Prevalence of Amyotrophic Lateral Sclerosis — United States, 2010–2011
Corresponding author: Paul Mehta, MD, Division of Toxicology and Human Health Sciences, Agency for Toxic Substances and Disease Registry. Telephone: 770-488-0556; E-mail: pum4@cdc.gov.
Abstract
Problem/Condition: Amyotrophic lateral sclerosis (ALS), commonly known as Lou Gehrig's disease, is a progressive and fatal neuromuscular disease for which no cure has been identified. Although ALS has no known definitive cause, familial ALS (a hereditary form) occurs in 5%–10% of cases. Many hypotheses have been formulated about what causes ALS, including chemical exposures, occupational exposure, military service, infectious agents, nutritional intake, physical activity, and trauma. Worldwide, ALS affects white males aged >60 years more often than any other group. In the United States, ALS surveillance is necessary to estimate the incidence and prevalence of ALS and collect data on risk factors. ALS is not a nationally notifiable condition in the United States (i.e., it is not a reportable condition in all jurisdictions), and individual state reporting requirements differ, with Massachusetts being the only state that mandates reporting.
Period Covered: October 19, 2010–December 31, 2011.
Description of System: In 2009, the federal Agency for Toxic Substances and Disease Registry (ATSDR) implemented the National ALS Registry to collect and analyze data regarding persons with ALS in the United States. The main goals of the Registry, as defined by the 2008 ALS Registry Act, are to describe the incidence and prevalence of ALS better, examine risk factors such as environmental and occupational exposures, and characterize the demographics of those living with ALS. The Registry uses a two-pronged approach to identify all cases of ALS. The first approach uses four existing national administrative databases (maintained by Medicare, Medicaid, the Veterans Health Administration, and the Veterans Benefits Administration) to identify prevalence of ALS. The second approach uses a secure web portal (http://www.cdc.gov/als) that was launched to the public on October 19, 2010, to identify cases not included in the four national administrative databases and to collect risk-factor data on known ALS cases. ALS patients who have registered via the web portal can complete brief risk-factor surveys online that are intended to attain a better understanding of ALS (e.g., genetics and environmental and occupational exposures) and help determine disease progression.
Results: During October 19, 2010–December 31, 2011, a total of 12,187 persons meeting the surveillance case definition of definite ALS were identified by the Registry, for a prevalence of 3.9 cases of ALS per 100,000 persons in the U.S. general population. Incidence cannot be measured because the date of diagnosis was not noted in all patient records. Overall, ALS was more common among white males, non-Hispanics, and persons aged 60–69 years. The age groups with the lowest number of persons with ALS were age 18–39 years and age >80 years. Males had a higher prevalence rate of ALS than females overall and across all data sources.
Interpretation: This is the first (and to date the only) effort to estimate the national prevalence of ALS in the United States. Using the combined approach of the national databases and the web-based portal enables researchers to estimate ALS prevalence more accurately. Registry findings for the prevalence of ALS are consistent with findings from long-established ALS registries in Europe and from smaller-scale epidemiologic studies conducted previously in the United States. Although incidence cannot be measured with Registry data at this time, incidence is being measured in smaller geographic areas that have participated in ATSDR's State and Metropolitan Area ALS surveillance projects.
Public Health Actions: Data collected by the National ALS Registry are being used to better describe the prevalence of ALS in the United States and to help facilitate research. The combined approach of using national administrative databases and a self-enrollment web portal to collect data is novel and potentially could be used for other non-notifiable diseases such as Parkinson's disease or multiple sclerosis. ATSDR is working closely with ALS advocacy and support groups, researchers, health-care professionals, and others to promote the National ALS Registry in order to capture all cases of ALS. To further enhance and strengthen the Registry, ATSDR is 1) adding new modules to the portal to examine other potential risk factors, 2) launching a feasibility study for a novel ALS biorepository (available at http://wwwn.cdc.gov/als/ALSBioRegistry.aspx) linked to the Registry that would potentially provide biologic specimens from patient enrollees to help researchers learn more about disease etiology, 3) engaging in surveillance activities in selected states and large metropolitan areas to help test the completeness of the Registry as well as calculating incidence in these areas, and 4) using the Registry to recruit patient enrollees for new clinical trials and epidemiologic studies. Additional information about the National ALS Registry is available at http://www.cdc.gov/als or by calling toll-free at 1-877-442-9719.
Introduction
Amyotrophic lateral sclerosis (ALS), commonly known as Lou Gehrig's disease, is a progressive and fatal neuromuscular disease. Most persons die within 2–5 years of receiving a diagnosis of ALS (1). There is no known definitive cause of ALS, but a hereditary form of the disease, familial ALS, occurs in 5%–10% of cases (1). No cure has been identified. Riluzole (brand name Rilutek) is the only drug that has been approved by the U.S. Food and Drug Administration (FDA) to treat ALS. Riluzole has been demonstrated to slow ALS progression; however, it does not demonstrate marked improvement in ALS symptoms and increases survival time only minimally (2). Although there is no blood test for ALS, the diagnosis of the disease has evolved. Diagnosis is based on signs and symptoms as well as on neurophysiologic tests, primarily electromyograms. The El Escorial Criteria, a set of clinical and diagnostic features that aim to rule out nonmotor neuron diseases and other motor neuron diseases with restricted presentations (i.e., those presenting as either upper or lower motor neuron diseases but not both) (1,3) are used to classify ALS patients for research studies. Most persons who receive an initial diagnosis of these other motor neuron diseases ultimately will progress to include both upper and lower motor neurons and thus will receive an ALS diagnosis (1,4).
ALS affects persons of all races and ethnicities. Several potential risk factors for ALS have been identified. Whites, males, those aged >60 years, and those with a family history of the disease are more likely to develop ALS (1). Previous exposure to heavy metals (e.g., lead) also has been associated with an increased risk for ALS (5–7). Certain occupations (e.g., military service) have been identified as possible risk factors (8–10). Nutritional intake, exposure to infectious agents, physical activity, and trauma also have been identified as possible risk factors (11–17). However, most risk-factor studies have had small sample sizes or have been conducted in limited geographic areas in populations that might not be representative of the U.S. population.
Background
ALS is not a nationally notifiable condition, and national disease surveillance systems collect data related primarily to infectious diseases (18). On the basis of studies mostly from Europe (19–27), the incidence rate of ALS across all ages is estimated to be 1.6 persons per 100,000 population, and the rate increases to five persons per 100,000 population in the seventh decade of life (28). An estimated 5,000 persons receive a diagnosis of ALS each year in the United States, with an estimated prevalence of 12,000 cases (28). ALS is more prevalent in men than in women, with a typical age of diagnosis at age 55–75 years (1). Further analysis is necessary to determine if the incidence or prevalence of ALS in the United States is rising or declining for all age groups and to assess the role of environmental or occupational risk factors.
During 2006–2009, four ATSDR-funded pilot projects were conducted to evaluate the feasibility of identifying cases of ALS by matching data from national administrative databases to site-specific administrative and clinical databases (e.g., ALS clinic records) for January 1, 2001–December 31, 2005 (29). The national administrative databases included those maintained by Medicare, Medicaid, the Veterans Heath Administration, and the Veterans Benefits Administration; these systems incorporate data on approximately 90 million persons. Participating sites included the Mayo Clinic (Rochester and Olmsted County, Minnesota), Emory University (Atlanta, Georgia), the South Carolina Budget and Control Board, and nine members of the Health Management Organization Research Network in seven states (California, Massachusetts, Michigan, New Mexico, Oregon, Pennsylvania, and Wisconsin) (29).
In 2008, the U.S. Congress passed the ALS Registry Act (30). ATSDR was designated to create and maintain the National ALS Registry because of its previous pilot work on ALS, because ALS has a potential environmental risk factor link, and because of ATSDR's experience with designing other health registries (e.g., the World Trade Center Health Registry). The main goals of the Registry, as defined by the 2008 ALS Registry Act, are to describe the incidence and prevalence of ALS better, examine risk factors such as environmental and occupational factors, and characterize the demographics of persons living with ALS. ATSDR's National ALS Registry will contribute critical data for further analysis of incidence, prevalence, and causal risk factors. The Registry is also important as an innovative use of administrative and self-reported data to identify cases; traditionally, noncommunicable disease registries rely on data from health-care providers to identify cases.
The purpose of this report is to describe the first (and to date the only) effort to estimate the national prevalence of ALS in the United States and selected descriptive risk factors. This report is intended for public health officials, clinicians, and researchers working to better understand and address the needs of persons with ALS and their families.
Methods
The National ALS Registry is the first national registry to use existing administrative data (from Medicare, Medicaid, the Veterans Heath Administration, and the Veterans Benefits Administration) as a major source of case ascertainment. An algorithm was developed and used to identify persons with ALS on the basis of encounter* codes such as having ALS listed as a code in the visit record or having such a code and having seen a neurologist, a death certificate listing ALS as a cause or contributing cause of death, and prescription for Riluzole (Box). Data from October 19, 2010–December 31, 2011 are presented. All activities involving human subjects were reviewed and approved by CDC's Institutional Review Board (IRB).
Description of Registry
A health registry is a system containing uniform information about persons that is collected in a systematic and comprehensive way to serve a predetermined purpose (31). These data typically are acquired, maintained, and updated over a prolonged period of time, usually measured in years. Registry activities might range from only listing exposed persons with associated contact information to serving as a research repository of information (e.g., demographics, exposure, and health information). A public health registry is a registry that is set up to accomplish a public health goal or activity, including obtaining information on persons who have either a particular disease, a condition (e.g., a risk factor) that predisposes them to the occurrence of a health-related event, or prior exposure to substances or circumstances known or suspected to cause adverse health effects.
The National ALS Registry uses a two-pronged approach to identify cases of ALS in the United States. These cases are identified for surveillance purposes and not for diagnosis or treatment. The first approach uses records from four existing national administrative databases (Medicare, Medicaid, the Veterans Heath Administration, and the Veterans Benefits Administration) to identify ALS cases on the basis of an algorithm developed through pilot projects (29). The second approach, which was launched to the public on October 19, 2010, uses a secure web portal (http://www.cdc.gov/als) to capture cases that are not included in the national administrative databases. This approach allows patients to self-identify, enroll in the National ALS Registry if screening criteria are met, and take brief surveys regarding risk factors (32). The unit of analysis for the Registry is a person with a single (i.e., unique) record. Merging records for persons identified as having ALS from the administrative databases with those persons who enrolled in the National ALS Registry web portal creates a unique record after data are de-duplicated by using a combination of the last five digits of the person's Social Security number, sex, month and year of birth, and first and last name. This ensures that persons who are identified in both the administrative databases and the web portal are not counted twice.
Misclassification of age at diagnosis is likely in the administrative data because the date of diagnosis is not included and must be estimated on the basis of the first date of a medical service encounter. Because of the individual variation in the time lag between diagnosis and benefit approval, using narrow age categories increases the likelihood of miscategorization. For this reason, standard 10-year age categories were used to reduce the potential for misclassification of age at diagnosis.
National Administrative Databases
Medicare is a U.S. government-provided health-care insurance program for persons aged ≥65 years, some disabled persons aged <65 years, and persons of all ages with end-stage renal disease. Persons approved for the Social Security Administration Disability Insurance Benefit or Supplemental Security Income because of ALS can begin receiving Medicare without a 24-month waiting period. Medicaid is the U.S. health program for persons and families with low incomes and limited resources; Medicaid data include inpatient, outpatient, and pharmacy records for persons receiving this benefit. Veterans Health Administration data include inpatient, outpatient, and pharmacy records for veterans receiving health-care benefits. Approximately 20% of veterans qualify for this benefit. Veterans Benefits Administration data include records for veterans receiving pensions or compensation for service-related disabilities. In 2008, the Veterans Administration determined that ALS is to be considered a service-connected condition regardless of when it occurred in relationship to military service and anyone who served at least 90 days of continuous active duty in the U.S. military may qualify for this benefit (33).
Algorithm Used to Identify Cases
The National ALS Registry used an existing algorithm to identify persons with ALS (Figure 1 and Box). On the basis of knowledge of ALS and findings from other studies (34–36), individual and combined variables were entered into the algorithm. The variables included International Classification of Diseases Ninth Revision (ICD-9) 335.20 and VBA 8017 codes, a prescription for Riluzole, and the frequency of visits to a neurologist. Only data for persons categorized as "definite ALS" are included in this report. Those determined to be "possible ALS" will be reevaluated as additional years of data become available.
The algorithm was developed initially during the pilot projects and categorized persons as either "definite ALS," "possible ALS," or "not ALS," with a sensitivity of 87% and specificity of 85% (29). During the pilot projects this algorithm identified persons with ALS using data from the national databases by identifying individual patient encounters (records of health-care services) with an ICD-9 code for any motor neuron disease (335.2–335.29) and Veterans Benefits Administration–specific codes for any motor neuron disease for the specific project catchment area. ATSDR then provided individual encounter data from the national databases, including full name and social security number, to the pilot project sites (29). In addition, pilot sites examined site specific administrative and clinical databases to identify any cases that might have been missed during review of the national databases' encounter data. Pilot sites completed a standardized spreadsheet for each person found in either a national or pilot site database and combined all encounter records into one record per person by database (29). This step was not repeated during the current study period (i.e., case classification relied only on the use of the algorithm) because the coding scheme most likely to identify those persons who had definite ALS had been determined during the pilot study. At that time, researchers had abstracted accessible medical records by using a standardized medical records abstraction form designed by the project neurologists for signs and symptoms of ALS and electromyography results, when available. Using these data, a neurologist had assigned diagnoses by using the El Escorial Criteria (3), a tool to characterize the certainty of an ALS diagnosis in clinical practice and research, which was considered the "gold standard." Therefore, the sensitivity and specificity of both individual and combined variables were examined by comparing what was documented in the administrative databases against the El Escorial Criteria classification assigned by the neurologist. ATSDR then combined the data from the four pilot projects (29).
Self-Identification Through a Secure Web Portal
Not all persons with ALS can be identified through the existing national databases because of eligibility requirements for each of the sources as well as potential obstacles to applying for benefits (e.g., economic or educational disadvantage). Therefore, a secure web portal was created to help identify ALS cases not found in the administrative databases. To enter the National ALS Registry through its web portal, patients must answer a series of validation (screening) questions. These validation questions were obtained from the Veterans Administration's ALS Registry (which is no longer enrolling persons with ALS) and were found to be very accurate; 93.4% of those who passed the screening questions were determined by a neurologist to have ALS/motor neuron disease (37). Those persons who do not "pass" the ATSDR screening questions cannot register and are instructed to call Registry staff for more information.
Prevalence Calculation
The prevalence of ALS was calculated from the Registry by using the de-duplicated total number of persons with ALS identified through administrative data and those who self-identified for the numerator. The 2011 Census was used for the denominator (38).
Survey Modules and Risk-Factor Definitions
During October 19, 2010–December 31, 2011, the Registry web portal had seven brief risk-factor survey modules available that persons with ALS could use to help researchers learn more about the disease. These modules captured the following current and historic information about enrollees: sociodemographics, occupational history, military history, physical activity, alcohol consumption and cigarette smoking, and family history of neurodegenerative diseases. These topics are mentioned in the literature as potentially being associated with the disease. The seventh module collected information on a person's disease progression (i.e., ALS Functional Rating Scale). Patients answer all modules only once except for the module pertaining to disease progression, which is offered for completion multiple times per year. More risk-factor modules (e.g., detailed residential history and residential pesticide use) have come online since January 1, 2012, and results will be described in subsequent reports.
Current smokers were defined as persons reporting having smoked one or more cigarettes per day for ≥6 months and currently smoking. Former smokers were defined as persons reporting having smoked one or more cigarettes per day for ≥6 months but not currently smoking. Nonsmokers were defined as persons reporting never having smoked one or more cigarettes per day for ≥6 months. Current alcohol drinkers were defined as persons reporting having consumed alcoholic beverages at least once a month for ≥6 months and currently drinking alcohol. Former drinkers were defined as persons reporting having consumed alcoholic beverages at least once a month for ≥6 months and not currently drinking alcohol. Nondrinkers were defined as persons reporting not having consumed any alcoholic beverages in a month for ≥6 months. Military-service history was defined as ever having been a member of the armed forces. Titles of longest-held jobs and corresponding industries were selected by respondents from predetermined drop-down menus.
Results
A total of 12,187 persons were identified as "definite ALS" across the four national databases and through web portal registration during October 19, 2010–December 31, 2011 (Table 1). The overall prevalence rate of ALS was 3.9 per 100,000 persons and increased as age increased. The age group 18–39 years had the lowest prevalence rate (0.5 per 100,000 persons), and the age group 70–79 years had the highest prevalence rate (17.0 per 100,000 persons). When the self-enrollment portal data were analyzed alone, the age group 50–59 years had the highest prevalence rate of ALS. ALS patients identified in the national databases tended to be older than those identified through the web portal. Males had a higher overall prevalence rate (4.8 per 100,000 persons) than females (3.0 per 100,000 persons); across each data source individually and overall. The ratio of males to females was 1.56. The prevalence rate for whites was twice that of blacks; whites had a prevalence rate of 4.2 per 100,000 persons compared with 2.0 per 100,000 persons for blacks (Table 2). Prevalence rates by age group, sex, and race are provided (Figures 2–4).
For the 10,971 patients for whom race was known, whites accounted for 79.1% of all cases identified when all data sources were combined, whereas blacks accounted for 6.5%. The proportion of whites who registered was higher in the web portal when compared with the national databases (Table 2) and therefore might not be representative of all persons with ALS.
Of the 1,647 respondents who provided information on smoking history, approximately half of the respondents were either former or current smokers while half were nonsmokers. Of the 1,640 respondents who provided information on alcohol use, fewer respondents identified themselves as current drinkers, more identified themselves as former drinkers, and a similar percentage identified themselves as nondrinkers when compared with national estimates (39). Of the 1,828 respondents who provided information on educational attainment, a total of 71% of respondents reported some college or higher education. Of the 1,651 respondents who provided information on military service, nearly one quarter (23%) of respondents reported a history of military service. A summary of descriptive risk-factor data is provided (Table 3).
The current employment status of the majority of the 1,711 respondents who provided information on employment status was either disabled (45%) or retired (31%). Only 15% of respondents were currently employed full-time. A diverse range of job titles and industries in which persons with ALS ever worked were reported. Educators and health-care professionals represented the job titles held the longest by respondents. The three industries in which persons with ALS worked the longest were professional, scientific, and technical services, followed by health care and social assistance, and then education services. These groups represented 30% of industries in which persons with ALS worked the longest. The longest-held occupation by years of employment ranged from 15% for <10 years to 29% for those who worked 20–30 years. A summary of occupational history risk-factor data is provided (Table 4).
Discussion
The National ALS Registry is collecting national data on disease prevalence, assessing risk factors for the development of ALS, and exploring ways of facilitating research on ALS. The collection of data from national administrative databases and the web portal will be a continual process, and future annual reports will examine data collected in 2012 and beyond. During October 19, 2010–December 31, 2011, a total of 12,187 persons were identified with ALS across the four national databases and through web portal registration for a prevalence rate of 3.9 cases of ALS per 100,000 persons in the United States. This is consistent with the prevalence rates for ALS reported in the United States and other countries, including previously reported prevalence rates from ALS registries in Europe (19–27). Identifying the cases of ALS using a two-pronged methodology is a feasible approach. The proportion of cases identified via the national databases is larger than that of patients identified by the web portal (in which participation is voluntary). The two age groups with the highest prevalence rates were 60–69 years and 70–79 years. ALS is more common above age 50 years (1). Men have a higher prevalence than females across all age groups and data sources. The ratio of males to females is consistent with other published data on ALS (28). Whites have a higher prevalence of ALS than blacks. The reason for this difference in prevalence by race is unknown and needs to be investigated further. Nevertheless, these differences in prevalence by race and by sex are consistent with other studies (19–28).
Calculating the incidence of ALS from the National ALS Registry data is difficult. One hindrance is the determination of the actual date of diagnosis for ALS. The national databases do not report an actual diagnosis date. In the portal, persons with ALS do indicate a date of diagnosis, but this information is self-reported and has the potential for errors. However, the state- and metropolitan-level surveillance initiatives that are a part of the National ALS Registry will allow for population-based estimates of ALS incidence in smaller defined geographic areas of the United States (see Registry Enhancements).
Why ALS affects whites and non-Hispanics at a higher rate than it does members of any other groups is unknown. White males receive a diagnosis at a higher rate than white females. Factors such as occupational history and environmental exposures might be associated with this finding. The age groups 60–69 years and 70–79 years are the most common ages of onset, which is consistent with the reported literature (1,40,41). Patients who receive a diagnosis at an earlier age have a slightly better prognosis although the average survival time after onset of symptoms is approximately 3 years; however, only a small proportion of patients survive beyond 5 years (1). To identify possible risk factors for the disease above and beyond what already is being captured and analyzed by the Registry, ATSDR is funding institutions to collect and analyze data from ALS patients. More information is available at http://wwwn.cdc.gov/ALS/ALSExternalResearchfundedbyRegistry.aspx.
The reporting of risk factors in this report is for descriptive purposes only, and inferences should be made with caution. As the Registry matures, it might be possible to draw more conclusions from the data collected through the risk-factor modules. Insufficient information is available from the portal data to determine if either smoking or alcohol use is a risk factor in ALS susceptibility. However, compared with national estimates (39), more respondents identified themselves as nonsmokers than as current or former smokers. The portal data indicate that persons with a higher level of educational attainment tend to have a higher rate of ALS. This finding is based on self-reporting by registrants and does not indicate that having a college degree increases ALS susceptibility. Persons with a higher level of educational attainment tend to use the Internet more often than those who do not have a higher education (42) and might be more proactive about searching for information on their diagnosis than persons who have high school only or less education. In addition, persons participating in the portal tend to be younger than persons in the national databases. In general, persons in this younger demographic group also report a higher Internet usage than older persons (42). The percentage of patients who reported having served in the military is 23.5% compared with the national average of 9.1% in 2011 (43). Military service has been associated as a potential risk factor for ALS (44). However, no definitive etiologies related to military service have been identified, and further analysis is required. Finally, self-selection bias might have played a role. Members of the military might have been more likely to volunteer to enroll in the Registry or might have had a higher awareness of the Registry through specific channels such as the Veterans Administration compared with those registrants who have not served in the military.
Portal respondents self-reported their employment status, job title held longest, industry in which they worked, and years of employment. More than three quarters (76%) of ALS patients reported that they were either retired or disabled. Because of the disease's debilitating characteristics and mobility issues caused by the disease, this figure is not surprising. Portal data also indicated that persons who held their occupations for 20–30 years were professionals in the health-care and education fields. This does not necessarily indicate a correlation between specific occupations or industries and ALS but might rather reflect a bias toward more educated persons self-registering. Additional research is necessary in this area.
ATSDR has begun a further analysis of the risk-factor surveys currently available to web portal registrants. During the study period for this report, risk-factor surveys included sociodemographic characteristics, occupational history (most recent and longest-held jobs), military history, cigarette smoking and alcohol consumption, physical activity, family history of neurodegenerative diseases, and disease progression. Since 2012, the following surveys were added: clinical data (e.g., medical devices used and body onset), open-ended questions (i.e., thoughts on ALS etiology), lifetime residential history, lifetime occupational history, and residential pesticide use. Future surveys to be brought online include hobbies involving toxic exposures, trauma (e.g., traumatic brain injury and electric shocks), caffeine consumption, reproductive history (for females only), and health insurance information. Surveys are intended to help researchers identify risk factors and their relationship, or lack thereof, to ALS.
In 2006, Medicare Part D (which provides coverage for prescription medications) became available to Medicare recipients. The inclusion of prescription use of Riluzole among Medicare recipients will enhance case identification. It will be important to evaluate the completeness of the Registry. Information will be captured each time a case is identified either through the national databases or self-registration so that capture-recapture statistical techniques can be used to estimate the number of persons missed (42,43). This information will be used to determine if specific subgroups are underrepresented and can be targeted for outreach. It is also important to identify the degree of medical specialty of the source (e.g., primary practice or neurologist) for each identified record as this assists in the evaluation of the reliability of the diagnosis. If the evaluation identifies groups of persons who are underrepresented in the Registry, additional case-finding strategies will be developed.
Because the Registry also is being used to facilitate ALS research, ATSDR, through the Registry's research notification mechanism, has sent approximately 13,000 e-mail messages to portal registrants about clinical trials and studies in which they are eligible to participate. These studies have ranged from randomized control trials/studies of drugs, psychological well-being, treatment of muscle cramps, and risk-factor analysis. ATSDR anticipates that additional researchers will apply for and use this research mechanism to increase patient recruitment. A complete listing of the studies that have used the Registry for recruitment purposes is available at http://wwwn.cdc.gov/ALS/ALSResearchNotificationClinicalTrialsStudies.aspx.
Limitations
The findings in this report are subject to at least four limitations. First, because ALS is not a notifiable disease in the United States (except for Massachusetts), ensuring that all newly diagnosed and prevalent ALS cases in the United States are captured in the Registry is challenging; therefore, the possibility of underascertainment exists. However, the large administrative database methodology ATSDR is using was vetted through a pilot effort and will allow for the majority of ALS cases in the United States to be identified, given its sensitivity and specificity of 87% and 85%, respectively. In addition, ATSDR is using national stakeholders to promote the Registry to patients so they can self-enroll though the Registry's web portal. ATSDR endeavors to improve both the completeness and representativeness of the data over time.Second, although every attempt was made to de-duplicate the files, differences in fields collected by the different sources, misspellings of names, and data entry errors could have prevented records from merging correctly. Additional reviews of the records by individual variables indicate that the overall conclusions probably are unaffected because data entry errors are unlikely to have occurred in any substantial number of records. Third, the calculation of ALS incidence is not possible at this time because the date of diagnosis is not captured through the large administrative database approach, and cases without a date of diagnosis comprise 68% of cases in the Registry. However, incidence is being calculated in select states and metropolitan areas and will allow for population-based estimates of ALS incidence in smaller defined geographic areas of the United States. Finally, the Registry has been officially active since October 2009 and is still maturing (i.e., it is continuing to add more participants and might not be representative of all ALS patients). As more persons with ALS enroll and complete surveys, a better understanding of possible risk factors might emerge.
Promoting the Registry
To inform persons with ALS about the Registry, ATSDR has partnered with external nonprofit organizations such as the ALS Association (ALSA) (http://www.alsa.org), the Muscular Dystrophy Association (MDA) (http://www.mda.org/disease/amyotrophic-lateral-sclerosis), and the Les Turner ALS Foundation (http://www.lesturnerals.org), among others. These organizations have chapters, offices, and clinics located throughout the United States that provide support and care to persons with ALS and their families. They also encourage persons with ALS to become contributing members of the Registry. These organizations increasingly are using tablet personal computers and portable hotspots to aid in enrollment of those patients who are homebound, lack computer or internet access, or are otherwise immobile. In addition, ATSDR uses several tools to promote the Registry, including posts in social media channels such as Facebook and Twitter, advertisements in print and electronic professional magazines, and participation in nationwide events such as scientific conferences and meetings of patient support groups.
Registry Enhancements
In addition to enrolling persons with ALS in the Registry, ATSDR is taking steps to enhance the Registry's usefulness and utility. One such enhancement is undertaking active state and metropolitan level surveillance initiatives that will allow for timely population-based case estimates of ALS in smaller defined geographic areas. Three states (Florida, New Jersey, and Texas) and eight metropolitan areas (Atlanta, Georgia; Baltimore, Maryland; Chicago, Illinois; Detroit, Michigan; Las Vegas, Nevada; Los Angeles, California; Philadelphia, Pennsylvania; and San Francisco, California) have participated. These local surveillance activities have actively identified neurologists who diagnose ALS or provide care for persons with ALS and asked them to report persons with ALS under their care during a specified period. This process is helping ATSDR evaluate the Registry's completeness by comparing state and local data to data from the same areas collected in the Registry. If some areas or groups are found to be underrepresented in the Registry, ATSDR will work with external partners to find ways to reach these populations.
Another Registry enhancement includes the development of a research notification mechanism to inform persons with ALS about new research studies in which they might be able to participate. When researchers send ATSDR information about their studies, a review panel evaluates the project and verifies that the study has been approved by the researcher's IRB. ATSDR then sends information about the study via e-mail to Registry enrollees who have agreed to be contacted about such projects. Registrants then can contact the researcher if they want to take part in the study. ATSDR does not provide identifiable information to researchers at any point in this process. Since the research notification mechanism's May 2012 deployment, approximately 96% of enrollees in the National ALS Registry have elected to be notified about ALS research opportunities (available at http://wwwn.cdc.gov/ALS/ALSClinicalResearch.aspx).
Finally, ATSDR is funding a feasibility study for the creation of a national biorepository collecting biologic specimens (blood and tissue) from persons in the ALS Registry. Biorepositories (or biobanks) are "libraries" in which biospecimens are stored and made available for scientists to study for clinical or research purposes. A biorepository can help researchers learn more about the cause(s) of ALS and can provide data that can be studied along with demographic and other environmental as well as occupational data currently being provided by persons with ALS (available at http://wwwn.cdc.gov/als/ALSBioRegistry.aspx). Linking the specimens to the information collected from Registry participants is a novel approach that will make the Registry even more useful.
Conclusion
Surveillance is important to monitor changes in incidence and prevalence of a condition. Surveillance data also can be used in planning for health-care needs, detecting changes in health practices, and assessing the burden of disease. For chronic diseases, monitoring the burden of disease (morbidity, disability, and mortality) is important (47). To date, national disease surveillance systems have been related primarily to infectious diseases. In 1992, directors of the World Health Organization noncommunicable disease collaborating centers and key officials in centers for noncommunicable diseases advocated increased surveillance of noncommunicable diseases; this recommendation was made on the basis of the lack of incidence data for noncommunicable diseases (48). In addition, registries can be used to identify patients with specific conditions who can be asked to participate in research studies.
This is the first prevalence estimate of ALS for the United States that is based on national data. The establishment of the National ALS Registry will allow for analysis of prevalence of this disease as well as continuing research on risk factors. Using existing database resources from Medicare, Medicaid, the Veterans Heath Administration, and the Veterans Benefits Administration as well as the self-reported web-based portal will allow for a more accurate estimate of disease burden and could be a model for other non-notifiable diseases such as Parkinson's disease or multiple sclerosis. ATSDR endeavors to improve both the completeness and representativeness of the data over time to ensure completeness and accuracy of the Registry. Furthermore, identifying possible risk factors for ALS through close partnerships with academic researchers and enrolling persons with ALS in the Registry will facilitate further exploration of ALS etiology.
Acknowledgments
Frank Bove, ScD, Division of Toxicology and Human Health Sciences, Agency for Toxic Substances and Disease Registry, and James J. Sejvar, MD, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC, provided scientific review of this report.
References
- Mitsumoto H, Chad DA, Pioro EP. Amyotrophic lateral sclerosis. Philadelphia, PA: F.A. Davis Company; 1998.
- Carlesi C, Pasquali L, Piazza S, et al. Strategies for clinical approach to neurodegeneration in amyotrophic lateral sclerosis. Arch Ital Biol 2011;149:151–67.
- Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–9.
- Norris F, Shepherd R, Denys E, et al. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci 1993;118:48–55.
- Armon C, Kurland LT, Daube JR, O'Brien PC. Epidemiologic correlates of sporadic amyotrophic lateral sclerosis. Neurology 1991;41:1077–84.
- Kamel F, Umbach DM, Munsat TL, Shefner JM, Hu H, Sandler DP. Lead exposure and amyotrophic lateral sclerosis. Epidemiology 2002;13:311–9.
- Roelofs-Iverson RA, Mulder DW, Elveback LR, Kurland LT, Molgaard CA. ALS and heavy metals: a pilot case-control study. Neurology 1984;34:393–5.
- Nicholas JS, Lackland DT, Dosemeci M, et al. Mortality among US commercial pilots and navigators. J Occup Environ Med 1998;40:980–5.
- Schulte PA, Burnett CA, Boeniger MF, Johnson J. Neurodegenerative diseases: occupational occurrence and potential risk factors, 1982 through 1991. Am J Public Health 1996;86:1281–8.
- Sutedja NA, Fischer K, Veldink JH, et al. What we truly know about occupation as a risk factor for ALS: a critical and systematic review. Amyotroph Lateral Scler 2009;10:295–301.
- Okamoto K, Kihira T, Kondo T, et al. Lifestyle factors and risk of amyotrophic lateral sclerosis: a case-control study in Japan. Ann Epidemiol 2009;19:359–64.
- Wang H, O'Reilly EJ, Weisskopf MG, et al. Vitamin E intake and risk of amyotrophic lateral sclerosis: a pooled analysis of data from 5 prospective cohort studies. Am J Epidemiol 2011;173:595–602.
- Beghi E, Logroscino G, Chio A, et al. Amyotrophic lateral sclerosis, physical exercise, trauma and sports: results of a population-based pilot case-control study. Amyotroph Lateral Scler 2010;11:289–92.
- Fang F, Chen H, Wirdefeldt K, et al. Infection of the central nervous system, sepsis and amyotrophic lateral sclerosis. PLoS ONE 2011;6:e29749.
- Okamoto K, Kihira T, Kobashi G, et al. Fruit and vegetable intake and risk of amyotrophic lateral sclerosis in Japan. Neuroepidemiology 2009;32:251–6.
- Piazza O, Siren AL, Ehrenreich H. Soccer, neurotrauma and amyotrophic lateral sclerosis: is there a connection? Curr Med Res Opin 2004;20:505–8.
- Woolsey PB. Cysteine, sulfite, and glutamate toxicity: a cause of ALS? J Altern Complement Med 2008;14:1159–64.
- CDC. Summary of notifiable diseases. MMWR 2013;60(No. 53).
- Anonymous. The Scottish Motor Neuron Disease Register: a prospective study of adult onset motor neuron disease in Scotland. Methodology, demography and clinical features of incident cases in 1989. J Neurol Neurosurg Psychiatry 1992;55:536–41. Available at http://www.ncbi.nlm.nih.gov/pubmed/1640227.
- Bettoni L, Bazzani M, Bortone E, Dascola I, Pisani E, Mancia D. Steadiness of amyotrophic lateral sclerosis in the province of Parma, Italy, 1960–1990. Acta Neurol Scand 1994;90:276–80.
- Chiò A, Cucatto A, Calvo A, Terreni AA, Magnani C, Schiffer D. Amyotrophic lateral sclerosis among the migrant population to Piemonte, northwestern Italy. J Neurol 1999;246:175–80.
- Guidetti D, Bondavalli M, Sabadini R, et al. Epidemiological survey of amyotrophic lateral sclerosis in the province of Reggio Emilia, Italy: influence of environmental exposure to lead. Neuroepidemiology 1996;15:301–12.
- Huber S, Henn V. Unchanged incidence and prevalence of amyotrophic lateral sclerosis in the canton of Zurich. Schweiz Arch Neurol Psychiatr (Bucur) 1995;146:52–4.
- Mandrioli J, Faglioni P, Merelli E, Sola P. The epidemiology of ALS in Modena, Italy. Neurology 2003;60:683–9.
- Mitchell JD, Gibson HN, Gatrell A. Amyotrophic lateral sclerosis in Lancashire and South Cumbria, England, 1976–1986: a geographical study. Arch Neurol 1990;47:875–80.
- Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Incidence and prevalence of ALS in Ireland, 1995–1997: a population-based study. Neurology 1999;52:504–9.
- Tysnes OB, Vollset SE, Aarli JA. Epidemiology of amyotrophic lateral sclerosis in Hordaland county, western Norway. Acta Neurol Scand 1991;83:280–5.
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders? Neurology 2007;68:326–37.
- Kaye WE, Sanchez M, Wu J. Feasibility of creating a National ALS Registry using administrative data in the United States. Amyotroph Lateral Scler Frontotemporal Degener 2014 Mar 6 [Epub ahead of print].
- US Public Health Service. ALS Registry Act. Washington, DC: 110th Congress. Public Law 2008;122 Stat 4047:110–373. Available at http://wwwn.cdc.gov/als/Download/ALS%20Registry%20Act%20(Public%20Law%20110-373).pdf.
- Brooke EM. The current and future use of registers in health information systems. Pub. No. 8, Geneva, Switzerland: World Health Organization; 1974.
- Antao VC, Horton DK. The National Amyotrophic Lateral Sclerosis (ALS) Registry. J Environ Health 2012;75:28–30.
- US Department of Veteran Affairs. Federal benefits for veterans, dependents and survivors. Chapter 2: service-connected disabilities. Available at http://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp.
- Fisher ES, Baron JA, Malenka DJ, Barrett J, Bubolz TA. Overcoming potential pitfalls in the use of Medicare data for epidemiologic research. Am J Public Health 1990;80:1487—90.
- Pope GC, Urato CJ, Kulas ED, Kronick R, Gilmer T. Prevalence, expenditures, utilization, and payment for persons with MS in insured populations. Neurology 2002;58:37–43.
- Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol 2000;55:929–37.
- Allen KD, Kasarskis EJ, Bedlack RS, et al. The national registry of veterans with amyotrophic lateral sclerosis. Neuroepidemiology 2008;30:180–90.
- US Census Bureau. Annual estimates of the resident population for the United States, regions, states, and Puerto Rico: April 1, 2010 to July 1, 2013 (NST-EST2013–01). Washington, DC: US Census Bureau; 2013.
- Schiller JS, Lucas JW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2011. National Center for Health Statistics. Vital Health Stat 2012;10(256).
- Chiò A. Risk factors in the early diagnosis of ALS: European epidemiological studies. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1(Suppl 1):S13–8.
- Eisen A. Amyotrophic lateral sclerosis is a multifactorial disease. Muscle Nerve 1995;18:741–52.
- Pew Research Center. Internet user demographics. Pew Internet and American Life Project 2013. Washington, DC: Pew Research Center; 2014. Available at http://pewinternet.org/data-trend/internet-use/latest-stats.
- US Census Bureau. 2011 American Community Survey 1-year estimates. B21001–sex by age by veterans status for civilian population 18 years plus. Washington, DC: US Census Bureau; 2011. Available at http://factfinder2.census.gov/bkmk/table/1.0/en/ACS/11_1YR/B21001.
- Weisskopf MG, O'Reilly EJ, McCullough ML, et al. Prospective study of military service and mortality from ALS. Neurology 2005;64:32–7.
- Coffman CJ, Horner RD, Grambow SC, Lindquist J. Estimating the occurrence of amyotrophic lateral sclerosis among Gulf War (1990–1991) veterans using capture-recapture methods. Neuroepidemiology 2005;24:141–50.
- Preux PM, Druet-Cabanac M, Couratier P, et al. Estimation of the amyotrophic lateral sclerosis incidence by capture-recapture method in the Limousin region of France. J Clin Epidemiol 2000;53:1025–9.
- Thacker SB, Stroup DF, Rothenberg RB. Public health surveillance for chronic conditions: a scientific basis for decisions. Stat Med 1995;14:629–41.
- World Health Organization. Shanghai declaration on non-communicable diseases. BMJ 1993;306:588.
*An encounter is a record of a health-care service (e.g., a visit to a physician, hospitalization, x-ray, or laboratory test).
FIGURE 1. National Amyotrophic Lateral Sclerosis (ALS) Registry methodology
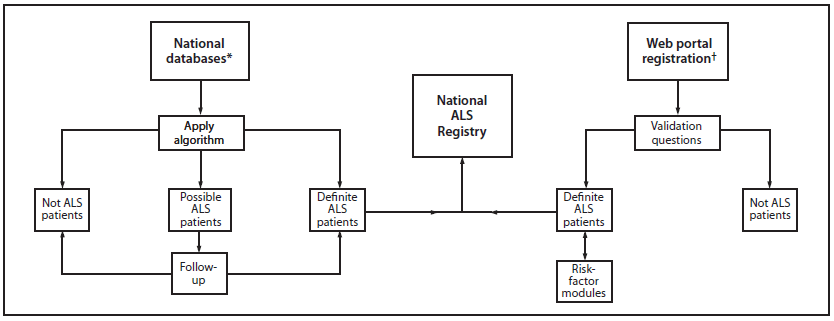
* Maintained by Medicare, Medicaid, the Veterans Health Administration, and the Veterans Benefits Administration.
† Available at http://www.cdc.gov/als.
Alternate Text: This figure shows the protocol used by the National Amyotrophic Lateral Sclerosis (ALS) Registry to identify ALS patients in the United States.
FIGURE 2. Prevalence rates* for cases of amyotrophic lateral sclerosis (ALS), by age group — National ALS Registry, United States, October 19, 2010–December 31, 2011
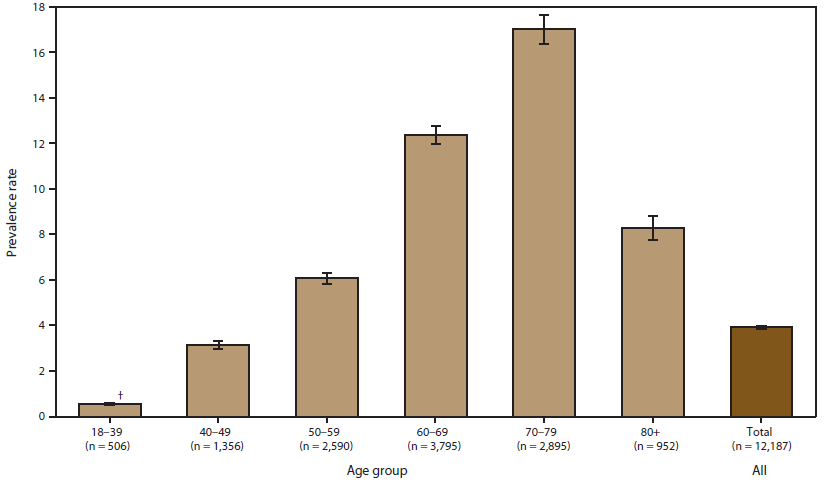
* Per 100,000 population.
† 95% confidence interval.
Alternate Text:The figure shows prevalence rates per 100,000 population for cases of amyotrophic lateral sclerosis in the United States, by age group, on the basis of data from the National ALS Registry for October 19, 2010-December 31, 2011. Prevalence rates were highest for persons aged 70-79 years and lowest for those aged 18-39 years.
FIGURE 3. Prevalence rates* for cases of amyotrophic lateral sclerosis (ALS), by sex — National ALS Registry, United States, October 19, 2010–December 31, 2011
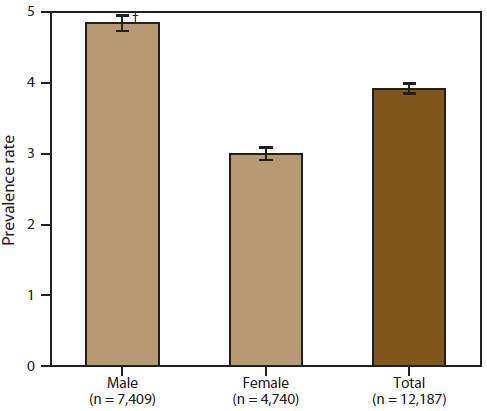
* Per 100,000 population.
† 95% confidence interval.
Alternate Text: The figure shows prevalence rates per 100,000 population for cases of amyotrophic lateral sclerosis in the United States, by sex, on the basis of data from the National ALS Registry for October 19, 2010-December 31, 2011. Prevalence rates were higher for males than for females.
FIGURE 4. Prevalence rates* for cases of amyotrophic lateral sclerosis (ALS), by race — National ALS Registry, United States, October 19, 2010–December 31, 2011
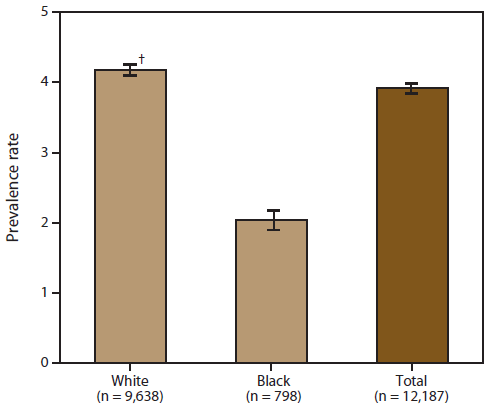
* Per 100,000 population.
† 95% confidence interval.
Alternate Text: The figure shows prevalence rates per 100,000 population for cases of amyotrophic lateral sclerosis in the United States, by race, on the basis of data from the National ALS Registry for October 19, 2010-December 31, 2011. Prevalence rates were higher for whites than for blacks.
|
TABLE 4. (Continued) Risk factors for amyotrophic lateral sclerosis (ALS) of registrants in secure web portal, by employment status, occupation,* and length of employment — National ALS Registry, United States, October 2010–December 2011 |
||
|---|---|---|
|
Characteristic |
No. |
(%) |
|
Industry worked in for longest period of time |
||
|
Agriculture, forestry, fishing and hunting |
30 |
(1.8) |
|
Mining |
6 |
(0.4) |
|
Utilities |
33 |
(1.9) |
|
Construction |
102 |
(6.0) |
|
Manufacturing (food, textile, apparel) |
31 |
(1.8) |
|
Manufacturing (paper, printing, chemicals, petroleum, leather, lumber, stone) |
71 |
(4.1) |
|
Manufacturing (metal, electrical, transport, professional) |
126 |
(7.4) |
|
Wholesale trade |
22 |
(1.3) |
|
Retail trade I (cars, gas, furniture, electronics, food-beverage, clothing) |
113 |
(6.6) |
|
Retail trade II (sporting goods, books, music) |
17 |
(1.0) |
|
Transportation and warehousing I (air, rail, water, ground, pipeline) |
47 |
(2.7) |
|
Transportation and warehousing II (postal, courier, warehouse) |
21 |
(1.2) |
|
Information |
57 |
(3.3) |
|
Finance and insurance |
96 |
(5.6) |
|
Real estate and rental and leasing |
23 |
(1.3) |
|
Professional, scientific, and technical services |
201 |
(11.7) |
|
Management of companies and enterprises |
28 |
(1.6) |
|
Administrative and support and waste management and remediation services |
6 |
(0.4) |
|
Educational services |
172 |
(10.0) |
|
Health care and social assistance |
180 |
(10.5) |
|
Arts, entertainment, and recreation |
27 |
(1.6) |
|
Accommodation and food services |
39 |
(2.3) |
|
Other services (except public administration) |
112 |
(6.5) |
|
Public administration |
60 |
(3.5) |
|
Employment at longest-held occupation (yrs) |
||
|
<10 |
260 |
(15.2) |
|
10–19 |
488 |
(28.5) |
|
20–29 |
497 |
(29.0) |
|
≥30 |
415 |
(24.2) |
|
* Respondents with missing occupational information were excluded: employment status, 3 (0.2%); job title held for the longest time, 36 (2.1%); industry worked in for the longest time, 94 (5.5%); and employment at longest held occupation, 54 (3.2%). One respondent was not included in occupational analyses because of incomplete data. |
||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


