Diagnosis of Tuberculosis in Three Zoo Elephants and a Human Contact — Oregon, 2013
Please note: An erratum has been published for this article. To view the erratum, please click here.
, MPH1; , MD1; , MPH1; , MPH2; , MPH2; , MD1; , DVM3; 4; 4; , DVM3; , DVM2
In 2013, public health officials in Multnomah County, Oregon, started an investigation of a tuberculosis (TB) outbreak among elephants and humans at a local zoo. The investigation ultimately identified three bull elephants with active TB and 118 human contacts of the elephants. Ninety-six (81%) contacts were evaluated, and seven close contacts were found to have latent TB infection. The three bulls were isolated and treated (elephants with TB typically are not euthanized) to prevent infection of other animals and humans, and persons with latent infection were offered treatment. Improved TB screening methods for elephants are needed to prevent exposure of human contacts.
In May 2013, a routine annual culture of a sample from a trunk washing on elephant A, an Asian elephant aged 20 years at a zoo in Oregon's Multnomah County, yielded Mycobacterium tuberculosis, indicating active, potentially infectious disease. Bidirectional transmission of M. tuberculosis between elephants and humans has been documented (1). Assuming that elephant A was not infectious at the time of his previous negative trunk wash sample culture, the infectious period was defined as the 12 months preceding the positive results of the May 2013, trunk wash sample (May 2012–May 2013) (2). The Multnomah County Health Department (MCHD) investigated close and casual contacts of elephant A. Close contacts were defined as persons with any presence in the 8,300–square-foot elephant barn or who had been within 15 feet (4.6 m) of any of the eight elephants in the enclosed outdoor area at least weekly during the past 12 months. Casual contacts included zoo employees or volunteers who might have been exposed to elephant trunk secretions or fecal matter (3), but who had not had close contact with elephant A. Human contacts were evaluated with either a tuberculin skin test (TST) or interferon gamma release assay (IGRA). For close contacts, TST conversions were defined as indurations of ≥5 mm (rather than ≥10 mm used in TB screening) (4) within 2 years of the most recent negative TB screening test, and were considered indicative of infection with M. tuberculosis. Historical annual TB screening test results for close contacts were obtained from the zoo's occupational health providers. Historical test results were unavailable for other contacts. TB test results reported for contacts were documented at the initial evaluation and at ≥8 weeks after the last known exposure. Contacts whose first test occurred at least 8 weeks following the last exposure had only one TST or IGRA.
The zoo identified 19 close contacts, all of whom had TSTs at ≥8 weeks after exposure; 13 were negative. Six persons with no previous positive TST and at least one negative TST during the past 2 years had positive TSTs (Figure 1). None of the contacts with positive TSTs had spent time in TB-endemic countries, or had other risk factors for TB, such as a history of homelessness or injection-drug use or diagnosis of human immunodeficiency virus. All had chest radiographs and were evaluated for symptoms; none had active disease. Among close contacts, the number and percentage of conversions from negative TST to positive within 2 years (31.6%) was higher than expected, given the baseline of 4% of the U.S. population having latent infection on the basis of a single ≥10 mm skin test result (5).
Because of the positive test results among close contacts, MCHD expanded the investigation to identify 39 casual contacts. A third group of 20 contacts was identified among persons who had attended special events at which elephant A sprayed paint with his trunk onto canvases behind attendees, potentially exposing them to aerosolized M. tuberculosis. Among all 59 casual and special event contacts, exposure to elephant A was approximately <30 minutes and at a distance of ≥25 feet. Among the 59 casual and special event contacts identified, 48 (81%) were fully evaluated; none had a positive TST or IGRA (Figure 1).
Before diagnosis of TB in elephant A, elephants were routinely screened for TB by annual cultures of samples collected from trunk washings, with samples collected from each elephant on 3 consecutive days. Following diagnosis of TB in elephant A, the zoo increased the frequency of trunk washings to once a month for infected elephants and once every 3 months for uninfected elephants. Serologic screenings were conducted once or twice a year to identify infected, but culture-negative, elephants. During the course of the investigation, antibodies to M. tuberculosis were detected in the serum of elephant A's father (elephant B), aged 51 years. Subsequently, in October 2013, culture of a trunk wash sample from elephant B was positive. The other seven elephants in the herd, including elephant A, had negative trunk washings at that time. Elephant B's close human contacts were identical to those of elephant A, with the exception of one new employee, whose TB screen was negative when he began employment.
In October 2013, another local public health department discovered that patient A, who had completed treatment for culture-confirmed pleural TB in the fall of 2012, had also been a casual contact of elephant A. Upon receiving notification for routine annual TB screening from the zoo, patient A had sought guidance from the health department regarding documentation of TB status. Patient A had worked at the zoo intermittently during 2012, but had limited contact with elephants (1 hour cumulative presence in the elephant barn). Given the pleural (sputum-culture-negative) nature of patient A's disease, patient A was most likely noninfectious.
The Oregon Health Authority had reviewed patient A's M. tuberculosis isolate's genotype in 2012, and found no matches in Oregon. When patient A's zoo work history was revealed in October 2013, well into the contact investigation for elephant A, the Oregon Health Authority reviewed the genotypes of the isolates of patient A and elephant A, and found that they differed by only one locus in the 24-locus mycobacterial interspersed repetitive units (MIRU) pattern (Figure 2). Isolates from patient A and elephant A were analyzed at CDC using whole genome sequencing. Comparison of the assembled genomes from the two isolates identified no differences. Although this result is consistent with transmission, it does not indicate direction of transmission, and does not provide information about how patient A or the elephant contracted TB. Elephant B's isolate was genotyped, and spacer oligonucleotide typing (spoligotype) from this isolate matched those of patient A and elephant A (Figure 2).
In May 2014, a third bull elephant, elephant C, aged 44 years, was found to be infected with M. tuberculosis by a positive culture from a trunk washing sample. Elephant C's isolate was not whole genome sequenced; all of this elephant's human contacts were the same as those of elephant B. None of the three elephants had shown signs of illness, although elephant B had experienced temporary weight loss. All three elephants' isolates were susceptible to first-line M. tuberculosis drugs. Each bull has received different and changing regimens; treatment is ongoing and guided by drug levels and tolerance.
Because the strain isolated from patient A matched that from elephant A, MCHD personnel searched for an unidentified, common human source and explored the possibility that the elephants might have been previously transmitting TB despite negative trunk washings. During the summer of 2014, the investigation was expanded to include two additional groups: 1) all current and former employees who had worked at the zoo since January 1, 2010, and who met the definition of close contacts, and 2) persons who participated in the same February 2012 zoo orientation as patient A, which was the time when patient A had the most contact with elephants (Figure 1, Figure 3). Among the 28 persons who participated in the 2012 zoo orientation (including patient A), 18 had a negative TST; nine persons no longer worked at the zoo and could not be reached. MCHD concluded that persons who participated in the same orientation as patient A were likely not infected with TB in the course of their orientation. MCHD uncovered no evidence of a previously unidentified human case in the zoo orientation cohort that could have infected other humans or elephant A during this time. As of April 2015, reports from CDC's TB Genotyping Information Management System revealed that the isolates from elephant A and from patient A have unique genotypes (spoligotype + 24-locus MIRU), not matched locally or nationally.
Final results of the investigation of all 31 close contacts since 2010 identified one additional positive TST result from July 2011 (induration = 19 mm); this is close to the zoo's baseline of 0–1 conversions per year (Figure 1). On the basis of these findings, shedding of M. tuberculosis by elephants before elephant A's diagnosis was deemed unlikely.
Throughout the investigation, MCHD worked with the zoo and the Oregon Health Authority to ensure the safety of staff members, animals, and the public. Close and prolonged contact, including spending multiple hours indoors with infected elephants, was associated with TB transmission in this investigation. Continuing routine protocols for annual TB screening of humans who work with elephants is warranted, as is a heightened screening recommendation for the closest contacts until summer 2016. In addition to other administrative and environmental controls, all current close contacts wear a fit-tested N-95 respirator or higher level of protection when in the elephant barn or in contact with any potentially infectious elephant. Close contacts will continue to receive a TST every 6 months until summer 2016, at which point the exposure control plan will be reevaluated. Close contacts with previous positive test results will have a periodic TB symptom screen rather than a TST.
Once all elephants complete treatment for active TB, the Oregon Health Authority, MCHD, and the zoo veterinarians will decide whether to modify the exposure control plan. The elephants will continue to be screened at regular intervals according to Department of Agriculture guidelines (2). Because of the absence of guidance on determining when an elephant is no longer infectious, the zoo and state and local public health professionals defined an infectious elephant as one that 1) has had M. tuberculosis isolated from a culture of a trunk washing sample, 2) has not received at least 2 months of adequate TB treatment, and 3) has not had at least three consecutive negative findings from cultures of monthly trunk washing samples; or that is not responding to treatment, has a worsening serologic picture,* or might otherwise pose a risk to the herd, zoo personnel, or the public. On the basis of the contact investigation results, MCHD has advised that outdoor contact with infectious elephants for <30 minutes and at a distance of ≥25 feet posed minimal risk for TB transmission.
MCHD also worked with zoo veterinarians and the state public health veterinarian to develop guidelines for safe public elephant viewing. Although the contact investigation suggested minimal risk, all infectious elephants were removed from general display and public viewing within 100 feet. Routine indoor and outdoor public viewing of noninfectious elephants is considered safe.
Discussion
In North America, approximately 5% of captive Asian elephants are infected with M. tuberculosis, on the basis of positive cultures of trunk washing samples or necropsy results (6). The U.S. Department of Agriculture's Animal and Plant Health Inspection Service has developed guidelines for the screening and diagnosis of TB in captive elephants, including annual trunk wash samples for mycobacterial culture (2). However, trunk-wash sample cultures, the standard for diagnosing active TB in elephants, are insensitive, and some cases of TB might be missed. Serologic screening is used in some settings to identify elephants with TB infection (7), but is controversial among elephant veterinarians and is subject to false-positive results (7).
Although MCHD's investigation did not suggest previously unrecognized shedding of M. tuberculosis by the elephants, annual personnel screening is an important component of occupational safety, given the potential risk for TB exposure to staff members as well as the risk to elephants of transmission from humans with undiagnosed TB. Organizations that conduct TB testing for employees should have a mechanism for tracking results and investigating when TST conversions are elevated above the annual baseline. In addition, better understanding of modes of TB transmission between humans, elephants, and other animals might lead to more comprehensive guidelines for prevention of TB transmission in high-risk settings (8). Genotyping surveillance, in conjunction with epidemiologic investigation, might also be effective in linking human and non-human TB cases and evaluating unrecognized transmission, especially if the strains are rare. Collaboration between public health, veterinary medicine, and occupational health experts would allow for better understanding of the risks for and prevention of zoonotic transmission of M. tuberculosis.
Acknowledgments
Lauren Cowan, Division of Tuberculosis Elimination, Mycobacteriology Laboratory Branch CDC; Brian Baker, Division of Tuberculosis Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; Bob Lee, Oregon Zoo.
1Multnomah County Health Department, Oregon; 2Public Health Division, Oregon Health Authority; 3Oregon Zoo; 4Washington County Department of Health and Human Services, Oregon.
Corresponding author: Amy Zlot, amy.zlot@multco.us, 503-988-3406.
References
- Michalak K, Austin C, Diesel S, Bacon MJ, Zimmerman P, Maslow JN. Mycobacterium tuberculosis infection as a zoonotic disease: transmission between humans and elephants. Emerg Infect Dis 1998;4:283–7.
- Animal and Plant Health Inspection Service. Guidelines for the control of tuberculosis in elephants. Washington, DC: US Department of Agriculture, Animal and Plant Health Inspection Service; 2012. Available at http://www.usaha.org/Portals/6/Committees/tuberculosis/TB%20Guidelines%202012%20Draft%20revision%2020April2012.pdf.
- Murphree R, Warkentin JV, Dunn JR, Schaffner W, Jones TF. Elephant-to-human transmission of tuberculosis, 2009. Emerg Infect Dis 2011;17:366–71.
- National Tuberculosis Controllers Association; CDC. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005;54(No. RR-15).
- CDC. Latent tuberculosis infection: a guide for primary health care providers. Atlanta, GA: US Department of Health and Human Services, CDC; 2013. Available at http://www.cdc.gov/tb/publications/ltbi/pdf/targetedltbi.pdf.
- Feldman M, Isaza R, Prins C, Hernandez J. Point prevalence and incidence of Mycobacterium tuberculosis complex in captive elephants in the United States of America. Vet Q 2013;33:25–9.
- Lyashchenko KP, Greenwald R, Esfandiari J, et al. Tuberculosis in elephants: antibody responses to defined antigens of Mycobacterium tuberculosis, potential for early diagnosis, and monitoring of treatment. Clin Vaccine Immunol 2006;13:722–32.
- Stephens N, Vogelnest L, Lowbridge C, et al. Transmission of Mycobacterium tuberculosis from an Asian elephant (Elephas maximus) to a chimpanzee (Pan troglodytes) and humans in an Australian zoo. Epidemiol Infect 2013;141:1488–97.
* Serologic tests can be used as indicators of active infection in elephants or to assess an elephant's response to infection and treatment.
Summary
What is already known on this topic?
In North America, approximately 5% of captive Asian elephants are infected with Mycobacterium tuberculosis. Bidirectional spread of M. tuberculosis between elephants and humans has been documented.
What is added by this report?
Investigation of a tuberculosis (TB) outbreak among three elephants at an Oregon zoo identified multiple close, casual, and spectator contacts. One hundred and eighteen contacts were identified, 96 of these contacts were screened, and seven close contacts (six recent conversions and one earlier positive test) were found to have latent, noninfectious TB. Whole-genome sequencing revealed that one elephant's M. tuberculosis isolate identically matched the isolate of a person with pleural TB who attended a zoo orientation in 2012. The lack of guidance about how to manage captive, TB-infected elephants complicated the decision-making process for protection of zoo contacts, other animals at the zoo, and the general public.
What are the implications for public health practice?
Collaboration between public health, veterinary medicine, and occupational health experts could lead to better understanding about associated risks, and could help prevent zoonotic transmission of M. tuberculosis. The development of improved TB screening methods for elephants is needed to prevent exposure to humans with close and prolonged contact.
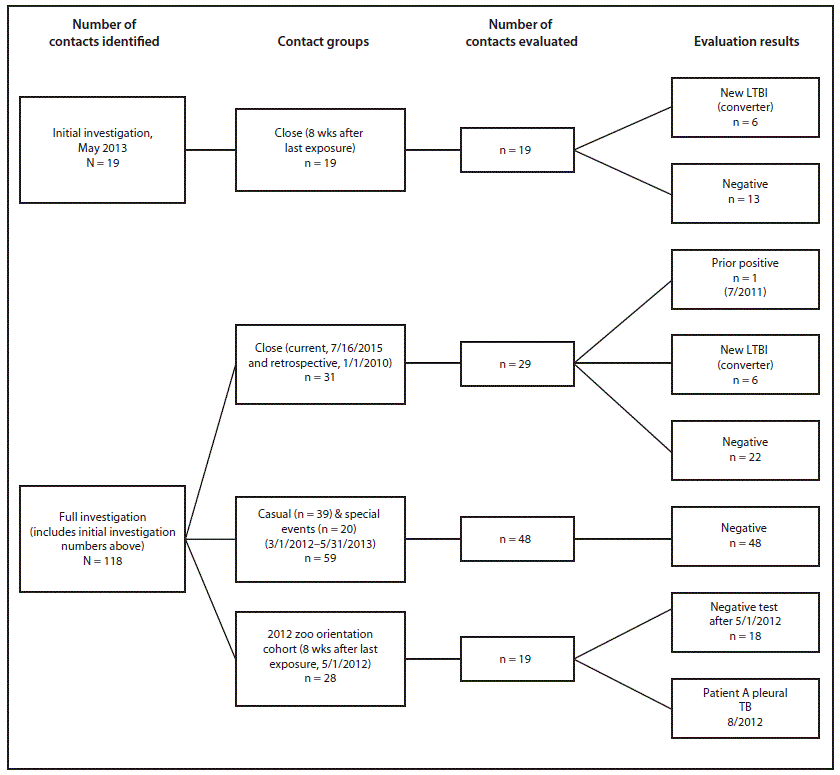
Abbreviations: LTBI = latent tuberculosis infection; TB = tuberculosis.
Alternate Text: The figure above is a diagram showing a contact investigation of elephants with tuberculosis at an Oregon zoo in 2013.
FIGURE 2. Genotyping analysis of M. tuberculosis isolates from patient A and elephant A* — Oregon, 2013
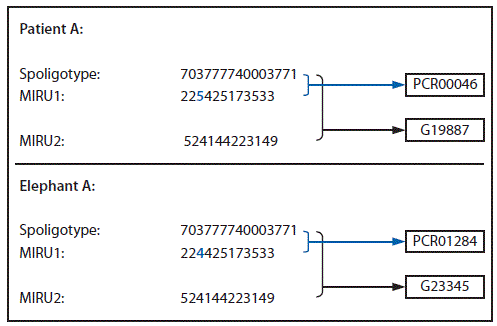
* Patient A and elephant A have slightly different genotypes (spoligotype+MIRU1+MIRU2), differing by only one locus.
Alternate Text: The figure above is a genotyping analysis of Mycobacterium tuberculosis isolates from patient A and elephant A from an Oregon zoo tuberculosis outbreak in 2013.
FIGURE 3. Timeline of tuberculosis diagnoses in three elephants and a casual contact at a zoo — Oregon, 2013*
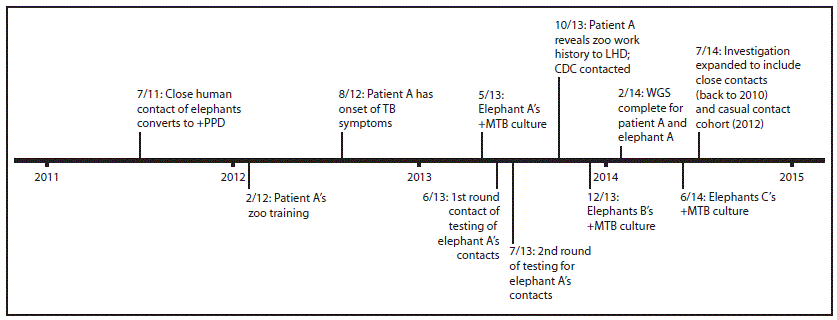
Abbreviations: LHD = local health department; +Mtb = positive for Mycobacterium tuberculosis; +PPD = positive purified protein derivative test (tuberculin skin test); TB = tuberculosis; WGS = whole genome sequencing.
* Current contacts (as of May 2013) of Elephant A during March 1, 2012–May 13, 2013 were initially investigated; in July 2014, the investigation was expanded to include close contacts back to January 1, 2012 and a casual (zoo orientation) cohort in February 2012.
Alternate Text: The figure above is a timeline of tuberculosis diagnoses in three elephants and a casual contact at an Oregon zoo in 2013.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


