Methods
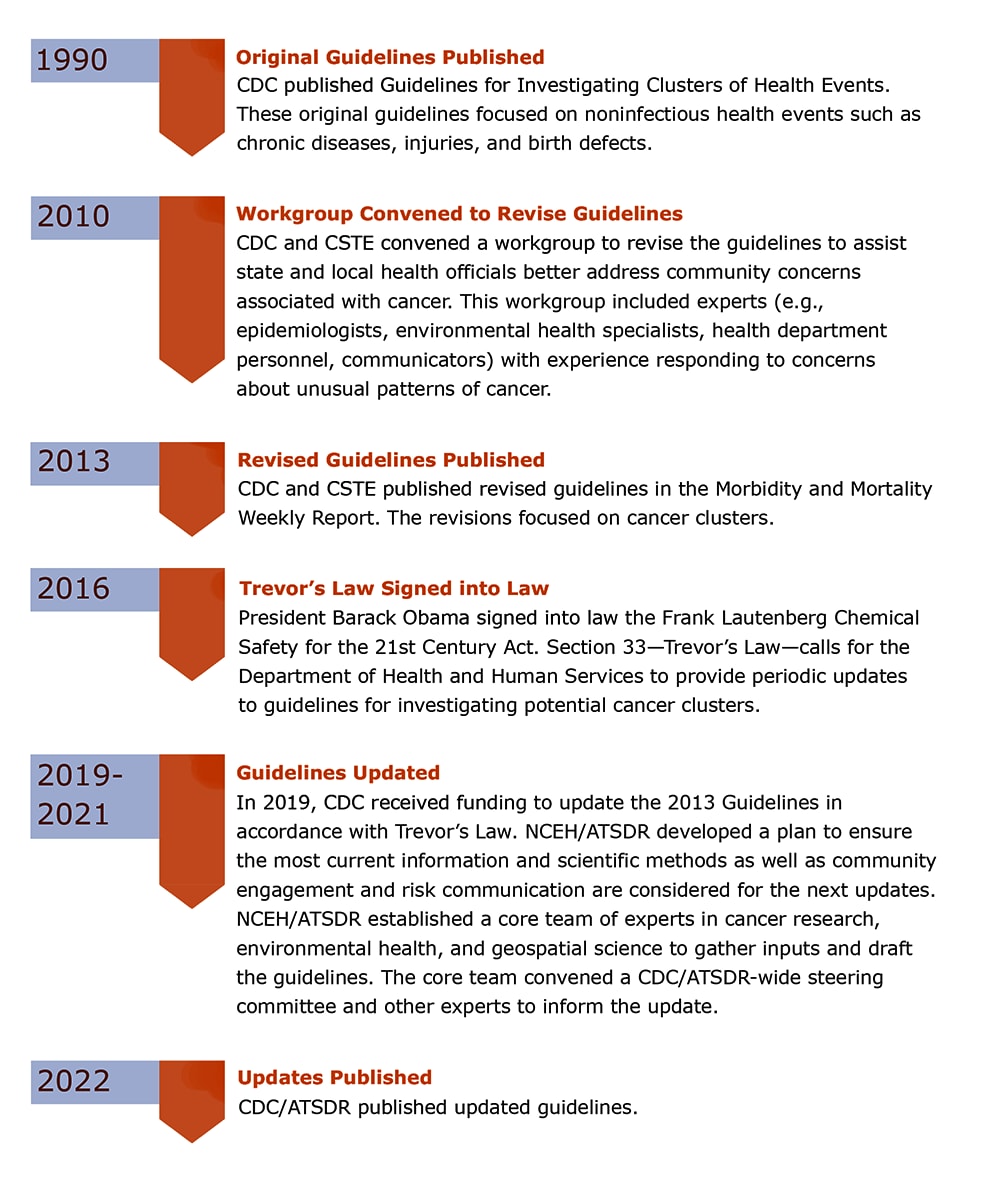
CDC Guidelines for investigating clusters have been transformed over the past few decades. The timeline in Figure 1 summarizes the history and highlights events that helped prompt revisions.
A variety of inputs informed the update:
- Literature reviews
- Media scan
- Subject matter expert input from academic partners, non-governmental organizations, the steering committee, and other federal partners
- Input from state, tribal, local, and territorial (STLT) partners
- Input from members of the public and advocacy groups that have been involved with cancer concerns in their communities
CDC/ATSDR also evaluated advances in the fields of environmental epidemiology, geospatial methods, cancer genomics, and community engagement strategies to determine the feasibility of incorporating these elements to enhance the guidelines.
Literature Review
The literature review and search criteria were designed in collaboration with CDC’s Stephen B. Thacker library. CDC/ATSDR reviewed peer-reviewed articles published since the literature review for the 2013 Guidelines, January 2010–April 2021. Because not all publications of cancer investigations conducted by STLT departments appear in peer-reviewed literature, reports published on STLT health websites but not in peer-reviewed journals (gray literature) were also reviewed.
The literature review focused on the following focus areas:
- Epidemiologic investigations of cancer clusters in community and residential settings
- Geospatial and temporal methods to evaluate clusters
- Rare-event and small-area-estimation statistical methods
- Novel approaches for grouping cancers by molecular characteristics
- Approaches for engaging, educating, and communicating with affected communities
Spatial statisticians and geospatial epidemiologists from ATSDR’s Geospatial Research Analysis and Service Program (GRASP) and the NCEH National Environmental Public Health Tracking Program (Tracking Program) established a geographic information systems (GIS) workgroup to focus on literature specific to spatial cluster methods and GIS cluster resources.
Media Scan
A media scan assessed the communication landscape related to environmental hazards and excess cancer. The media scan employed a two-phase approach to comprehensively review media around specific cancer clusters.
Subject Matter Expert Input
CDC/ATSDR convened a panel with external scientific experts to provide subject matter expertise on how to update the 2013 Guidelines, address any gaps in the guidelines, and incorporate new approaches, particularly regarding methods of statistical analysis and community engagement. Experts included representatives from STLT public health agencies, public health partner organizations, and academia. Subject matter expertise included the following: assessing unusual patterns of cancer, environmental epidemiology, environmental risk assessment, geospatial methods and statistics, cancer registry data, pediatric oncology, community outreach, and risk communication.
Experts in cancer genomics from academia and the National Cancer Institute provided input on the current state of the science in cancer genomics and applicability to evaluations of unusual patterns of cancer. Results from subject matter expert discussions and the literature review indicate the need for ongoing research in cancer genomics before these advances may be feasibly integrated into the guidelines. CDC/ATSDR will continue to evaluate advancements in cancer genomics and provide updates to the guidelines when appropriate.
STLT Survey
CDC/ATSDR developed a survey instrument to request information on STLT public health agency’s approach, best practices, and capacity for addressing local cancer inquiries (OMB Control No. 0920-1879, Expiration Date: 01/31/2021). The goal of the survey was to understand current and best practices associated with the existing guidelines and to identify strengths, weaknesses, suggested revisions, and resource needs for STLT public health officials to conduct investigations associated with local cancer concerns. Members of the expert panel who currently or formerly served as state health officials pilot-tested the instrument. The survey was sent to members of the Council of State and Territorial Epidemiologists (CSTE), state Environmental Health Directors (SEHD) of the Association of State and Territorial Health Officials (ASTHO), and Tribal Epidemiology Centers. The survey also asked STLT partners to submit cancer cluster protocols and reports if available to include in the gray literature review. The response rate from state health department partners was one hundred percent. Responses were also submitted by two U.S. territories and the District of Columbia.
STLT Focus Groups
A series of focus groups with STLT public health agency professionals who respond to unusual patterns of cancer in their communities (OMB Control No. 0920-1879, Expiration Date: 01/31/2021) provided additional feedback for improving the guidelines and allowed discussion amongst STLT officials regarding approaches to cancer cluster inquiries. Focus group questions primarily focused on best practices, limitations, and common needs when addressing cancer cluster inquiries.
CSTE and ASTHO Workgroups
CSTE and ASTHO convened workgroups to review the 2013 Guidelines and provide feedback and recommendations on facilitators and barriers to implementing the guidelines and specific tools, trainings, and non-financial resources to enable STLT agencies to better implement the guidelines. Workgroups were composed of individuals who were either previous or current state public health officials routinely involved in addressing community concerns about unusual patterns of cancer. CDC and ATSDR worked with CSTE and ASTHO to gain broad geographic representation among members.
Federal Register Notice Commentary
A request for public comment was released as a Federal Register Notice to solicit input from the public, including individuals, community groups, and scientific and medical professionals (2). The notice was open from May 15, 2019, through July 15, 2019, for public comments. Public comments are available in the federal docket.
A second FRN was published May 25, 2022. This solicitation was open for public comment for 60 days. CDC/ATSDR received 46 sets of comments from state health departments, community members, academicians, clinicians, cancer registries, non-governmental organizations, and private consultants on behalf of trade associations. Modifications were made to the draft guidelines after careful consideration and are summarized in the FRN announcement (https://www.regulations.gov; Docket No. CDC-2022-0070) of the final publication of the guidelines.
Stakeholder Meeting
NCEH/ATSDR conducted a stakeholder meeting in April 2021 to obtain input from non-governmental organizations, academicians, clinicians, and community members regarding the role their organizations, connections, and similar organizations might play in responding to concerns about unusual patterns of cancer. Stakeholder meeting participants were also asked to suggest the names of individuals who might be willing to participate in community focus groups held in the summer and fall of 2021.
Community Focus Groups and Interviews
Several methods helped to identify participants for community focus groups and interviews:
- Results of the media scan
- Input from the stakeholder-meeting attendees
- Suggestions from the STLT survey respondents
- Community members that have been involved in or are aware of excess cancer concerns or investigations in their communities
- Community members representing environmental justice communities and tribes
- Academicians involved with cancer research in community settings
The goal of the focus groups and interviews was to gather feedback for ways to improve public health and/or environmental officials’ communication and engagement with individuals and communities concerned about environmental hazards and unusual patterns of cancer. Seven virtual community focus groups and three interviews were convened throughout the summer and fall of 2021 (OMB Control No. 0923-0047, Expiration Date: 02/28/2022).
Internal and External Reviews
The draft guidelines underwent a series of internal and external federal agency reviews as well as an external peer review. The guidelines were resubmitted for a final, rigorous internal CDC review and approval process following revisions to address comments received from the 2022 FRN publication.