Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Multistate Outbreak of Salmonella Serotype Bovismorbificans Infections Associated with Hummus and Tahini — United States, 2011
On September 27, 2011, three clinical isolates of Salmonella enterica serotype Bovismorbificans with indistinguishable pulsed-field gel electrophoresis (PFGE) patterns were identified by the District of Columbia Public Health Laboratory (PHL). Human infection with S. Bovismorbificans is rare in the United States. Through query of PulseNet, the national molecular subtyping network for foodborne disease surveillance, six additional cases with indistinguishable PFGE patterns were identified in three states (Maryland, Michigan, and Virginia) during the prior 60 days. All nine patients had eaten at restaurants in the District of Columbia (DC) or northern Virginia <2 weeks before illness onset. This report summarizes the investigation led by the DC Department of Health (DOH), in which 23 cases of S. Bovismorbificans infections were identified among persons from seven states and DC, with illness onset during August 19–November 21, 2011. On May 30, 2012, traceback indicated that contaminated tahini (sesame seed paste) used in hummus prepared at a Mediterranean-style restaurant in DC was a plausible source of Salmonella infections. DOH restricted the sale of hummus and prohibited the use of hummus ingredients in other food items at implicated restaurants to prevent further illness. This investigation also illustrates challenges associated with ingredient-driven outbreaks and the value of PulseNet for identifying clusters of cases that are geographically dispersed.
Epidemiologic Investigation
PulseNet* was used throughout the investigation to monitor the outbreak PFGE pattern, a pattern new to PulseNet. Cases were defined as laboratory-confirmed S. Bovismorbificans infection with the PFGE pattern of the outbreak strain in a person anywhere in the United States with illness onset during August 2011–January 23, 2012.
State and local health departments, CDC, and the Food and Drug Administration (FDA) collaboratively investigated the outbreak. A total of 23 culture-confirmed cases with PFGE patterns indistinguishable from the outbreak strain were identified. Illness onsets occurred during August 19–November 21, 2011, and peaked during September 8–October 12 (Figure 1). The majority of cases were identified in the mid-Atlantic region of the United States: DC (eight), Maryland (seven), and Virginia (three). One case per state was identified in California, Delaware, Michigan, New Hampshire, and New Jersey (Figure 2). State health department staff members conducted open-ended interviews and obtained information about 22 patients. These interviews indicated that most of the patients had eaten at restaurants in the DC metropolitan area. A structured questionnaire was used to reinterview eight of the patients in three states and DC to assess exposures to approximately 500 types of restaurants, foods, and animals during the week before illness onset. Mediterranean-style food and restaurants commonly were mentioned. Three patients were reinterviewed with a targeted questionnaire that focused on Mediterranean-style restaurant and food exposures. Interview methodologies varied during the course of the investigation and among health departments (e.g., exposure data are missing for some patients who were not asked or did not report exposure to specific foods).
Among the 22 patients with exposure information, 20 (91%) reported eating at a restaurant in the DC metropolitan area (Table). Among 15 patients asked about Mediterranean-style restaurant exposure, 14 (93%) indicated that they had eaten at a Mediterranean-style restaurant in the DC metropolitan area, including six restaurants in DC and two in northern Virginia. Through either open-ended or targeted interviews, nine (69%) of 13 patients reported eating at restaurants A, B, or C, and three of eight reported eating at DC restaurants, before symptom onset. Sixteen (84%) of 19 patients reported eating Mediterranean-style food; 10 (67%) of 15 patients reported eating hummus. Other commonly reported foods eaten were lettuce (11 of 14; 79%), chicken (11 of 15; 73%), tomato (11 of 15; 73%), and cucumber (nine of 11; 82%).
Median age of the 23 patients was 27 years (range: 20–87 years); 13 (57%) were female. One patient was asymptomatic. Among the 22 symptomatic patients, 21 (96%) had one or more symptoms consistent with Salmonella infection: 21 reported diarrhea (defined as three or more loose stools during 24 hours), 16 (73%) reported abdominal cramps, 16 (73%) reported nausea, 15 (68%) reported fatigue, 13 (59%) reported fever, seven (32%) reported bloody diarrhea, and four (18%) reported vomiting. All 23 patients received outpatient medical care. No hospitalizations or deaths were reported.
Environmental Investigation
During November 7–8, DOH visited restaurants A and B to collect food samples; 15 prepared foods, including hummus and hummus ingredients (e.g., tahini), were collected for laboratory testing. Investigators learned that restaurants A, B, and C had the same owner. Restaurant A performed all food preparation for restaurants B and C; specific food items, including hummus and tzatziki sauce, were prepared at restaurant A and delivered to restaurants B and C for sale to customers.
DC PHL isolated S. Bovismorbificans from the hummus sample collected from restaurant A. The two enzymes (Xba1 and Bln1) PFGE pattern of this isolate was indistinguishable from that of the cases (patterns TDFX.0108 and TDFA26.0006, respectively). All other food items tested negative for Salmonella. An additional 18 samples of ingredients and prepared foods were collected during inspections of restaurants A, B, and C during November 16–17. S. Bovismorbificans with a PFGE pattern indistinguishable from the outbreak strain was isolated from one sample of hummus collected from each of restaurants A and C; all other food samples tested negative for Salmonella. DOH also cited restaurants A, B, and C for multiple food safety violations, including inadequate food temperature control, insufficient hand washing, and the presence of insects and other pests.
On November 18, DOH issued an embargo on hummus and hummus ingredients (tahini, chickpeas, garlic, lemon juice, salt, and olive oil) from restaurant A. DOH ordered the restaurants' owner to restrict the preparation of hummus and use of hummus ingredients in other foods (e.g., tahini used in falafel), the delivery of hummus to restaurants B and C, and the sale of hummus at all three restaurants until the source of contamination was identified. On November 14, the Virginia Department of Health visited the two northern Virginia Mediterranean-style restaurants specifically mentioned by patients to collect food samples and obtain food supplier information. Tracebacks performed for restaurants A and B and one Virginia restaurant revealed multiple food items purchased from a common distributor in northern Virginia that provided bulk food items for restaurants. The Virginia Department of Agriculture and Consumer Services visited the warehouse distributor to obtain lot numbers of hummus ingredients (tahini and chickpeas) and to collect food samples. All food samples collected from the northern Virginia restaurants and from the distributor tested negative for Salmonella.
On November 30, FDA and PHL conducted environmental sampling at restaurant A. Seven food handlers, including five kitchen staff members and two delivery drivers, worked at restaurant A; none reported gastrointestinal symptoms during the prior month. On December 7, stool specimens were collected from all five kitchen staff members, but not the two drivers who delivered prepared food from restaurant A to restaurants B and C; the drivers reportedly had limited contact with foods. Salmonella was not isolated from any of the restaurant A environmental samples or food handler stool specimens.
DOH lifted the embargo on hummus and hummus ingredients at restaurants A, B, and C during February 2012 because no additional cases had been reported and food safety violations had been corrected. Additional food samples were collected, including hummus; no Salmonella was isolated.
On May 30, 2012, traceback by FDA suggested that tahini (sesame seed paste) used in hummus was a plausible source for Salmonella infections. The traceback revealed tahini used at the different restaurants in the DC metropolitan area came from a common foreign manufacturer from Lebanon associated with recent Salmonella outbreaks in Canada. FDA issued a mandate that all products imported from this manufacturer undergo Salmonella testing before entry into the United States. At the time of this report, FDA recommended coordination with Canadian officials to conduct a foreign inspection of the tahini manufacturing plant.
Reported by
Reginald Blackwell, Morris Blaylock, PhD, Sosina Merid, District of Columbia Public Health Laboratory; John Davies-Cole, PhD, Arian Gibson, Daniella Herdman, MPH, Robert Sudler, MS, District of Columbia Dept of Health. Hannah Lee, MPH, Maryland Dept of Health and Mental Hygiene. Kate Corvese, MPH, Seth Levine, MPH, Virginia Dept of Health. Karen Blickenstaff, MS, Food and Drug Administration. Joanna Gaines, PhD, Leslie Hausman, MPH, Div of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases; Tiana A. Garrett, PhD, EIS Officer, CDC. Corresponding contributor: Tiana A. Garrett, vid6@cdc.gov, 202-442-9065.
Editorial Note
Since 2001, S. Bovismorbificans has been identified in only five other foodborne outbreaks in the United States (1). These outbreaks have been linked to alfalfa sprouts, homemade cheese, pasta salad, striped bass, and one unknown source (1). During 2009, S. Bovismorbificans infections accounted for 62 (0.15%) of 40,828 Salmonella cases reported nationally (2). During the previous decade, S. Bovismorbificans outbreaks in Finland, Germany, and Australia have been linked to vegetables (3–5).
During this multistate outbreak, 23 cases of S. Bovismorbificans infection with matching PFGE patterns were identified. Tahini used in hummus prepared at restaurant A, a Mediterranean-style restaurant in DC, was a plausible source of S. Bovismorbificans infection for at least 10 of the patients. The sale of this hummus by two additional DC restaurants (restaurants B and C) broadened the opportunity for exposure.
This outbreak illustrates the challenges associated with ingredient-driven outbreaks and the importance of PulseNet in reporting cases in different states. Only 10 of 15 patients with information about hummus exposure reported eating hummus before illness onset. However, patients who did not report eating hummus might have eaten Mediterranean-style foods prepared with tahini, even if they did not recall eating Mediterranean-style food. Other commonly reported foods, particularly lettuce, chicken, tomato, and cucumber, were consumed before illness onset, but consumption was not limited to Mediterranean-style restaurants.
This is the first report of S. Bovismorbificans associated with tahini in the United States. Sesame seeds used to make tahini are high in fats, similar to peanuts. Salmonella species can survive for long periods in high fat foods (e.g., peanut butter), and if seeds or nuts are improperly processed (e.g., roasted at inadequate temperatures). Recalls of tahini for possible Salmonella contamination have occurred in the United States, but an outbreak as a result of tahini consumption has never been reported (6,7). The brand of tahini implicated in this outbreak previously was recalled in Canada for contamination with Salmonella Cubana (September 2011) (8) and Salmonella Seftenberg (February 2012) (9). How this tahini brand was contaminated with three different Salmonella serotypes requires further investigation.
The findings in this report are subject to at least two limitations. For this study, interviewing methodology across jurisdictions was inconsistent. Not all patients were interviewed with the structured or targeted questionnaire, and several patients were not available for follow-up. Second, Salmonella was not detected in the samples of tahini tested, but was determined through traceback to be a plausible source.
Determining the source of any ingredient-driven outbreak is challenging. Public health officials should 1) routinely perform PFGE and report cases to PulseNet to identify cases outside of a geographic cluster, 2) identify frequently reported restaurants, or restaurant clusters, to obtain information on specific food items that are served at more than one restaurant, and 3) inquire about the shelf-life and turnover rates of products and ingredients used in restaurants to help to determine the product's exposure time window during the outbreak. In addition, public health officials and consumers should be informed that products made from imported sesame seed paste have been associated with Salmonella outbreaks and should be considered as possible sources for foodborne illness in the United States.
Acknowledgments
Alpha Diallo, PhD, District of Columbia Public Health Laboratory; Ivory Cooper, Denise Tyree, MS, District of Columbia Dept of Health. Jeffrey Higa, MPH, Akiko Kimura, MD, California Dept of Public Health. Stephanie Belinkse, Delaware Dept of Health and Social Svcs. Alvina Chu, MHS, Maryland Dept of Health and Mental Hygiene. Sally A. Bidol, MPH, Susan Bohms, MPH, Katherine Arends, MPH, Michigan Dept of Community Health. Allison Wellman, Virginia Dept of Health; Ato Atughonu, Christy Brennan, Matthew Ettinger, Virginia Dept of Agriculture and Consumer Svcs. Elizabeth Daly, MPH, New Hampshire Dept of Health and Human Svcs. Janice Gironda, New Providence Health Dept. Thomas Hill, MPH, Susan Lance, DVM, PhD, Christine Smith, Food and Drug Administration. Sheryl Lyss, MD, Scientific Education and Professional Development Program Office, CDC.
References
- CDC. National Foodborne Disease Outbreak Surveillance System. Atlanta, GA: US Department of Health and Human Services, CDC. Available at http://wwwn.cdc.gov/foodborneoutbreaks/default.aspx. Accessed May 3, 2012.
- CDC. National Salmonella surveillance annual summary 2009. Table 3. Atlanta, GA: US Department of Health and Human Services, CDC, 2009. Available at http://www.cdc.gov/ncezid/dfwed/pdfs/salmonellaannualsummarytables2009.pdf. Accessed May 3, 2012.
- Rimhanen-Finn R, Niskanen T, Lienemann T, et al. A nationwide outbreak of Salmonella Bovismorbificans associated with sprouted alfalfa seeds in Finland, 2009. Zoonoses Public Health 2011;58:589–96.
- Gilsdorf A, Jansen A, Alpers K, et al. A nationwide outbreak of Salmonella Bovismorbificans PT24, Germany, December 2004–March 2005. Euro Surveill 2005;10:pii=2667.
- Stafford RJ, McCall BJ, Neill AS, et al. A statewide outbreak of Salmonella Bovismorbificans phage type 32 infection in Queensland. Commun Dis Intell 2002;26:568–73.
- Food and Drug Administration. Premier Organics issues nationwide recall for Artisana raw tahini because of possible health risk. September 4, 2009. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2009. Available at http://www.fda.gov/safety/recalls/ucm181619.htm. Accessed May 3, 2012.
- Food and Drug Administration. nSpired Natural Foods issues nationwide recall of Maranatha sesame tahini because of possible health risk. May 25, 2007. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2007. Available at http://www.fda.gov/Safety/Recalls/ArchiveRecalls/2007/ucm112209.htm. Accessed May 3, 2012.
- Canadian Food Inspection Agency. Health hazard alert: certain Alkanater brand tahina may contain Salmonella bacteria. Ottawa, Ontario, Canada: Canadian Food Inspection Agency; 2011. Available at http://www.inspection.gc.ca/english/corpaffr/recarapp/2011/20110923e.shtml. Accessed November 15, 2012.
- News Desk. Salmonella concern prompts tahini recall in Canada. Food Safety News. February 2, 2012. Available at http://www.foodsafetynews.com/2012/02/tahini-recalled-in-canada-for-salmonella. Accessed May 3, 2012.
* Additional information available at http://www.cdc.gov/pulsenet.
What is already known on this topic?
Salmonella is the most commonly reported cause of bacterial enteric infections in the United States, but determining the cause of an ingredient-driven outbreak is challenging.
What is added by this report?
In September 2011, three clinical isolates of Salmonella enterica serotype Bovismorbificans with indistinguishable pulsed-field gel electrophoresis (PFGE) patterns were identified in the District of Columbia (DC). Through PulseNet, the national molecular subtyping network for foodborne disease surveillance, six additional recent cases with indistinguishable PFGE patterns were identified. All nine patients had eaten at one of three restaurants in DC. Investigation led to 23 cases with the same PFGE pattern in seven states and DC. Imported tahini (sesame seed paste) used in hummus prepared at a Mediterranean-style restaurant in DC was a plausible source of the infections.
What are the implications for public health practice?
Public health officials and consumers should know that products made from imported sesame seed paste have been associated with Salmonella outbreaks and should be considered as possible sources for foodborne illness in the United States. For ingredient-driven outbreaks, public health officials should use PulseNet to link geographically dispersed cases and attempt to identify clusters to obtain information on specific exposures common to patients.
FIGURE 1. Number of culture-confirmed cases (n = 23) with infections of outbreak strain of Salmonella enterica serotype Bovismorbificans, by date of illness onset or stool culture* — United States, August 19–November 21, 2011
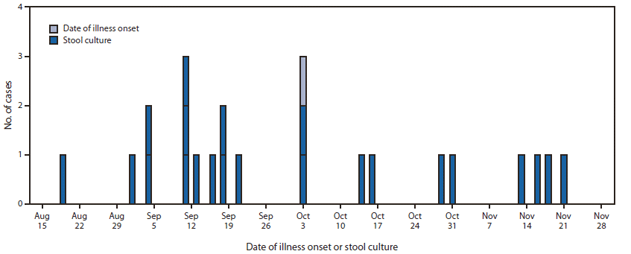
* For one asymptomatic case.
Alternate Text: The figure above shows the number of culture-confirmed cases (n = 23) with infections of outbreak strain of Salmonella enterica serotype Bovismorbificans, by date of illness onset or stool culture in the United States, during August 19-November 21, 2011. A total of 23 culture-confirmed cases with PFGE patterns indistinguishable from the outbreak strain were identi¬fied. Illness onsets occurred during August 19-November 21, 2011, and peaked during September 8-October 12.
FIGURE 2. Number and location of Salmonella enterica serotype Bovismorbificans case detections — United States, August–December 2011*
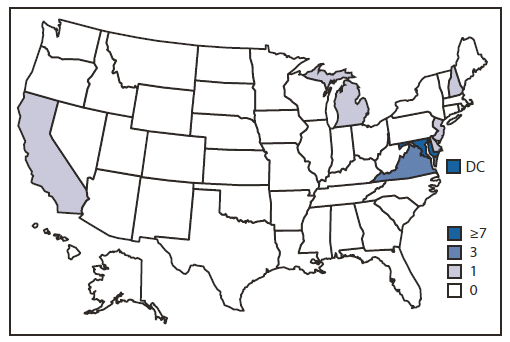
* A total of 23 persons infected with the outbreak strain of S. Bovismorbificans were reported from seven states and the District of Columbia, including eight in the District of Columbia, seven in Maryland, three in Virginia, and one each in Delaware, California, Michigan, New Hampshire, and New Jersey.
Alternate Text: The figure above shows the number and location of Salmonella enterica serotype Bovismorbificans case detections in the United States, during August- December 2011. The majority of cases were identified in the mid-Atlantic region of the United States: District of Columbia (eight), Maryland (seven), and Virginia (three). One case per state was identified in California, Delaware, Michigan, New Hampshire, and New Jersey.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


