Chapter 10: Pertussis
Manual for the Surveillance of Vaccine-Preventable Diseases
Authors: Amy Blain, MPH; Tami Skoff, MS; Pam Cassiday, MS; Maria Lucia Tondella, PhD; Anna Acosta, MD
Disease Description
Pertussis, more commonly known as whooping cough, is a contagious, respiratory disease caused by the bacterium Bordetella pertussis. The illness is typically characterized by a prolonged paroxysmal cough that is often accompanied by an inspiratory whoop. Disease presentation can vary with age and history of previous exposure or vaccination. Young infants may present to a clinic or hospital with apnea and no other disease symptoms. Adults and adolescents with some immunity may exhibit only mild symptoms or have the typical prolonged paroxysmal cough. In all persons, cough can continue for months.
Pertussis rarely causes severe complications among healthy, vaccinated persons. Infants, however, are at greatest risk for pertussis-related complications and mortality. Pneumonia is the most common complication in all age groups; seizures and encephalopathy are rare and generally occur only among very young infants. Death is infrequent and most likely to occur in unvaccinated infants, although fatalities are occasionally reported among older children and adults with serious underlying health conditions.[1]
In addition to B. pertussis, 3 other Bordetella species can cause disease in humans: B. parapertussis, B. holmesii, and B. bronchiseptica. B. parapertussis causes a pertussis-like illness that is generally milder than pertussis, likely because the bacteria do not produce pertussis toxin. Co-infection of B. pertussis and B. parapertussis can occur but is uncommon. Disease attributable to Bordetella species other than B. pertussis is not reportable to the Centers for Disease Control and Prevention (CDC).
Background
In the pre-vaccine era, pertussis was a common childhood disease and a major cause of child and infant mortality in the United States. Routine childhood vaccination led to a reduction in disease incidence from an average of 150 reported cases per 100,000 persons between 1922 and 1940, to 0.5 cases per 100,000 persons in 1976.[2] The incidence of reported pertussis began increasing in the 1980s, however, and significant peaks in disease have been observed in recent years. In 2012, 48,277 cases were reported nationwide, exceeding levels observed since 1955. Reported pertussis cases have decreased since 2012, with 18,975 cases reported during 2017; however, levels remain significantly increased compared to those observed during the 1990s and early 2000s. Multiple factors have likely contributed to the increase, including waning immunity from acellular pertussis vaccines, heightened provider and public awareness, improved diagnostic testing, and possibly molecular changes within the pertussis bacterium. The incidence of pertussis remains highest among young infants. From 2012 through 2017, 66.7%, of all pertussis-related deaths (n = 72) reported to CDC were among infants less than two months of age, who were too young to have received DTaP vaccine. As of 2017, the second highest incidence of pertussis continues to occur among school-aged children and adolescents.[3–5]
Importance of Rapid Case Identification
Early diagnosis and treatment of pertussis might limit its spread to other susceptible people. When pertussis is strongly suspected, attempts to identify and provide chemoprophylaxis to household and other close contacts at high risk should proceed without waiting for laboratory confirmation. When suspicion of pertussis is low, the investigation can be delayed until there is laboratory confirmation of the diagnosis. However, chemoprophylaxis of pregnant women and infants, as well as their household contacts, should not be delayed.
Importance of Surveillance
Surveillance data collected through case investigations are used to assess the impact of disease and monitor changes in epidemiology over time. Surveillance data are also used to guide public health policy and development of prevention and control strategies. CDC uses surveillance data to monitor national trends in pertussis and identify populations at risk. Local and state health departments use surveillance data to identify clusters of related cases that might indicate an outbreak.
Laboratory surveillance to monitor changes in the B. pertussis organism is also important. Isolates of B. pertussis collected through routine surveillance have provided researchers with the resources necessary to identify such changes, including recent changes in the organism at the molecular level.[6–9] This information is vital to understanding the evolution of B. pertussis and how those changes may impact the current pertussis vaccination program and other prevention strategies. See Section VII, “Laboratory Testing” for more details.
Disease Reduction Goals
As part of the Healthy People 2020 project, disease reduction goals of 2,500 indigenous pertussis cases per year in children <1 year of age and 2,000 cases per year among adolescents 11 through 18 years of age were proposed.[10] In 2017, 2,276 cases were reported among infants less than 1 year of age, while 6,171 cases were observed among adolescents 11 through 18 years of age.[5]
Case Definitions
The following case definition for pertussis was approved by the Council of State and Territorial Epidemiologists (CSTE) in June 2019 and went into effect January 1, 2020.[11]
Clinical case definition
In the absence of a more likely diagnosis, a cough illness lasting ≥2 weeks with at least one of the following signs or symptoms:
- Paroxysms of coughing, OR
- Inspiratory “whoop,” OR
- Posttussive vomiting, OR
- Apnea (with or without cyanosis)
Laboratory criteria for diagnosis
-
- Isolation of B. pertussis from a clinical specimen
- Positive polymerase chain reaction (PCR) for B. pertussis
Epidemiologic linkage
- Contact with a laboratory-confirmed case of pertussis.
Case classification
Probable:
- In the absence of a more likely diagnosis, illness meeting the clinical criteria,OR
- Illness with cough of any duration, with
- At least one of the following signs or symptoms:
- Paroxysms of coughing, or
- Inspiratory “whoop”, or
- Posttussive vomiting, or
- Apnea (with or without cyanosis)
AND
- Contact with a laboratory confirmed case (epidemiologic linkage)
- At least one of the following signs or symptoms:
Confirmed:
- Acute cough illness of any duration with
- Isolation of B. pertussis from a clinical specimen, OR
- PCR positive for B. pertussis
Collection of epidemiologic and clinical data is essential for reporting cases that meet the clinical case definition. Investigators should make every attempt to collect information on paroxysms of cough, whoop, posttussive vomiting, apnea, and duration of cough, as these variables are required to determine whether an individual meets the pertussis clinical case definition. When feasible, case investigations initiated shortly after cough onset should include follow-up calls to collect information on cough duration of at least 14 days. Follow-up is essential regardless of confirmatory test results so that cases meeting the clinical case definition can be reported. Both probable and confirmed pertussis cases should be reported to the National Notifiable Diseases Surveillance System (NNDSS) by the state health department via the National Electronic Telecommunications System for Surveillance (NETSS) or National Electronic Disease Surveillance System (NEDSS).
Laboratory confirmation of pertussis is important because other pathogens can cause symptoms similar to pertussis. All patients with cough and a positive B. pertussis culture or PCR should be reported as confirmed, even those with cough lasting less than 14 days.
Laboratory Testing
Refer to Chapter 22, “Laboratory Support for Surveillance of Vaccine-Preventable Diseases” for detailed information on laboratory testing for pertussis and for specific information on specimen collection and shipment.
Specimen collection
Specimen collection and shipping are important steps in obtaining laboratory diagnosis or disease confirmation. Guidelines have been published for specimen collection and handling of microbiologic agents. Information is also available on using CDC laboratories as support for reference and disease surveillance; this includes
- a central website for requesting lab testing,
- the form [2 pages, 2.80 MB] required for submitting specimens to CDC (See Appendix 23, Form # CDC 50.34),
- information on general requirements for shipment of etiologic agents (Appendix 24 [4 pages]) — although written to guide specimen submission to CDC, this information may be applicable to submission of specimens to other laboratories; and
- the CDC Infectious Diseases Laboratories Test Directory, which not only contains a list of orderable tests for that institution, but also detailed information on appropriate specimen types, collection methods, specimen volume, and points of contact.
Determining who has pertussis and who does not can be difficult. Whenever possible, a nasopharyngeal swab or aspirate should be obtained from all persons with suspected pertussis. A properly obtained nasopharyngeal swab or aspirate is essential for optimal laboratory diagnosis. Health department personnel and other healthcare practitioners who are asked to obtain these specimens should receive training and supervision from persons experienced in collection of nasopharyngeal specimens. CDC has developed two short training videos for collection of nasopharyngeal aspirate and swab specimens, which can be accessed on the CDC pertussis website.
Reporting and Case Notification
Case reporting within a jurisdiction
Each state and territory (jurisdiction) has regulations or laws governing the reporting of diseases and conditions of public health importance. [12] These regulations and laws list the diseases to be reported and describe those persons or institutions responsible for reporting, including healthcare providers, hospitals, laboratories, schools, daycare and childcare facilities, and other institutions. Persons reporting should contact the jurisdiction/state health department for jurisdiction-specific reporting requirements.
Case notification to CDC
Notifications by jurisdiction/state health departments of all probable and confirmed pertussis cases should be sent to CDC using event code 10190 through NNDSS via NETSS or NEDSS. When provisional information is reported to NNDSS, NETSS and NEDSS reports can be updated as additional information is collected. NETSS and NEDSS accept information about clinical symptoms, laboratory confirmation, and vaccination history; this information is included in the Pertussis Surveillance Worksheet (Appendix 11 [2 pages]) available for reference and use in case investigation. Case notifications should not be delayed because of incomplete information or lack of confirmation. Data can be updated electronically as more information becomes available.
Information to collect
Case investigation should include collection of the epidemiologic information listed on the CDC pertussis surveillance worksheet (see Appendix 11). State health departments often supplement the suggested CDC investigation questions with additional information relevant to cases in their communities. The jurisdiction in which the patient resides at the time of diagnosis should submit the case notification to CDC.
Comments on reporting
When laboratory testing is not completed or is negative, but the individual has symptoms consistent with pertussis, it is important to determine duration of cough—specifically, whether it lasts 14 days or longer—in order to determine if a person’s illness meets the definition of a clinical case. If the first interview is conducted within 14 days of cough onset and cough is still present at the time of interview, it is important to follow up at 14 days or later after cough onset.
Pertussis case investigation methods vary across state and local health jurisdictions; CDC is committed to helping improve standardization of surveillance practices for pertussis. Please refer to Appendix 11 for a detailed instruction sheet describing each data element outlined in the pertussis surveillance worksheet.
Vaccination
For specific information about the use of pertussis vaccines, refer to The Pink Book, which provides general recommendations, including vaccine use and scheduling, immunization strategies for providers, vaccine content, adverse events and reactions, vaccine storage and handling, and contraindications and precautions.
Enhancing Surveillance
A number of surveillance activities can improve detection and reporting of cases as well as the completeness and accuracy of the case report form information reported. In addition to those outlined below, Chapter 19, “Enhancing Surveillance,” lists activities that might be applicable to pertussis surveillance.
Assuring regular and appropriate diagnostic testing
It is important that available diagnostic tests are used appropriately. To ensure pertussis diagnosis and reporting is optimized, reporting jurisdictions should assess the timing of diagnostic testing relative to cough onset for cases identified through routine surveillance. Bacterial culture for pertussis is most useful during the first 2 weeks of cough and prior to antibiotic use. PCR may effectively diagnose pertussis from 2–4 weeks of cough onset, although sensitivity declines after 3 weeks. Appropriate serologic assays are most useful in the 2–8 weeks following cough onset; however, this testing is not confirmatory for the purpose of case reporting. Diagnostic testing at inappropriate times may result in both false-positive and false-negative results, reducing the overall quality of pertussis diagnosis, and ultimately, pertussis surveillance.
Optimal Timing in Weeks for Diagnostic Testing
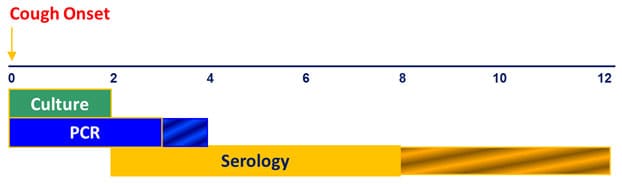
Unlike many other vaccine-preventable diseases of childhood, pertussis remains endemic in the United States. Cases are expected to occur in all communities; a period of several years in which no cases are reported from a jurisdiction likely reflects failures to diagnose and/or report disease rather than an absence of disease. The level of diagnostic testing being undertaken can be evaluated by reviewing the number of pertussis diagnostic tests (e.g., cultures or PCR results) ordered by physicians within a jurisdiction.
Monitoring surveillance indicators
Regular monitoring of surveillance indicators might identify specific areas of the surveillance and reporting system that need improvement. Some suggested surveillance indicators to monitor include:
- Completeness of key data elements collected during pertussis case investigations. Information on clinical presentation, antibiotic treatment, vaccination history, and epidemiologic data are of particular importance and should be collected to the fullest extent possible.
- The proportion of cases reported among infants, children, adolescents, and adults. Jurisdictions with reported pertussis cases heavily weighted toward infants are likely missing a significant proportion of pertussis disease in their community.
- The proportion of cases diagnosed solely with DFA or serologic assays. A high proportion of non-confirmatory laboratory testing for pertussis may be an indication of the need for increased education and promotion of proper pertussis diagnostic testing practices.
- The median interval between onset of cough and notification of state or local public health authorities in probable and confirmed cases.
Expanding pertussis data collection
CDC has partnered with 7 states (CO, CT, GA, MN, NM, NY, and OR) participating in the Emerging Infections Program (EIP) Network to conduct enhanced surveillance of pertussis (EPS) and other Bordetella species. EPS is characterized by enhanced case ascertainment and augmented data collection that goes beyond what is requested nationally through NNDSS. Participating sites collect isolates and specimens, when available, for further characterization at the CDC Pertussis and Diphtheria Laboratory. EPS sites also provide the infrastructure for conducting pertussis special studies including those aimed at evaluating pertussis prevention and control strategies.
Other states interested in collecting additional pertussis surveillance information may consider adding the following data elements that are not currently included on the national pertussis surveillance worksheet:
- Cyanosis (Did the patient experience cyanosis during his/her pertussis infection?)
- Healthcare personnel status (Was the patient employed as healthcare personnel during his/her pertussis infection?)
- Pregnancy status of female patient at cough onset (Was the patient pregnant or post-partum at time of cough onset?)
- For patients <1 year of age:
- Mother’s Tdap vaccination history (Did the mother receive Tdap prior to, during, or after her pregnancy with the infant case-patient?)
- Gestational age (in weeks) at time of birth
Completing the pertussis death worksheet
- At present, there are no standard reporting guidelines for pertussis-related deaths. To improve understanding of the characteristics associated with fatal pertussis infections, CDC developed a Pertussis Death Worksheet during 2018. The worksheet is intended to capture clinical, laboratory, and epidemiologic information for all laboratory diagnosed or epidemiologically linked pertussis cases that resulted in death, whether or not the decedent meets the CSTE pertussis case definition requirements for reporting. Reporting jurisdictions with cases of fatal pertussis should ensure that the CDC Pertussis Death Worksheet is completed and returned via fax to Amy Blain at 404-235-1822. Detailed instructions are provided in the Manual for Surveillance of VPDs Appendices.
Streamlining reporting using electronic methods
Although many surveillance systems still rely on paper and pencil for data collection, use of data from sources such as electronic medical records, electronic case reporting [13–19], and clinical laboratory information systems (LIMS) can significantly improve reporting speed, enhance data quality, and reduce workload.
Case Investigation
Case investigations generally include reviews of laboratory, hospital, and clinic records, as well as immunization registries, which are the best sources for information about diagnoses and immunization histories. Investigations also include interviews of patients, which are necessary to identify sources of infections and contacts at risk. Investigations can include treatment of patients and chemoprophylaxis and or vaccination of contacts.
Treatment and chemoprophylaxis
Antimicrobial treatment does not generally lessen the severity of disease unless it is begun early in the course of illness, prior to paroxysmal coughing. [20] Early treatment reduces transmission and is essential for disease control. The spread of pertussis can be limited by decreasing the infectivity of the patient and by protecting close contacts.[21] Persons with pertussis are infectious from the onset of symptoms through the third week after the onset of paroxysms or until 5 days after the start of effective antimicrobial treatment. The recommended antimicrobial agents and doses are the same for treatment and chemoprophylaxis.[20]
Three macrolides (azithromycin, erythromycin, clarithromycin) are recommended for treatment of pertussis. Azithromycin is most popular because it is given in a short, simple regimen of 1 dose each day for 5 days. It is the preferred antimicrobial for use in infants younger than 1 month of age. For infants younger than 1 month of age, macrolides should be used with caution: some studies have demonstrated an association between erythromycin and azithromycin with infantile hypertrophic pyloric stenosis (IHPS). However, infants younger than 1 month of age are at increased risk of developing severe pertussis and life-threatening complications. These risks outweigh the potential risk of IHPS that has been associated with macrolide use. Resistance of B. pertussis to macrolides is rare, and antimicrobial susceptibility testing is not routinely recommended. Testing is appropriate in some circumstances and is recommended when treatment failure is suspected. Refer to Section VII, “Laboratory Testing” for information on how to contact the CDC Pertussis and Diphtheria Laboratory to discuss susceptibility testing. If resistance to macrolides is suspected or if their use is contraindicated, it is recommended to treat with trimethoprim–sulfamethoxazole (TMP-SMZ). TMP-SMZ should not be used to treat infants younger than 2 months of age.[20]
CDC recommends administration of chemoprophylaxis to contacts at high risk and household members of a pertussis patient. For more specific information on chemoprophylaxis, please see the Outbreak Control section below.
Limited available data suggest B. parapertussis is less susceptible to antibiotics than pertussis, although some studies indicate that erythromycin, azithromycin, clarithromycin, TMP-SMZ, and ciprofloxacin have activity against B. parapertussis.[20-29] Because data on the clinical effectiveness of antibiotic treatment are limited, treatment decisions should be based on clinical judgment with particular attention towards special populations, including infants, elderly, and immunocompromised persons; treatment may be warranted to prevent severe outcomes and decrease duration of illness.
Vaccination
During the course of a pertussis investigation, under-vaccinated contacts of pertussis cases may be identified. Contacts who have not received the recommended number of pertussis-containing vaccinations (i.e., DTaP, Tdap) should follow the age appropriate catch-up immunization schedule.[30,31] Vaccination is not a substitute for chemoprophylaxis and is unlikely to prevent illness in a person who has already been infected with B. pertussis.[30-33]
Outbreak Control
Pertussis outbreaks can be difficult to identify and manage. Other respiratory pathogens often cause clinical symptoms similar to pertussis, and co-circulation with other pathogens does occur. To respond appropriately (e.g., provide appropriate chemoprophylaxis), it is important to confirm that B. pertussis is circulating in the outbreak setting and to determine whether other pathogens are contributing to the outbreak. Because culture is the most specific test for pertussis, confirmation by culture for at least one suspected pertussis case is recommended any time there is suspicion of a pertussis outbreak.
To reduce the risk of pertussis in new mothers and their infants, especially those too young to be vaccinated, ACIP recommends that pregnant women receive a dose of Tdap vaccine during each pregnancy. During outbreaks, prevention measures should focus on efforts to improve Tdap coverage during pregnancy to reduce severe illness and possible deaths in vulnerable infants.
Pertussis incidence remains elevated in the United States, and community transmission is widespread. Thus, extensive contact tracing and broad-scale use of chemoprophylaxis among contacts may not be an effective use of limited public health resources. While antibiotics may prevent pertussis disease if given prior to symptom onset, there are no data to indicate that widespread use of chemoprophylaxis among contacts effectively controls or limits the scope of pertussis outbreaks. Another important consideration is the overuse of antibiotics; CDC promotes the judicious use of antibiotics among healthcare providers and patients.[34] Given these considerations, CDC supports targeting chemoprophylaxis through the following measures to persons at high risk of developing severe pertussis and to persons who will have close contact with those at high risk of developing severe pertussis:
- Providing chemoprophylaxis to all household contacts of a pertussis case. Within families, secondary attack rates have been demonstrated to be high, even when household contacts are up-to-date with immunizations.[35] Administration of antimicrobial prophylaxis to asymptomatic household contacts within 21 days of cough onset in the index patient can prevent symptomatic infection.
- Providing chemoprophylaxis within 21 days of exposure to contacts of a pertussis case who are at high risk of severe illness. These include:
- Infants and women in their third trimester of pregnancy—severe and sometimes fatal pertussis-related complications occur in infants <12 months of age, especially among infants <4 months of age. Women in their third trimester of pregnancy may be a source for transmission of pertussis to their newborn infant.
- All persons with pre-existing health conditions that may be exacerbated by a pertussis infection (e.g., immunocompromised persons and patients with moderate to severe medically treated asthma).
- Contacts who themselves have close contact with either infants <12 months of age, pregnant women, or individuals with pre-existing health conditions at risk for severe illness or complications.
- All contacts in high-risk settings that include infants <12 months of age or women in the third trimester of pregnancy, which include but are not limited to, neonatal intensive care units, childcare settings, and maternity wards.
A broader use of chemoprophylaxis may be appropriate in limited closed settings, when the number of identified cases is small, and when a community-wide outbreak is not ongoing. However, when continued transmission of pertussis is evident, multiple rounds of antibiotics would not be recommended. Rather than repeating a course of antibiotics, contacts should be monitored for onset of signs and symptoms of pertussis for 21 days.
Active screening for symptomatic patients with suspected pertussis can be considered during outbreaks in settings such as schools, daycare centers, and hospitals. Active screening for suspected cases potentially reduces exposure to persons with pertussis, encourages timely medical evaluation and treatment of cases, and promotes prompt administration of antibiotics to close contacts at high risk for infection or complications due to infection.[35-42]
References
- Vitek CR, Pascual FB, Baughman AL, Murphy TV. Increase in deaths from pertussis among young infants in the United States in the 1990s. Pediatr Infect Dis J 2003;22(7):628–34.
- Davis SF, Strebel PM, Cochi SL, Zell ER, Hadler SC. Pertussis surveillance—United States, 1989–1991. MMWR Surveill Summ 1992;41(SS-8):11–19.
- CDC. Pertussis—United States, 2001–2003. MMWR Morb Mortal Wkly Rep 2005;54(50):1283–6.
- Guris D, Strebel PM, Bardenheier B, , et al. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clin Infect Dis 1999;28(6):1230–7. DOI: 10.1086/514776.
- National Notifiable Diseases Surveillance System, 1990–2015. Division of Health Informatics and Surveillance , Center for Surveillance, Epidemiology, and Laboratory Services, Office of Public Health Scientific Services, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, Atlanta, GA 30329.
- Schmidtke AJ, Boney KO, Martin SW, Skoff TH, Tondella ML, Tatti KM. Population diversity among Bordetella pertussis isolates, United States, 1935–2009. Emerg Infect Dis 2012;18(8):1248–55. DOI: 10.3201/eid1808.120082.
- Pawloski LC, Queenan AM, Cassiday PK, et al. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin Vaccin Immunol 2014;21:119–25. DOI: 10.1128/CVI.00717-13
- Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, et al. Pertactin-negative B. pertussis strains: evidence for a possible selective advantage. Clin Infect Dis 2015;60(2):223–7. DOI: 10.1093/cid/ciu788.
- Cassiday PK, Skoff TH, Jawahir S, Tondella ML. Changes in predominance of pulsed-field gel electrophoresis profiles of Bordetella pertussis isolates, United States, 2000–2012. Emerg Infect Dis 2016;22(3):442–8. DOI: 10.3201/eid2203.151136
- U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington, DC.
- CSTE. Revision to the case definition for national pertussis surveillance. CSTE position statement 19-ID-08: Atlanta, GA: CSTE; 2019.
- Adams DA, Thomas KR, Jajosky R, et al. Summary of notifiable infectious diseases and conditions—United States, 2014. MMWR Morb Mortal Wkly Rep 2016;63(54):1–152 DOI: 10.15585/mmwr.mm6354a1
- CDC. Progress in improving state and local disease surveillance—United States, 2000–2005. MMWR Morb Mortal Wkly Rep 2005;54(33):822–5.
- CSTE. Improving public health practice by enhancing the public health community’s capability for electronic information exchange using HL7 CDA [5 pages]. CSTE position statement 13-SI-03; Atlanta, GA: CSTE; 2013.
- CSTE. Common data structure for national notifiable diseases. CSTE position statement 15-EB-01 [6 pages]. Atlanta, GA: CSTE; 2015.
- Smith PF, Hadler JL, Stanbury M, Rolfs RT, Hopkins RS; CSTE Surveillance Strategy Group. “Blueprint version 2.0”: updating public health surveillance for the 21st century. J Public Health Manag Pract 2013 May–Jun;19(3):231–9. doi: 10.1097/PHH.0b013e318262906e
- CSTE. Review of and recommendations for the National Notifiable Disease Surveillance System: a state and local health department perspective [49 pages].
- CSTE. 2004–2010 National assessments of electronic laboratory reporting in health departments: findings and recommendations [4 pages]. [assessment brief]. Atlanta, GA: CSTE; 2012.
- Mac Kenzie WR, Davidson AJ, Wiesenthal A, et al. The promise of electronic case reporting. Public Health Rep 2016;131(6):742–6. https://journals.sagepub.com/doi/10.1177/0033354916670871
- CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Recomm Rep 2005;54(RR-14):1–16.
- American Academy of Pediatrics. Pertussis (Whooping Cough). In: Kimberlin DW, editor. Red Book: 2015 Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics;2015:608–21.
- Mastrantonio P, Stefanelli P, Giuliano M, et al. Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. J Clin Microbiol 1998;36(4):999–1002.
- Hoppe JE, Tschirner T. Comparison of media for agar dilution susceptibility testing of Bordetella pertussis and Bordetella parapertussis. Eur J Clin Microbiol Infect Dis 1995;14(9):775–9.
- Hoppe JE, Bryskier A. In vitro susceptibilities of Bordetella pertussis and Bordetella parapertussis to two ketolides (HMR 3004 and HMR 3647), four macrolides (azithromycin, clarithromycin, erythromycin A, and roxithromycin), and two ansamycins (rifampin and rifapentine). Antimicrob Agents Chemother 1998;42:965–6.
- Hoppe JE, Tschirner T. Comparison of Etest and agar dilution for testing the activity of three macrolides against Bordetella parapertussis. Diagn Microbiol Infect Dis 1997;28(1):49–51. DOI: 10.1016/S0732-8893(97)89160-2
- Hoppe JE, Rahimi-Galougahi E, Seibert G. In vitro susceptibilities of Bordetella pertussis and Bordetella parapertussis to four fluoroquinolones (levofloxacin, d-ofloxacin, ofloxacin, and ciprofloxacin), cefpirome, and meropenem. Antimicrob Agents Chemother 1996;40(3):807–8.
- Hoppe JE, Simon CG. In vitro susceptibilities of Bordetella pertussis and Bordetella parapertussis to seven fluoroquinolones. Antimicrob Agents Chemother 1990;34(11):2287–8. DOI: 10.1128/AAC.34.11.2287
- Hoppe JE, Eichhorn A. Activity of new macrolides against Bordetella pertussis and Bordetella parapertussis. Eur J Clin Microbiol Infect Dis 1989;8(7):653–4. DOI: 10.1007/BF01968151
- Watanabe M, Haraguchi Y. In vitro susceptibility of Bordetella parapertussis to various antimicrobial agents. Antimicrob Agents Chemother 1989;33(6):968–9. DOI: 10.1128/AAC.33.6.968
- CDC. Catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind—United States, 2017 [8 pages]. [updated 2017 Feb 6; cited 2017 Mar 14].
- CDC. Recommended immunization schedule for adults aged 19 years or older, by vaccine and age group—United States, 2017 [6 pages]. [updated 2017 Feb 6; cited 2017 Mar 14].
- Bisgard KM, Christie CD, Reising SF, Sanden GM, Cassiday PK, Gomersall C, et al. Molecular epidemiology of Bordetella pertussis by pulsed-field gel electrophoresis profile: Cincinnati, 1989–1996. J Infect Dis 2001;183:1360–7. DOI: 10.1086/319858
- Kurzynski TA, Boehm DM, Rott-Petri JA, et al. Antimicrobial susceptibilities of Bordetella species isolated in a Multicenter Pertussis Surveillance Project. Antimicrob Agents Chemother 1988;32(1):137–40. DOI: 10.1128/AAC.32.1.137
- CDC. Get smart: know when antibiotics work. Atlanta GA [updated: 2013 Oct 23; cited 2014 Jan 20].
- Sprauer MA, Cochi SL, Zell ER. Prevention of secondary transmission of pertussis in households with early use of erythromycin. Am J Dis Child 1992;146(2):177–81. DOI: 10.1001/archpedi.1992.02160140043018
- Dodhia H, Miller E. Review of the evidence for the use of erythromycin in the management of persons exposed to pertussis. Epidemiol Infect 1998;120(2):143–9.
- Halperin SA, Bortolussi R, Langley JM, et al. A randomized, placebo-controlled trial of erythromycin estolate chemoprophylaxis for household contacts of children with culture-positive Bordetella pertussis infection. Pediatrics 1999;104(4):e42
- von König CH. Use of antibiotics in the prevention and treatment of pertussis. Pediatr Infect Dis J 2005;24(5 Suppl):S66–8.
- Alexander EM, Travis S, Booms C, Kaiser A, Fry NK, Harrison TG, et al. Pertussis outbreak on a neonatal unit: identification of a healthcare worker as the likely source. J Hosp Infect 2008;69(2):131–4. DOI: 10.1016/j.jhin.2008.02.011
- Elumogo TN, Booth D, Enoch DA, Kuppuswamy A, Tremlett C, Williams CJ, et al. Bordetella pertussis in a neonatal intensive care unit: identification of the mother as the likely source. J Hosp Infect 2012;82(2):133–5. DOI: 10.1016/j.jhin.2012.07.012
- CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep 2005;54(RR14):1–16.
- Clark TA. Responding to pertussis. J Pediatr 2012;161(6):980–2.