FIGURE 1. Incidence of Kaposi's Sarcoma (KS), Pneumocystis carinii Pneumonia (PCP), and other opportunistic infections --- United States, 1979--1981
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
AIDS: the Early Years and CDC's Response
Corresponding author: James W. Curran, MD, Rollins School of Public Health, Emory University, 1518 Clifton Road, NE, Room 8011, Atlanta, GA 30322; Telephone: 404-727-8720; Fax 404-712-8879; E-mail: jcurran@emory.edu.
Initial Reports
The MMWR description of five cases of Pneumocystis carinii pneumonia (PCP) among homosexual men in Los Angeles was the first published report about an illness that would become known as acquired immunodeficiency syndrome (AIDS) (1). Appearing 4 months before the first peer-reviewed article (2), the timeliness of the report can be credited to the astute clinical skills and public health sensitivity of Dr. Michael Gottlieb and his colleagues at the University of California, Los Angeles, School of Medicine and Cedars-Sinai Hospital, who worked closely with Dr. Wayne Shandera, the CDC Epidemic Intelligence Service (EIS) officer assigned to the Los Angeles County Department of Health Services.
The Parasitic Diseases Division of CDC's Center for Infectious Diseases already had become concerned about other reports of unusual cases of PCP. The Division housed the Parasitic Disease Drug Service, which administered the distribution of pentamidine isethionate for PCP treatment. Because PCP was rare and pentamidine was not yet licensed in the United States, it was available only from CDC. A review of requests for pentamidine had documented that PCP in the United States was almost exclusively limited to patients with cancer or other conditions or treatments known to be associated with severe immunosuppression (3). Recent requests for this drug from physicians in New York and California to treat PCP in patients with no known cause of immunodeficiency had sparked the attention of Division staff.
Shortly after the first report, additional cases of other life-threatening opportunistic infections (OIs) and a malignancy, Kaposi sarcoma (KS), were reported (4). After learning of these first cases, CDC, under the leadership of its Director, Dr. William Foege, formed a Task Force on Kaposi's Sarcoma and Opportunistic Infections to begin surveillance and conduct epidemiologic investigations. Despite the fiscal constraints at the time, approximately 30 CDC EIS officers and staff participated in the Task Force during the summer of 1981.
The first step for the Task Force was to establish a case definition for surveillance and investigation of the outbreak. The key underlying factor for the disease appeared to be severe suppression of the cellular immune system. The OIs initially reported were life-threatening and often fatal. Although KS was a known but infrequent cancer in the United States, the classical form of the disease was rarely life-threatening and typically occurred among elderly men. Another epidemiologic form of KS was seen among immunosuppressed renal transplant recipients.
To track KS/OI, the surveillance case definition had to emphasize specificity and accuracy of diagnosis. Thus, the original CDC case definition included 1) biopsy-proven KS among persons <60 years of age or biopsy- or culture-proven life-threatening or fatal OIs and 2) no known underlying illness (e.g., cancer or immune deficiency disease) or history of immunosuppressive therapy. This definition was soon adopted both in the United States and worldwide and was used for surveillance in countries where diagnostic capacities were available. The CDC case definition for what came to be called AIDS was modified in 1985 (5), 1987 (6), and 1993 (7). The World Health Organization employed a modified case definition for use in settings with limited diagnostic capacity (usually developing countries).
By the end of 1981, 159 cases of KS and OIs had been reported in the United States, with the earliest cases retrospectively identified in 1978 (8). By month of illness onset, cases demonstrated a clear increase over time (Figure 1). About half of the reports were for KS alone and 40% for PCP alone; 10% of patients were reported with both KS and PCP. Seventy-five percent of cases were reported from New York City or California, and all but one case were in men. Within 6 months, it was clear that a new, highly concentrated epidemic of life threatening illness was occurring in the United States. The co-occurrence of KS and OIs suggested that the epidemic was one of immunosuppression and that KS or OIs were a consequence of the immunosuppression.
Although the case definition's specificity was crucial for identifying the emerging epidemic, it lacked sensitivity. In fact, the reported KS/OI cases were described as "the tip of the iceberg" of a spectrum of illness being seen by physicians in New York City and California. These illnesses included other cancers (particularly non-Hodgkin lymphoma); thrombocytopenic purpura; and notably, persistent, unexplained generalized lymphadenopathy. Dr. Donna Mildvan and her colleagues in New York City, assisted by EIS officer, Dr. Bess Miller, described 57 cases of unexplained lymphadenopathy among gay men (9). At the time, nearly one third of the reported persons with KS had a history of such lymphadenopathy. Since lymphadenopathy and other symptoms often waxed and waned, it was speculated that such findings represented a milder, if much more common, form of the syndrome.
Early in 1982, CDC conducted a national case--control study that included most living patients with KS/OIs reported in the United States. The 50 cases among gay men were compared with control gay men matched by city of residence, race, and age. The studies, led by Drs. Harold Jaffe and Martha Rogers, found that case-patients tended to be much more sexually active than controls and were more likely to have had other sexually transmitted infections (10,11).
In early 1982, Dr. David Auerbach, the EIS officer who had replaced Dr. Shandera in Los Angeles, was approached by a member of the local gay community about a possible sexual link between the still rare cases in southern California. In collaboration with Dr. William Darrow of the Task Force, Dr. Auerbach investigated 13 of the first 19 cases reported from Los Angeles and Orange counties and found that nine had reported sexual contact with another person reported with AIDS within 5 years before their onset of symptoms (12). Auerbach and Darrow then extended the epidemiologic investigation nationwide to 90 patients (approximately three quarters of reported cases among gay men alive at the time). Forty of the 90 patients in 10 cities were linked by sexual contact with another case-patient (13). These findings, along with the results from the case--control study, strongly suggested that the new syndrome was caused by a sexually transmissible infectious agent. Nonetheless, whether because of competing hypotheses or merely denial, many scientists and the public were skeptical of the infectious agent causation theory.
Then, in early summer 1982, an elderly man with severe hemophilia A was reported to have died from PCP. Shortly thereafter, two additional PCP cases were reported among young men with severe hemophilia from separate states. These latter cases were investigated in depth by Dr. Dale Lawrence of the Task Force, who determined that their PCP was accompanied by severe unexplained immunosuppression, and these patients had no history of homosexual contact or needle sharing (14). For more than a decade, persons with hemophilia in the United States had received reconstituted lyophilized clotting factor concentrates, derived from human plasma, to prevent the devastating effects of their disease. However, the concentrates were pooled from the plasma of >1,000 donors per lot and were known to transmit hepatitis viruses.
Within the next several months, AIDS cases also were reported among infants (15--17), female sex partners of men with, or at high risk for, AIDS (18,19), and an infant and adults who had received blood transfusions (20,21). Taken together, these cases provided strong evidence that AIDS was caused by an infectious agent that could be transmitted by blood and from mother to child, as well as through homosexual and heterosexual contact.
In the summer of 1982, life-threatening OIs and KS were also reported among 34 Haitian migrants to the United States (22). Most were reported to be heterosexual men with no known risk factors who had migrated from Haiti within the past 2 years. Cases of disseminated KS had been recently reported from Port-Au-Prince as well (23). These reports indicated an epidemiologically distinct pattern of illness that ultimately would be explained mostly by heterosexual transmission. The reporting of these cases as "Haitian entrants" was accurate and, these authors believe, necessary for public health purposes, but the stigma of "AIDS labeling" added to the already considerable burden for thousands of Haitian migrants fleeing poverty and political persecution (24).
Recommendations for Prevention
During the initial year after the first reports of AIDS, when the term "gay plague" was commonly used, the disease received relatively little attention from the mainstream media, the public, or politicians. By the end of 1982, however, it was clear that others were at risk for the disease, and what had been complacency turned into serious concern, even panic. Many persons caring for AIDS patients were concerned about their own safety and, in some cases, health-care workers refused to provide needed care. To provide guidance for protection of clinicians and laboratory workers managing patients with AIDS and their biologic specimens, CDC issued guidelines in November 1982 that were based on those previously recommended to protect against hepatitis B virus infection (25).
In March 1983, CDC, in conjunction with the Food and Drug Administration and the National Institutes of Health (NIH), issued interagency recommendations for the prevention of AIDS on the basis of the epidemiologic data (Table) (26). These recommendations, which were immediately endorsed by a variety of professional and community organizations, were developed before the cause of the syndrome was discovered and 2 years before antibody testing would be available for diagnostic testing of individuals or screening of blood donations. Yet, even in retrospect, the recommendations appear to have been essentially correct. They illustrate the power of epidemiologic investigation in understanding and preventing new diseases, even in the absence of an identified cause.
The causative retrovirus was described by Drs. Francois Barre-Sinoussi and Luc Montagnier and their colleagues from the French Pasteur Institute in May 1983 (27). Additional proof of causality, as well as the demonstration of sustained viral growth in vitro, was reported by Dr. Robert Gallo and colleagues at the U.S. National Cancer Institute, NIH, in 1984 (28). In 2008, Drs. Barre-Sinoussi and Montagnier were awarded the Nobel Prize in medicine for their discovery of human immunodeficiency virus (HIV).
The availability of laboratory reagents and techniques to identify HIV led to rapid scientific advances in understanding the natural history of the infection and AIDS. CDC's Dr. Paul Feorino and colleagues first demonstrated persistent HIV infection among seropositive blood donors who had transmitted HIV many years earlier, indicating that HIV-infected persons can be asymptomatic and viremic for many years and that seropositivity is equivalent to infection and infectivity (29). Dr. Harold Jaffe and colleagues from the San Francisco Health Department and CDC's Hepatitis Division reported a 6-year follow-up of a cohort of gay men originally recruited in 1978 for studies of hepatitis B virus infection in San Francisco (Figure 2) (30). By analyzing retrospectively obtained specimens, they found that at the time the first few AIDS cases were reported from the cohort in 1981, 30% of the 7,000 men were already infected with HIV. If these data were extrapolated nationally, as many as 200,000--300,000 gay men had already been infected in the United States at the time of the 1981 case reports. Using these natural history data, Dr. Meade Morgan projected that the cumulative incidence of AIDS would reach 270,000 by 1991 (31). These projections provided a wake-up call to those concerned about the future economic and human costs of the epidemic in this country.
By the mid-1980s, substantial concern existed about transmission of HIV through casual contact or by arthropods. Dr. Gerald Friedland and colleagues showed no evidence of transmission among close household contacts of HIV-infected patients in New York City (32). In a cover story for LIFE magazine, physicians from Florida had postulated that the high prevalence of AIDS in Belle Glade, a small town in southern Florida, resulted from insect transmission of HIV. Dr. Kenneth Castro and his colleagues published an extensive epidemiologic investigation in that community that did not support that hypothesis (33).
Drs. Steven McDougal and Linda Martin from CDC demonstrated that heat would inactivate HIV, providing a basis for production of clotting factor concentrate that would no longer transmit HIV infection (34). CDC laboratories also validated HIV antibody tests and provided proficiency testing materials for state public health laboratories and others. In the early HIV era, recommendations for HIV counseling and testing (35) and prevention of perinatal transmission (36) were made, and CDC provided resources and training for alternate testing and counseling sites (sites other than blood collection centers) (37). During the first 8 years of the epidemic, nearly 50 sets of recommendations and guidelines for AIDS were published in MMWR.
The Global Epidemic
By 1984, case reports described AIDS in Zaire (now the Democratic Republic of Congo), and a team of investigators including Dr. Joseph McCormick and Ms. Sheila Mitchell from CDC; Dr. Thomas Quinn from the National Institute of Allergy and Infectious Diseases, NIH; and Dr. Peter Piot from the Institute of Tropical Medicine in Belgium, made a joint visit to Kinshasa to verify the initial reports. Dr. Jonathan Mann was then recruited by CDC to establish a long-term project in Zaire, Project SIDA, in partnership with Dr. Bila Kapita from Mama Yemo Hospital in Kinshasa and colleagues from NIH and the Institute of Tropical Medicine. Projet SIDA would rapidly become the largest HIV/AIDS research project on the continent during the 1980s.
In 1985, CDC inaugurated and hosted in Atlanta the First International Conference on AIDS. The conference, chaired by Dr. Gary Noble of CDC, was attended by >2,000 registrants. At the conference, Dr. Mann delivered the first presentation about AIDS in Africa at a U.S. meeting and reported that the incidence of AIDS in Kinshasa was 15--30 times higher than in the United States (38).
Dr. Mann left Zaire to begin the Global Programme on AIDS at the World Health Organization. He was replaced as Project SIDA Director by Dr. Robert Ryder in 1986 and then by Dr. William Heyward in 1989. Dr. Kevin De Cock was the first Director of Projet Retro CI, CDC's second African research site, in Abidjan, C'ote D'Ivoire. Shortly thereafter, Dr. Bruce Weniger initiated CDC's HIV research site in Bangkok, Thailand.
Since the early days, CDC's response to the global HIV pandemic has been extensive. In the early 1980s, staff were detailed to, or volunteered from, many different parts of the agency. Initial CDC funding supported state and local health departments for surveillance and prevention activities, including HIV counseling and testing. In addition to these traditional CDC partners, hundreds of local and national community-based organizations were enlisted in the prevention efforts, and CDC provided support for school-based HIV education initiatives.
At CDC headquarters, the AIDS epidemic highlighted the need for behavioral and social scientists to participate in public health research.. Before 1981, only a handful of doctoral-trained behavioral and social scientists were on staff in Atlanta, but the numbers quickly grew. CDC's reputation and staff accomplishments also led to formation of the Global AIDS Program. Overall, many thousands of CDC staff have worked---and continue to work---with tens of thousands of colleagues throughout the world in the fight against AIDS. Well over 400 reports on HIV/AIDS have been published in MMWR since that first report in June 1981. The ongoing impact of CDC's scientific and programmatic contributions remains outstanding.
Lessons for the Future
In three decades, AIDS has emerged has a major global health problem. As the world faces the long struggle to combat the epidemic, several lessons from the early days emerge.
First, excellent surveillance of the initial AIDS cases was critical in responding to the epidemic. Surveillance was first needed to track the epidemic and direct etiologic investigations but later became critical in formulating early prevention and safety recommendations before HIV was discovered. Surveillance remains equally important now throughout the world to target resources and evaluate prevention efforts.
Second, the rapid identification of HIV as the causal agent of AIDS led to a much better understanding of transmission, natural history, and spectrum of illness. This understanding allowed for more targeted prevention efforts and paved the way for development of the first effective treatments.
Third, innovative science has in many ways exceeded the expectations of skeptics. These innovations include improvements in HIV diagnostics, such as rapid antibody testing and viral load assays; identification of zidovudine (AZT), the first antiretroviral (ARV) drug; use of ARVs to reduce perinatal transmission; effectiveness of prevention in many communities through counseling and testing, as well as behavior-based methods; and development of effective biomedical interventions, such as male circumcision, preexposure prophylaxis, and vaginal microbicides in addition to condom use and needle and syringe exchange. Finally, development of the three-drug ARV regimen (highly active antiroviral therapy [HAART]) has saved the lives of millions of persons with HIV infection in both the developed and developing worlds.
Fourth, as with most health problems where the etiology is well understood, prevention deserves primacy. Several million persons become newly infected with HIV each year, yet only approximately five to six million persons worldwide have been treated with HAART. The goal of universal HIV treatment cannot be met unless HIV incidence can be reduced. Furthermore, as long as the majority (or a substantial minority) of HIV-infected persons are unaware of their infection status, prevention and treatment efforts will be hampered.
Finally, committed persons have made---and will continue to make---the difference. Persons with HIV infection have played crucial roles in communities throughout the world by giving voice to their concerns and courageously advocating for HIV. Scientists in many disciplines who continue to discover breakthroughs for prevention and care provide hope for the future. Clinicians and caregivers and public health practitioners will pursue their work with an expanded science base.
The future of prevention and care for HIV means standing up to two societal foes, scarcity and discrimination, as much as the biologic challenge of the virus itself. Global resources for prevention and care for HIV remain severely short of the needs. Successful efforts for prevention must also include sustained and visible efforts to combat stigma and prevent discrimination.
This last lesson was emphasized by the late Jonathan Mann, who perished with his wife, HIV vaccine researcher, Mary Lou Clements-Mann, in a 1998 plane crash. Dr. Mann was the person most responsible for first calling world attention to AIDS and linking it to concerns about human rights. In one of his last public addresses, on the occasion of the 50th anniversary of the Universal Declaration of Human Rights, he stated, "Our responsibility is historic. For when the history of AIDS and the global response is written, our most precious contribution may well be that, at a time of plague, we did not flee, we did not hide, we did not separate ourselves" (39). The hope for the tens of millions affected by HIV currently and in the future will depend upon scientists, practitioners, and citizens working together.
References
- CDC. Pneumocystis pneumonia---Los Angeles. MMWR 1981;30:1--3.
- Hymes, KB, Greene JB, Marcus A, et al. Kaposi's sarcoma in homosexual men: a report of eight cases. Lancet 1981;318:598--600.
- Walzer PD, Perl DP, Krogstad DJ, Rawson PG, Schultz MG. Pneumocystis carinii pneumonia in the United States: epidemiologic, diagnostic, and clinical features. Ann Intern Med 1974;80:83--93.
- CDC. Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men---New York City and California. MMWR 1981;30:305--8.
- CDC. Revision of the case definition of acquired immunodeficiency syndrome for national reporting---United States. MMWR 1985;34:373--5.
- CDC. Revision of the CDC case definition for acquired immunodeficiency syndrome. MMWR 1987;36(Suppl):82--94.
- CDC. Impact of the expanded AIDS surveillance case definition on AIDS case reporting---United States, first quarter, 1993. MMWR 1993;42:308--10.
- CDC. Task Force on Kaposi's Sarcoma and Opportunistic Infections. Epidemiologic aspects of the current outbreak of Kaposi's sarcoma and opportunistic infections. N Engl J Med 1982;306:248--52.
- CDC. Persistent generalized lymphadenopathy among homosexual males. MMWR 1982;31:249--51.
- Jaffe HW, Choi K, Thomas PA, et al. National case control study of Kaposi's sarcoma and Pneumocystis carinii pneumonia in homosexual men: epidemiologic results. Ann Intern Med 1983;99:145--51.
- Rogers MF, Morens DM, Stewart JA, et al. National case-control study of Kaposi's sarcoma and Pneumocystis carinii pneumonia in homosexual men: Part 2. Laboratory results. Ann Intern Med 1983;99:151--8.
- CDC. A cluster of Kaposi's sarcoma and Pneumocystis carinii pneumonia among homosexual male residents of Los Angeles and Orange counties, California. MMWR 1982;31:305--7.
- Auerbach DM, Darrow WW, Jaffe HW, Curran JW. A cluster of cases of the acquired immune deficiency syndrome: patients linked by sexual contact. Am J Med 1984;76:487--92.
- CDC. Pnemocystis carini pneumonia among persons with hemophilia A. MMWR 1982;31:365--7.
- CDC. Unexplained immunodeficiency and opportunistic infections in infants---New York, New Jersey, California. MMWR 1982;31:665--7.
- Oleske J, Minnefor A, Cooper R, et al. Immune deficiency syndrome in children. JAMA 1983;249:2345--9.
- Rubenstein A, Sicklick M, Gupta A, et al. Acquired immunodeficiency with reversed T4/T8 ratios in infants born to promiscuous and drug-addicted mothers. JAMA 1983;249:2350--6.
- CDC. Immunodeficiency among female sexual partners of males with acquired immune deficiency syndrome (AIDS). MMWR 1983;31:697--8.
- Harris C, Small CB, Klein RS, et al, Immunodeficiency in female sexual partners of men with the acquired immunodeficiency syndrome. N Engl J Med 1983;308:1181--4.
- CDC. Possible transfusion-associated acquired immune deficiency syndrome (AIDS)---California. MMWR 1982;31:652--4.
- Curran JW, Lawrence DL, Jaffe HW, et al. Acquired immunodeficiency syndrome (AIDS) associated with transfusions. N Engl J Med 1984;310:69--75.
- CDC. Opportunistic infections and Kaposi's sarcoma among Haitians in the United States. MMWR 1982;31:353--61.
- Liautaud B, Laroche C, Duvivier J, et al. Le sarcoma de Kapsoi (maladie de Kaposi) est-il-frequent en Haiti? Presented at the 18th Congres des Medecins francophones de l'hemisphere Americain: Port-Au-Prince, Haita, April 1982.
- Farmer P. AIDS and accusation: Haiti and the geography of blame, Berkeley, CA: University of California Press; 1992.
- CDC. Acquired immune deficiency syndrome (AIDS): precautions for clinical and laboratory staffs. MMWR 1982;31:577--80.
- CDC. Prevention of acquired immune deficiency syndrome (AIDS): report of inter-agency recommendations. MMWR 1983;32:101--3.
- Barre-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983;220:868--71.
- Gallo RC, Salahuddin SZ, Popovic M, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 1984;224:500--3.
- Feorino PM, Jaffe HW, Palmer E, et al. Transfusion-associated acquired immunodeficiency syndrome (AIDS): evidence for persistent infection in blood donors. N Engl J Med 1985;312:1293--6.
- Jaffe HW, Darrow WW, Echenberg DF, et al. AIDS, AIDS-related conditions, and exposure to HTLV- III/LAV in a cohort of homosexual men: a 6-year follow-up study. Ann Intern Med 1985;103:210--4.
- Morgan WM, Curran JW. Acquired immunodeficiency syndrome (AIDS): current and future trends. Public Health Rep 1986;101:459--65.
- Friedland GH, Saltzman BR, Rogers MF, et al. Lack of transmission of HTLV-III/LAV infection to household contacts of patients with AIDS or AIDS-related complex with oral candidiasis. N Engl J Med 1986;314:344--50.
- Castro KG, Lieb S, Jaffe HW, et al. Transmission of HIV in Belle Glade, Florida: lessons for other communities in the United States. Science 1988;239:193--8.
- McDougal JS, Martin LS, Cort SP, Mozen CM, Heidebrant CM, Evatt BL. Thermal inactivation of the acquired immunodeficiency syndrome virus, human T-lymphotropic virus--III/lymphaderopathy-associated virus, with special reference to antihemophilic factor. J Clin Invest 1985;76:875--7.
- CDC. Public Health Service guidelines for counseling and antibody testing to prevent HIV infection and AIDS. MMWR 1987;36:509--15.
- CDC. Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR 1985;34:721--6.
- CDC. Human T-lymphotropic virus type III/lymphadenopathy associated virus antibody testing at alternate sites. MMWR 1986;35:284--7.
- Mann JM, Francis H, Asila PK, et al. Surveillance for acquired immunodeficiency syndrome in a central African city: Kinshasa, Zaire. JAMA 1986;255:3255--9.
- Mann JM. Presentation at XII International Conference on AIDS, June 28--July 3, 1998, Geneva, Switzerland.
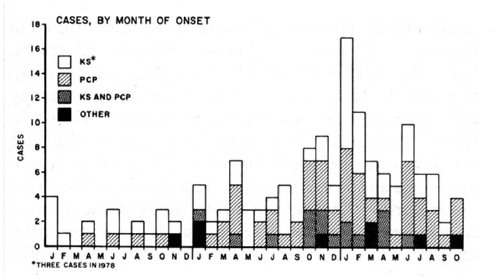
Source: Epidemiologic aspects of the current outbreak of Kaposi's sarcoma and opportunistic infections. N Engl J Med 1982;306:248--52. Reprinted with permission.
Alternate Text: The figure is a bar graph that presents the incidence of Kaposi's Sarcoma, Pneuocystis carinii pneumonia, and other opportunistic infections in the United States during 1979-1981.
FIGURE 2. Percent of men with human T-lymphotropic virus, type III/lymphadenopathy-associated virus antibody (seropositive) and number with acquired immunodeficiency syndrome (AIDS), by year of diagnosis, San Francisco City Clinic Cohort, 1978--1984

Source: Jaffe HW, Darrow WW, Echenberg DF, et al. The acquired immune deficiency syndrome in a context of homosexual men. A six-year follow-up study. Ann Intern Med 1985;103:210--4. Reprinted with permission.
Alternate Text: The figure is a line graph that presents the number of men with human T-lymphotropic virus, type III/lymphadenopathy-associated virus antibody and the number with AIDS, by year and diagnosis, in a San Francisco city clinic during 1978-1984.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


