Global Progress Toward Rubella and Congenital Rubella Syndrome Control and Elimination — 2000–2014
, MD1; , MD1; , PhD2; , MSc2; , MBChB2
Rubella virus usually causes a mild fever and rash in children and adults. However, infection during pregnancy, especially during the first trimester, can result in miscarriage, fetal death, stillbirth, or a constellation of congenital malformations known as congenital rubella syndrome (CRS). In 2011, the World Health Organization (WHO) updated guidance on the preferred strategy for introduction of rubella-containing vaccine (RCV) into national routine immunization schedules, including an initial vaccination campaign usually targeting children aged 9 months–15 years (1). The Global Vaccine Action Plan endorsed by the World Health Assembly in 2012 and the Global Measles and Rubella Strategic Plan (2012–2020) published by Measles and Rubella Initiative partners in 2012 both include goals to eliminate rubella and CRS in at least two WHO regions by 2015, and at least five WHO regions by 2020 (2,3). This report updates a previous report (4) and summarizes global progress toward rubella and CRS control and elimination during 2000–2014. As of December 2014, RCV had been introduced in 140 (72%) countries, an increase from 99 (51%) countries in 2000 (for this report, WHO member states are referred to as countries). Reported rubella cases declined 95%, from 670,894 cases in 102 countries in 2000 to 33,068 cases in 162 countries in 2014, although reporting is inconsistent. To achieve the 2020 Global Vaccine Action Plan rubella and CRS elimination goals, RCV introduction needs to continue as country criteria indicating readiness are met, and rubella and CRS surveillance need to be strengthened to ensure that progress toward elimination can be measured.
Immunization Activities
Data were obtained from the WHO and United Nations Children's Fund (UNICEF) Joint Reporting Form, which is used to collect information from countries on vaccination campaigns, vaccination schedules, and number of doses of RCV administered through routine immunization services, and from other WHO monitoring data (5). Data from 2000–2014 were analyzed to assess changes in rubella and CRS control activities.
According to data from 2014 (last updated in July 2015), RCV had been introduced in 140 (72%) of the 194 WHO countries, a 39% increase compared with the 99 (51%) countries that had introduced RCV in 2000, and a 6% increase over the 132 (68%) countries that had introduced RCV in 2012. RCV was introduced in seven (15%) countries in the African Region, 35 (100%) countries in the American Region, 15 (71%) countries in the Eastern Mediterranean Region, 53 (100%) countries in the European Region, six (55%) countries in the South-East Asia Region, and 24 (89%) countries in the Western Pacific Region (Table 1). The proportion of infants globally who received an RCV dose was 22% in 2000 and 46% in 2014.*
During 2000–2012, RCV was introduced into national immunization schedules in 33 countries. Among the 62 countries where RCV was not introduced by December 2012, RCV was introduced in eight countries during 2013–2014. During that period, 49 countries where RCV had not yet been introduced were eligible for Gavi Alliance immunization support,† and 13 countries were not eligible for Gavi support; RCV was introduced in seven of the Gavi-eligible countries during this period (Figure) (Table 2). A wide age-range campaign was part of the implementation for introduction in all eight countries (Table 2).
Among 140 countries where RCV has been introduced, the first RCV dose was provided with the first routine dose of measles-containing vaccine (MCV) in 137 (98%) countries. In 2014, the first RCV dose was administered at age 8–11 months in 15 (11%) countries, at age 12–18 months in 120 (85%) countries, and at age >18 months in five (4%) countries. RCV is provided in combination with measles vaccine only in 22 (19%) countries and in combination with measles and mumps vaccine (with or without varicella vaccine) in 117 (84%) countries; in one country, monovalent rubella vaccine is administered simultaneously with measles-mumps vaccine.
Surveillance Activities
Rubella and CRS surveillance are necessary to evaluate the disease burden before and after RCV introduction, to identify pregnant women infected with rubella virus who require follow-up to assess pregnancy outcomes, and to identify, diagnose, and medically manage CRS-affected infants. Countries report surveillance data, including cases of rubella and CRS, using standard case definitions§ and the WHO-UNICEF Joint Reporting Form (6); for this report, data from 2000–2014 were analyzed. The number of countries reporting rubella cases increased from 102 in 2000 to 172 in 2012 and then declined to 161 in 2014 (Table 1); the decline in countries reporting rubella cases from 2012 to 2014 was greatest in the European Region (47 to 37 countries), and the Western Pacific Region (23 to 16 countries). The number of countries reporting CRS cases increased from 75 in 2000 to 130 in 2012 and decreased to 114 in 2014. Of the 24 countries reporting rubella cases in 2012 but not in 2014, 21 are in regions with elimination goals. Of the 33 countries reporting CRS cases in 2012, but not in 2014, 18 are in regions with elimination goals. Of 140 countries where RCV was introduced by December 2014, 125 (89%) reported rubella cases, and 111 (79%) reported CRS surveillance results (either number of cases or zero reports) in 2014.
In 2014, a total of 33,068 rubella cases were reported to WHO from 161 countries, a 95% decrease from the 670,894 rubella cases reported in 2000 from 102 countries (Table 1). In the Americas, the last endemic rubella and CRS cases were reported in 2009, and the region was declared free of endemic rubella virus transmission in April 2015. The number of rubella cases decreased in the European Region from 621,039 in 41 countries in 2000 to 640 cases in 37 countries in 2014. In the Western Pacific Region, the number of cases increased from 5,475 in 15 countries in 2000 to 44,275 cases in 23 countries in 2012, before decreasing with improved reporting to 12,814 in 16 countries in 2014 with the end of a large outbreak in Japan. The number of rubella cases reported during 2000–2014 increased in the African region (from 865 cases in seven countries to 7,402 cases in 44 countries) and South-East Asia region (1,165 cases in 3 countries to 9,263 cases in 10 countries)(Table 1).
Discussion
Since the last progress report in 2012, which described the beginning of a new phase of accelerated rubella control and CRS prevention with updated WHO RCV introduction guidance (1) and Gavi Alliance funding for rubella vaccine introduction, countries have begun to increase introduction of RCV into immunization schedules, although greater efforts are needed to improve monitoring of elimination. RCV has been introduced into national immunization schedules in 41 countries since 2000, including eight countries with introduction during 2013–2014. RCV needs to be introduced in countries as WHO criteria (1) for introduction are met. Gavi Alliance funding support is instrumental in ensuring continued RCV introduction. Forty-two (78%) of the 54 countries where RCV is not in the national immunization schedule are eligible for Gavi Alliance funding support. Leadership, coordination, technical expertise, and financial resources provided by the Measles and Rubella Initiative partners also have provided critical support to accelerate RCV introduction and increase RCV coverage.
Recent and future RCV introductions provide an opportunity and motivation to establish and achieve regional rubella and CRS elimination goals. During 2012–2014, a rubella elimination goal was established in the Western Pacific Region, and a rubella and CRS control goal was established in the South-East Asia Region as an initial step toward establishing an elimination goal (7). The interruption of rubella virus transmission announced this year in the Region of the Americas provides evidence that rubella and CRS elimination can be achieved by introduction of rubella vaccine into routine infant vaccination schedules accompanied by a wide age range (i.e., infants to 15 years, and in some cases up to 39 years) immunization campaign. However, key challenges to achieving rubella elimination goals include civil unrest (Eastern Mediterranean Region), weak health care delivery systems with low routine vaccination coverage (African and South-East Asia Region), and vaccination hesitancy (European Region).
High-quality rubella and CRS surveillance is needed to monitor the impact of rubella vaccination programs, and verify achievement of rubella and CRS elimination goals. Guidelines for rubella and CRS surveillance (1), and a framework for verifying elimination of rubella and CRS have been published (8). Countries need to institute CRS surveillance and report both rubella and CRS cases in order to monitor the impact of the vaccination program on the epidemiology of both rubella and CRS. This need for reporting is especially true of countries with elimination goals and is necessary for the elimination verification process; the recent decrease in the number of countries reporting their rubella and CRS cases is particularly concerning regarding the attention given to monitoring elimination goals.
A vaccine delivery system that achieves and maintains high coverage with both RCV and MCV and integrated measles and rubella surveillance is a foundation for continued progress toward rubella and CRS control and elimination. Implementation of additional global WHO recommendations regarding the use of RCV can help countries that have introduced RCV optimize their use of the vaccine (9). The recommendations include the use of RCV when measles vaccine is administered in routine immunization services for vaccination of health workers; use of RCV for all measles campaigns; and a review of measles and rubella epidemiology to determine target age ranges. In addition, the recommendations improve monitoring of activities reflecting RCV use, including joint measles and rubella vaccination coverage surveys and regular analysis of measles and rubella surveillance data. Such analyses are needed to identify geographic areas and population groups with low immunity who are at greater risk for outbreaks, so that vaccination campaigns and other prevention and control measures can be directed toward them.
Immunization and surveillance activities are the foundation for rubella control and CRS prevention and reaching the Global Vaccine Action Plan goals. To reach regional elimination goals, countries at all levels need to follow the WHO recommendations for introducing RCV, strengthening routine immunization services, improving surveillance, and accelerating coordinated rubella control and elimination efforts.
1Global Immunization Division, Center for Global Health, CDC; 2Department of Immunization, Vaccines, and Biologicals, World Health Organization.
Corresponding author: Gavin B. Grant, gbgrant@cdc.gov.
References
- World Health Organization. Rubella vaccines. WHO position paper. Geneva, Switzerland: World Health Organization; 2011. Available at http://www.who.int/wer/2011/wer8629.pdf.
- World Health Organization. Global vaccine action plan. Geneva, Switzerland: World Health Organization; 2012. Available at http://apps.who.int/iris/bitstream/10665/78141/1/9789241504980_eng.pdf?ua=1.
- World Health Organization. Global measles and rubella strategic plan: 2012–2020. Geneva, Switzerland: World Health Organization; 2012. Available at http://reliefweb.int/sites/reliefweb.int/files/resources/Measles_Rubella_StrategicPlan_2012_2020.pdf.
- CDC. Rubella and congenital rubella syndrome control and elimination—global progress, 2000–2012. MMWR Morb Mortal Wkly Rep 2013;62:983–6.
- World Health Organization. Immunization, vaccines and biologicals: data, statistics and graphics. Geneva, Switzerland: World Health Organization; 2015. Available at http://www.who.int/immunization/monitoring_surveillance/data/en/.
- World Health Organization. WHO-recommended standards for surveillance of selected vaccine-preventable diseases. Geneva, Switzerland: World Health Organization; 2003. Available at http://apps.who.int/iris/bitstream/10665/68334/1/WHO_V-B_03.01_eng.pdf?ua=1.
- Regional Committee for South-East Asia Region. Measles elimination and rubella control. Available at http://www.searo.who.int/mediacentre/events/governance/rc/66/9.pdf.
- World Health Organization. Framework for verifying elimination of measles and rubella. Wkly Epidemiol Rec 2013;88:89–99.
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunizations, October 2014—conclusions and recommendations. Wkly Epidemiol Rec 2014;89:561–76.
* Estimate is based on the 2014 World Health Organization–United Nations Children's Fund joint estimate, adjusted for the 2014 United Nations Development Programme estimate of surviving infants per region.
† The Gavi Alliance provides support for low-income countries to introduce RCV into the national routine infant immunization schedule and to conduct vaccination campaigns for children aged 9 months–15 years if criteria indicating readiness for introduction are met.
§ Congenital rubella syndrome (CRS) is laboratory-confirmed in an infant who has a positive blood test for rubella-specific immunoglobulin M or, where available, detection of rubella virus in specimens from pharynx and urine. CRS is clinically confirmed in an infant if a qualified physician detects at least two of the following complications in the infant: cataracts, congenital glaucoma, congenital heart disease, loss of hearing, or pigmentary retinopathy, or one of those complications plus one of the following: purpura, splenomegaly, microcephaly, mental retardation, meningoencephalitis, radiolucent bone disease, or jaundice that begins within 24 hours after birth.
Summary
What is already known on this topic?
In 2011, the World Health Organization (WHO) updated guidance on the preferred strategy for introduction of rubella-containing vaccine into national routine immunization schedules, including an initial vaccination campaign for children aged 9 months–15 years. Global immunization targets five of six WHO regions to eliminate rubella and congenital rubella syndrome (CRS) by 2020.
What is added by this report?
During 2000–2014, reported rubella cases declined 95%, from 670,894 cases reported in 2000 in 102 countries to 33,068 cases reported in 2014 in 162 countries. As of December 2014, countries of four WHO regions had met rubella control and elimination goals (Western Pacific Region, Region of the Americas, European Region, and South-East Asia Region).
What are the implications for public health practice?
To achieve rubella elimination and control goals, a strong commitment is required at national and subnational levels in all countries to introduce rubella-containing vaccine, achieve high rubella vaccine coverage in routine immunization services, and conduct high-quality rubella and CRS surveillance.
FIGURE. Countries that have already introduced rubella-containing vaccine (RCV) and countries that have not introduced RCV, by eligibility status for Gavi Alliance support* — World Health Organization, 2015
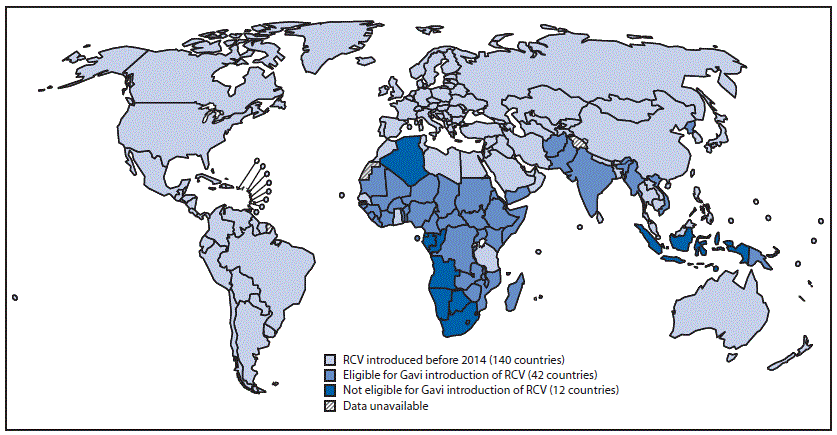
* The Gavi Alliance provides support for low-income countries, including support to introduce RCV into the national routine infant immunization schedule and to conduct vaccination campaigns for children aged 9 months–15 years if criteria indicating readiness for introduction are met.
Alternate Text: The figure above is a map of the world showing countries that have already introduced rubella-containing vaccine and countries that have not, by eligibility status for Gavi Alliance support, in 2015.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


