Notes from the Field: Carbapenem-resistant Enterobacteriaceae Producing OXA-48-like Carbapenemases — United States, 2010–2015
Please note: An erratum has been published for this article. To view the erratum, please click here.
, MD1,2; , PhD2; , MMSc2; , PhD2; , PhD2; , MD2
Carbapenem-resistant Enterobacteriaceae (CRE) are bacteria that are often resistant to most classes of antibiotics and cause health care–associated infections with high mortality rates (1). Among CRE, strains that carry plasmid-encoded carbapenemase enzymes that inactivate carbapenem antibiotics are of greatest public health concern because of their potential for rapid global dissemination, as evidenced by the increasing distribution of CRE that produce the Klebsiella pneumoniae carbapenemase and the New Delhi metallo-β-lactamase. Newly described resistance in Enterobacteriaceae, such as plasmid-mediated resistance to the last-line antimicrobial colistin, recently detected in China, and resistance to the newly approved antimicrobial, ceftazidime-avibactam, identified from a U.S. K. pneumoniae carbapenemase-producing isolate, highlight the continued urgency to delay spread of CRE (2,3). Monitoring the emergence of carbapenemases is crucial to limiting their spread; identification of patients carrying carbapenemase-producing CRE should result in the institution of transmission-based precautions and enhanced environmental cleaning to prevent transmission.* The OXA-48 carbapenemase was first identified in Enterobacteriaceae in Turkey in 2001 (2), and OXA-48-like variants have subsequently been reported around the world. The first U.S. reports of OXA-48-like carbapenemases were published in 2013 and included retrospectively identified isolates from 2009 (3) and two isolates collected in 2012 from patients in Virginia who had recently been hospitalized outside the United States (4). Although there are limited additional published reports from the United States (5), CDC continues to receive reprots of these organisms. This report describes patients identified as carrying CRE producing OXA-48-like carbapenemases in the United States during June 2010–August 2015.
CDC received reports of 52 CRE isolates producing OXA-48-like carbapenemases collected from 43 patients in 19 states during June 2010–August 2015 (Figure); seven of these isolates were identified retrospectively from six patients. Eight isolates from four patients were part of two different clusters in the United States during 2014. The number of patients from whom CRE isolates producing OXA-48-like carbapenemases were identified ranged from one in 2010 to 11 per year in 2013, 2014, and 2015. Thirty-five patients (81%) had OXA-48-like carbapenemase identified from K. pneumoniae isolates, seven (16%) from Escherichia coli isolates, one (2%) from an Enterobacter aerogenes isolate, and one (2%) from a Klebsiella ozaenae isolate; two of which were isolates of different species from a single patient. Isolates with both New Delhi metallo-β-lactamase and OXA-48-like carbapenemase genes were obtained from five patients (12%). The most common sources were urine (22 patients [51%]) and respiratory specimens (nine patients [21%]).
The median age among 35 (81%) patients for whom age was reported was 70 years (range = 29–91 years). Among 29 patients for whom a travel history was available, 19 (66%) had traveled internationally during the year before specimen collection, and 16 (55%) were hospitalized outside the United States for ≥1 night; these percentages increased to 76% and 64%, respectively, when cases associated with the two domestic clusters were excluded. India was the most frequently reported destination among patients with international travel (11 of 19 patients) and international hospitalization (nine of 16).
CRE producing OXA-48-like carbapenemases have demonstrated the ability to spread in other countries (6) and cause outbreaks in health care settings. Factors potentially contributing to the spread of these organisms include the high transfer efficiency of the plasmid containing OXA-48-like genes (6) and challenges in identifying these organisms. These challenges in identification occur because of the limited testing for CRE resistance mechanisms in U.S. clinical laboratories and the different susceptibility profiles of these organisms compared with other carbapenemase-producing CRE, making them difficult to differentiate from CRE that do not produce carbapenemases (organisms producing OXA-48-like carbapenemases might be susceptible to third generation cephalosporins). The modification of the CDC CRE surveillance definition in January 2015 to include organisms that are resistant to ertapenem or that possess a carbapenemase gene should improve sensitivity for detecting OXA-48-producing CRE (7).
Although clusters of CRE expressing OXA-48-like carbapenemases suggest transmission has occurred in the United States, the majority of identified patients were reported to have had exposure to health care outside the United States. This is consistent with recommendations in the CDC Health Advisory from February 2013 (8), which sought to prevent transmission of isolates producing non-K. pneumoniae carbapenemases by improving their detection in patients recently hospitalized outside the United States. Recommendations include determining the mechanism of resistance for any CRE isolated from such patients and considering CRE screening of these patients on admission.
1Epidemic Intelligence Service, CDC; 2Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, CDC.
Corresponding author: Meghan M. Lyman, mmlyman@cdc.gov, 404-639-4241.
References
- Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011;53:60–7.
- Liu Y, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet. Epub Nov 18, 2015.
- Humphries RM, Yang S, Hemarajata P, et al. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother 2015;59:6605–7.
- Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 2012;67:1597–606.
- Lascols C, Peirano G, Hackel M, Laupland KB, Pitout JD. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: first report of OXA-48-like enzymes in North America. Antimicrob Agents Chemother 2013;57:130–6.
- Mathers AJ, Hazen KC, Carroll J, et al. First clinical cases of OXA-48-producing carbapenem-resistant Klebsiella pneumoniae in the United States: the "menace" arrives in the new world. J Clin Microbiol 2013;51:680–3.
- Doi Y, O'Hara JA, Lando JF, et al. Co-production of NDM-1 and OXA-232 by Klebsiella pneumoniae. Emerg Infect Dis 2014;20:163–5.
- Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect 2014;20:821–30.
- CDC. FAQs about choosing and implementing a CRE definition. CDC Healthcare-associated infections. Atlanta, GA: US Department of Health and Human Services, CDC. Available at http://www.cdc.gov/hai/organisms/cre/definition.html.
- CDC Health Alert Network. New carbapenem-resistant enterobacteriaceae warrant additional action by healthcare providers. Atlanta, GA: US Department of Health and Human Services, CDC; 2013. Available at http://emergency.cdc.gov/han/han00341.asp.
* 2012 CRE Toolkit – Guidance for Control of Carbapenem-resistant Enterobacteriaceae. Available at http://www.cdc.gov/hai/organisms/cre/cre-toolkit/index.html.
FIGURE. Number of reported patients with carbapenem-resistant Enterobacteriaceae producing OXA-48-like carbapenemases (N = 43) — United States, June 2010–August 2015
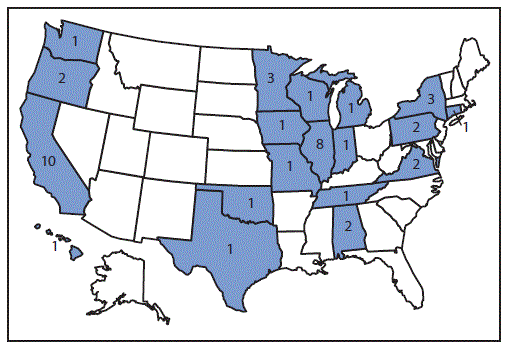
Alternate Text: The figure above is a map of the United States showing the number of reported patients with carbapenem-resistant Enterobacteriaceae producing OXA-48-like carbapenemases (N = 43) in the United States during June 2010-August 2015.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


