and Wisconsin in the Midwest and Colorado, Montana, Oregon, Utah, and Washington in the West).
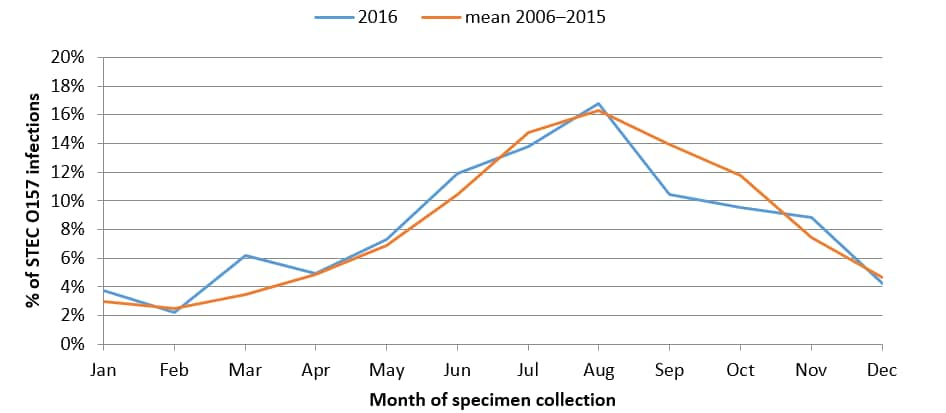
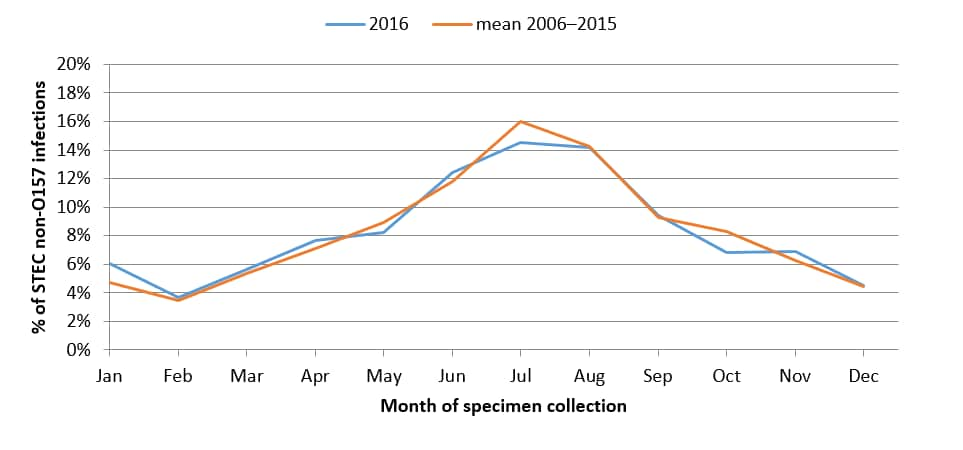
- The largest percentages of O157 and non-O157 isolates were reported in the summer months.
1 The practice of testing stool for Shiga toxin by culture-independent diagnostic tests (CIDT) without concomitant bacterial culture and serotyping has increased in recent years (2) (3). LEDS does not currently collect information on test type and cannot differentiate between STEC infection detected exclusively by CIDT and those that were culture-confirmed but reported without serogroup information.
2 Geographic regions in this report are consistent with those defined by the United States Census Bureau [PDF – 2 pages].
Figure 1. Incidence of human STEC infection reported to LEDS, by serogroup and year, United States, 1996–2016 1
Data table for Figure 1 [XLS – 1 page]
1 The “Total” category includes culture-confirmed infections of serogroup O157, non-O157 serogroups, and rough isolates.
2 The “Rough” category includes isolates with an O antigen that could not be determined because the strain autoagglutinated (agglutinated in all antisera and diluent). Strains behaving in this manner are often blocked in one or more steps of O antigen synthesis and typically appear flat with irregular edges when grown on solid media. These isolates could be O157 or non-O157 STEC.
3 The “Unknown” category includes STEC infections detected exclusively by CIDT and culture-confirmed STEC infections reported to LEDS without serogroup information. LEDS does not currently collect information on test type and cannot differentiate between these two types of reports.
| Rank | Serogroup | Number reported | Percent | Incidence |
|---|---|---|---|---|
| 1 | O157 | 2,323 | 42.7 | 0.72 |
| 2 | O26 | 868 | 16.0 | 0.27 |
| 3 | O103 | 848 | 15.6 | 0.26 |
| 4 | O111 | 557 | 10.2 | 0.17 |
| 5 | O121 | 258 | 4.7 | 0.08 |
| 6 | O145 | 123 | 2.3 | 0.04 |
| 7 | O45 | 106 | 1.9 | 0.03 |
| 8 | O118 | 57 | 1.0 | <0.01 |
| 9 | O186 | 38 | 0.7 | <0.01 |
| 10 | O71 | 29 | 0.5 | <0.01 |
| Non-ranked | Non-O157, other serogroups | 196 | 3.6 | 0.06 |
| Non-ranked | Non-O157, undetermined serogroup* | 24 | 0.4 | <0.01 |
| Subtotal, all non-O157 | 3,104 | 57.0 | 0.97 | |
| Rough** | 14 | 0.3 | <0.01 | |
| Total, All serogroups | 5,441 | 100.0 | 1.69 |
* The “Non-O157, undetermined serogroup” category includes isolates with an O antigen that could not be determined because the strain failed to agglutinate or agglutinated non-definitively in antisera against the currently recognized E. coli O antigens. We are unable at present to determine if this is due to inadequate expression of a known O antigen or expression of a new O antigen.
** The “Rough” category includes isolates with an O antigen that could not be determined because the strain autoagglutinated (agglutinated in all antisera and diluent). Strains behaving in this manner are often blocked in one or more steps of O antigen synthesis and typically appear flat with irregular edges when grown on solid media. These isolates could be O157 or non-O157 STEC.
| Age group, years | O157 (n = 2,237) | Non-O157 (n = 2,990) | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| <1 | 1.34 | 1.33 | 3.19 | 3.44 |
| 1-4 | 3.47 | 3.43 | 3.7 | 4.65 |
| 5-9 | 1.45 | 1.5 | 1.04 | 1.2 |
| 10-19 | 0.95 | .94 | 1.29 | 1.44 |
| 20-29 | 0.75 | 0.52 | 1.46 | 0.87 |
| 30-39 | 0.46 | 0.37 | 0.85 | 0.36 |
| 40-49 | 0.34 | 0.18 | 0.41 | 0.24 |
| 50-59 | 0.27 | 0.16 | 0.49 | 0.23 |
| 60-69 | 0.52 | 0.24 | 0.58 | 0.29 |
| 70-79 | 0.5 | 0.27 | 0.55 | 0.52 |
| ≥80 | 0.63 | 0.35 | 0.41 | 0.61 |
| Overall | 0.75 | 0.64 | 0.99 | 0.87 |
4 Non-O157 category includes isolates with undetermined serogroup.
Percentage of culture-confirmed human STEC O157 infections reported to LEDS, by month of specimen collection, United States, 2016 and mean annual percentage during 2006–2016
Percentage of culture-confirmed human non-O157 STEC infections reported to LEDS, by month of specimen collection, United States, 2016 and mean annual percentage during 2006–20165
Data table for Figure 4 [XLS – 14 KB]
5 Non-O157 category Includes isolates with undetermined serogroup.
Additional data from this report are available in the report appendices.
- Centers for Disease Control and Prevention (CDC). National STEC Surveillance Overview. Atlanta, Georgia: US Department of Health and Human Services, CDC, 2012.
- Iwamoto, Martha, et al. Bacterial enteric infections detected by culture-independent diagnostic tests—FoodNet, United States, 2012-2014. MMWR Morb Mortal Wkly Rep. 2015 Mar 13;64(9):252–7. PubMed
- Marder EP, Cieslak PR, Cronquist AB, et al. Incidence and trends of infections with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013–2016. MMWR Morb Mortal Wkly Rep. 2017 Apr 21;66(15):397-403. DOI PubMed
Centers for Disease Control and Prevention (CDC). National Shiga toxin-producing Escherichia coli (STEC) Surveillance Annual Report, 2016. Atlanta, Georgia: US Department of Health and Human Services, CDC, 2018.