Update: Shortened Interval for Postvaccination Serologic Testing of Infants Born to Hepatitis B-Infected Mothers
Weekly
October 9, 2015 / 64(39);1118-20, MD1; , MD1; 2; , MD3; , MD1
Infants born to hepatitis B-infected mothers receive postexposure prophylaxis to reduce their risk for perinatal hepatitis B virus (HBV) infection (1). Postexposure prophylaxis consists of hepatitis B (HepB) vaccine and hepatitis B immune globulin administered within 12 hours of birth, followed by completion of the 3-dose or 4-dose HepB vaccine series (1). Postvaccination serologic testing (PVST) assesses an infant's response to HepB vaccination and has typically occurred at age 9–18 months (1). This report provides a CDC update recommending shortening the interval for PVST from age 9–18 months to age 9–12 months. Providers should order PVST (consisting of hepatitis B surface antigen [HBsAg] and antibody to HBsAg [anti-HBs]) for infants born to HBsAg-positive mothers at age 9–12 months (or 1–2 months after the final dose of the vaccine series, if the series is delayed). This recommendation was prompted by the discontinuation of production of Hib/HepB vaccine (Comvax) and new data from the Enhanced Perinatal Hepatitis B Prevention Program supporting PVST 1–2 months after receipt of the last HepB vaccine dose, and at age ≥9 months.
An estimated 25,000 infants are born to HBsAg-positive mothers each year in the United States (2). Perinatal HBV infection, acquired in utero or during delivery, results in chronic HBV infection in 90% of infected infants (1). Approximately 25% of infants with HBV infection acquired perinatally will die prematurely as a result of complications of cirrhosis or liver cancer (1). Before the widespread availability of postexposure prophylaxis, up to 90% of infants born to HBsAg-positive mothers developed HBV infection (1). Postexposure prophylaxis is highly effective in preventing perinatal HBV transmission. In recent years in the United States, approximately 1% of infants receiving postexposure prophylaxis develop infection (3).
PVST consists of two tests: measurement of HBsAg and anti-HBs (1). Infants born to HBsAg-positive mothers who are HBsAg negative with anti-HBs levels ≥10 mIU/mL after having received a complete, 3-dose or 4-dose HepB vaccine series are identified as vaccine responders and considered seroprotected (4,5). Infants who are HBsAg negative with anti-HBs levels <10 mIU/mL require revaccination with a second 3-dose HepB vaccine series, followed by retesting for anti-HBs 1–2 months after the final vaccine dose (4).
Postvaccination seroprotection is achieved in 98% of healthy full-term infants who received a 3-dose or 4-dose HepB vaccine series, although it is lower among infants with birthweights <4.4 lbs (<2,000 g) (5). Vaccine efficacy studies have demonstrated protection against acute and chronic hepatitis B disease in immunocompetent vaccine responders (6). Anti-HBs levels following vaccination decline over time (6). Immunocompetent persons who achieve an anti-HBs level ≥10 mIU/mL 1–2 months after a complete HepB series remain protected, even if anti-HBs levels decline to <10 mIU/mL beyond that time, presumably because of persistent cellular immunity (7).
HepB vaccine doses subsequent to the monovalent HepB vaccine birth dose are administered as either monovalent or combination vaccine (1). Before December 31, 2014, two combination vaccines containing recombinant HBsAg were available in the United States for infants aged ≥6 weeks: 1) Hib/HepB vaccine (Comvax, Merck and Co, Inc.) and 2) DTaP-HepB-IPV vaccine (Pediarix, GlaxoSmithKline Biologicals) (1). Hib/HepB vaccine (Comvax) production has been discontinued. For infants born to HBsAg-positive mothers, the final dose of the HepB vaccine series is administered at age 6 months when monovalent or DTaP-HepB-IPV vaccine (Pediarix) is used to complete the series (1). When Hib/HepB vaccine (Comvax) was used to complete the series, the final dose was administered at age 12–15 months (1).
The optimal timing for PVST to detect a vaccine response generally is 1–2 months after the final dose of the HepB vaccine series (1). Results of tests for HBsAg can be transiently positive for 1–18 days after vaccination. PVST should be performed no earlier than age 9 months to avoid detection of passive anti-HBs from hepatitis B immune globulin administered at birth and to maximize the likelihood of detecting late HBV infection (1).
In developing this update to shorten the interval for PVST to age 9–12 months, CDC subject matter experts reviewed the shortened interval with professionals from academia and public health and considered existing (8) and new data (9) on anti-HBs levels among infants born to HBsAg-positive mothers. Among 348 infants born to HBsAg-positive mothers enrolled in the Enhanced Surveillance: Perinatal Hepatitis B Program in Dallas County, Texas, PVST performed at 4–7 months and 8–11 months after the final vaccine dose was associated with lower anti-HBs levels (odds ratios = 1.8 and 4.4, respectively; 95% confidence intervals = 1.2–2.8 and 1.3–14.5, respectively), when compared with PVST 1–3 months after vaccination (8). In a study analyzing data collected from 8,105 HBsAg-negative infants born to HBsAg-positive mothers enrolled in the Enhanced Perinatal Hepatitis B Prevention Program in five U.S. jurisdictions during 2008–2013, the percentages of tested infants with anti-HBs levels <10 mIU/mL at ages 1–2 months, 3–4 months, 5–6 months, 7–8 months, 9–10 months, 11–12 months, 13–14 months and 15–16 months after the final HepB vaccine dose were 2% (31 of 1573), 2.8% (86 of 3,110), 5.1% (91 of 1,769), 7.8% (55 of 705), 9.3% (43 of 463), 13.3% (32 of 240), 16.3% (21 of 129) and 21.6% (25 of 116), respectively (p<0.01, Mantel-Haenszel chi-square) (Figure) (9). Nearly one fourth (22.3%) of infants underwent PVST >6 months after the final vaccine dose (9).
For most infants born to HBsAg-positive mothers, PVST at age 9–12 months provides opportunities for testing at two well-child visits (i.e., 9-month and 12-month visits). An added benefit of a shortened interval to PVST is a reduction in the period during which nonresponders are at risk for transmission from close contacts with HBV infection. Earlier PVST enables prompt revaccination of those infants needing revaccination with a second 3-dose HepB vaccine series to attain protective anti-HBs levels. A shortened interval might also increase adherence with recommendations for timely completion of PVST and conserve public health resources involved in providing case management services (1).
In light of the lower measured anti-HBs levels (but continued protection) with increasing time following vaccination, PVST occurring at increasing intervals after the final vaccine dose could result in misclassification of some infants as vaccine nonresponders and therefore lead to unnecessary revaccination (9). Because nonresponding infants receive a second 3-dose HepB vaccine series followed by retesting of anti-HBs, testing soon after completion of the initial vaccine series reduces the possibility for misclassification and unnecessary revaccination (9). The PVST interval after the final dose in the primary HepB series no longer needs to extend to 18 months to accommodate infants completing their vaccination series with Hib/HepB vaccine (Comvax). Although no data are available, CDC subject matter experts postulate that harm will not occur as a result of a shortened PVST interval. PVST (consisting of HBsAg and anti-HBs) for infants born to HBsAg-positive mothers should be performed at age 9–12 months, or 1–2 months after the final dose of the HepB vaccine series if completion of the series is delayed.
1Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; 2Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; 3Boston University School of Public Health and School of Medicine, Massachusetts.
Corresponding author: Sarah Schillie, sschillie@cdc.gov, 404-718-8608.
References
- Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(No. RR-16).
- Smith EA, Jacques-Carroll L, Walker TY, Sirotkin B, Murphy TV. The national Perinatal Hepatitis B Prevention Program, 1994–2008. Pediatrics 2012;129:609–16.
- Kubo A, Shlager L, Marks AR, et al. Prevention of vertical transmission of hepatitis B: an observational study. Ann Intern Med 2014;160:828–35.
- CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008–2011. MMWR Morb Mortal Wkly Rep 2012;61:768–71.
- Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine 2013;31:2506–16.
- Schillie S, Murphy TV, Sawyer M, et al. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013;62(No. RR-10).
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011;53:68–75.
- Euler GL, Copeland JR, Rangel MC, Williams WW. Antibody response to postexposure prophylaxis in infants born to hepatitis B surface antigen-positive women. Pediatr Infect Dis J 2003;22:123–9.
- Ko SC, Schillie SF, Walker T, et al. Hepatitis B vaccine response among infants born to hepatitis B surface antigen-positive women. Vaccine 2014;32:2127–33.
Summary
What recommendations are being reviewed?
Postvaccination serologic testing (PVST) is recommended for infants born to hepatitis B surface antigen (HBsAg)-positive mothers at age 9–18 months. PVST consists of testing for HBsAg and antibody to HBsAg (anti-HBs).
Why are the recommendations being reviewed now?
With the discontinuation of Hib/HepB vaccine (Comvax), the hepatitis B vaccine series for infants born to HBsAg-positive mothers will usually be completed at age 6 months, allowing PVST at age 9–12 months. New data from the Enhanced Perinatal Hepatitis B Prevention Program are available that show that lower detectable levels of anti-HBs were associated with increased intervals between receipt of the last vaccine dose and PVST.
What is the new recommendation?
Considering the lower levels of anti-HBs with increasing time since completing vaccination and the extent of unnecessary revaccination, PVST, consisting of testing for HBsAg and anti-HBs, should be ordered at age 9–12 months (or 1–2 months after the final dose of the vaccine series, if delayed) for infants born to HBsAg-positive mothers.
FIGURE. Proportion of infants with anti-HBs ≥10 mIU/mL with increasing interval from final vaccine dose*
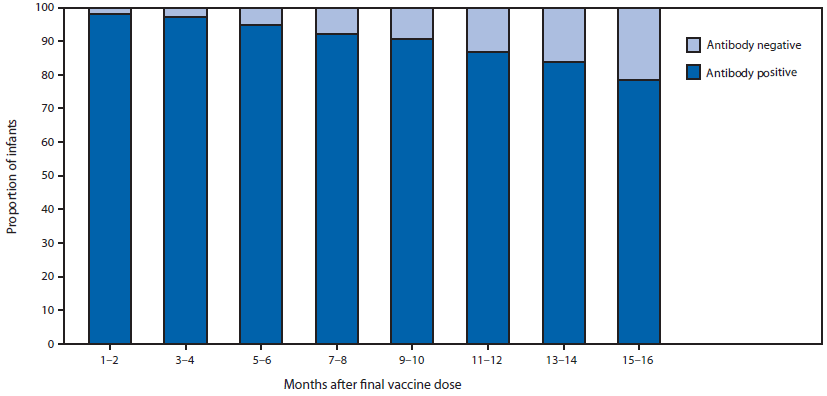
Source: Reprinted with permission of publisher from: Ko SC, Schillie SF, Walker T, et al. Hepatitis B vaccine response among infants born to hepatitis B surface antigen-positive women. Vaccine 2014;32:2127–33.
* p<0.01, Mantel-Haenszel chi square.
Alternate Text: The figure above is a bar chart showing the proportion of infants with anti-HBs ≥10 mIU/mL with increasing interval from final vaccine dose.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


