Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Progress Toward Poliomyelitis Eradication — Chad, January 2011–August 2012
In 1988, the World Health Assembly launched the Global Polio Eradication Initiative (GPEI) to interrupt transmission of wild poliovirus (WPV). By January 2012, indigenous WPV transmission had been interrupted in all countries except Afghanistan, Pakistan, and Nigeria (1). However, importation of WPV caused outbreaks in 29 and reestablished transmission in four, previously polio-free African countries during 2003–2011 (2,3). Transmission after WPV importation is considered reestablished when it continues for ≥12 months (2,3); in Chad, transmissions of WPV type 3 (WPV3) and WPV type 1 (WPV1) were reestablished. WPV3 was imported from Nigeria in 2007 and continued to circulate (2,3); the latest reported WPV3 case occurred on March 10, 2011. Transmission of WPV1 continued after a WPV1 case was imported from Nigeria in September 2010; the latest reported WPV1 occurred on June 14, 2012 (2). This report updates previous reports (1–3) and describes polio eradication activities and progress in Chad during January 2011–August 2012, as of October 2, 2012. Five WPV1 cases were reported during January–August 2012, compared with 111 WPV1 cases and three WPV3 cases reported during the same period in 2011. Five circulating type 2 vaccine-derived poliovirus (cVDPV2) cases occurred during July–August 2012. Current progress suggests that Chad could interrupt reestablished WPV transmission in 2012, although limitations in surveillance hamper the ability to detect ongoing transmission. Furthermore, with ongoing endemic WPV transmission in Nigeria (1,2), Chad remains at risk for new WPV importations. Efforts to strengthen surveillance and enhance routine and campaign immunization performance will need to continue in Chad to ensure interruption of reestablished WPV transmission, limit circulation after any WPV importation, and interrupt transmission of cVDPV.
Immunization Activities
In Chad, the estimated national routine immunization coverage of infants with 3 doses of oral polio vaccine (OPV) in 2011 was 31% (4). A surrogate measure of coverage by routine and supplementary immunization activities (SIAs)* can be obtained from parental recall of dose histories for children with acute flaccid paralysis (AFP) not attributed to polio (nonpolio AFP).† Nationally, 8.1% of children aged 6–35 months with nonpolio AFP did not receive any OPV doses ("zero-dose children").
During January 2011–August 2012, house-to-house SIAs targeted children aged 0–59 months using different OPV formulations, including trivalent (tOPV) and bivalent types 1 and 3 (bOPV). During this period, 11 national immunization days (including a child health day that included measles vaccination)§ and nine subnational immunization days were conducted; bOPV was used exclusively in 15 SIAs, and tOPV was used in three SIAs. In addition to programmatic limitations in many areas, including the capital area of N'Djamena, SIAs often were unable to reach children living in areas inaccessible because of large distances and lack of infrastructure. Several smaller-scale, focal SIAs (mop-ups) were conducted to vaccinate these children. Two short-interval, additional-dose SIAs¶ in 2012 targeted nomadic children aged <15 years. Additionally, after the 2012 outbreak in Lac (the Lake Chad region bordering northeast Nigeria), outbreak response targeted children aged <15 years for the first three SIAs.
AFP Surveillance
Standard indicators are used to monitor AFP surveillance performance (5).** In 2011, the annual national nonpolio AFP rate (per 100,000 population aged <15 years) was 5.7 (range: 3.1–12.5 among the 19 of 21 regions with more than 100,000 children aged <15 years) (Table). During 2011, 81% of AFP cases reported nationally had adequate stool specimens collected (range: 65%–100%), compared with 67% of AFP cases reported nationally during 2010 (5). In N'Djamena, 67% of stool specimens collected from children with AFP were adequate. The proportion of specimens arriving at the laboratory in good condition varied substantially by region. During this reporting period, 41 AFP cases could not be confirmed and were classified as polio-compatible, as of October 2, 2012, because of inadequate stool specimen collection. The laboratory processing stool specimens from Chad is located in Yaoundé, Cameroon. Transport of specimens from N'Djamena to Yaoundé frequently was delayed in 2010; however, no transport delays were reported during 2011–2012.
WPV and VDPV Incidence
In Chad, 132 WPV cases were reported in 2011 (129 WPV1 and three WPV3) (Table, Figure), compared with 26 WPV cases (11 WPV1 and 15 WPV3) in 2010. Five WPV cases (all WPV1) were reported in 2012 (through August), compared with 114 cases (111 WPV1 and three WPV3 cases) during the same period in 2011. The latest reported WPV3 case in Chad occurred in the Dar Sila region in eastern Chad in June 2011. During January 2011–August 2012, 91 (66.4%) WPV cases were among children aged <36 months. Of these 91 children, 14 (15.4%) received no OPV doses, 28 (30.8%) received 1–3 OPV doses, and 48 (52.7%) received ≥4 OPV doses (dose history was unknown for one child). During this period, WPV cases were reported in 18 (86%) of 21 regions. Distribution of WPV1 was widespread in 2011, sparing the two sparsely populated northern regions, with a concentration of cases clustered in Logone Orientale (Figure). During January–August 2012, five WPV1 cases were reported (two in Logone Orientale, one in Chari-Baguirmi, and two in Lac). During January 2011–August 2012, three WPV3 cases were reported, all in Dar Sila.
Five cVDPV2 cases from a single emergence have been confirmed during 2012 as of October 2 (Figure). Four of the cases were among children residing in N'Djamena, with onset from July 20 to August 18; the fifth related case was reported in a child residing in the eastern province of Ouaddaï, with onset on August 15. Immunization response activities are under way.
Reported by
World Health Organization Country Office, N'Djamena, Chad. World Health Organization Regional Office for Africa, Brazzaville, Republic of the Congo. Regional Reference Poliovirus Laboratory, Yaoundé, Cameroon. Global Polio Laboratory Network, Polio Eradication Dept, World Health Organization, Geneva, Switzerland. Div of Viral Diseases, National Center for Immunization and Respiratory Diseases; Global Immunization Div, Center for Global Health; Judy Kruger, PhD, Global Tobacco Control Br, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Corresponding contributor: Katrina Kretsinger, kkretsinger@cdc.gov, 404-639-6164.
Editorial Note
Transmission after importations of WPV3 and WPV1 from Nigeria in 2007 and 2010, respectively, became reestablished in previously polio-free Chad because of chronically low routine immunization coverage and low-quality SIAs. After years of persistent weaknesses in the polio program in Chad, progress toward eradication has been made; cases increased fourfold in 2011 compared with 2010, but then decreased 96% during January–August 2012 compared with the same period in 2011. Circulation of reestablished WPV3 might have been interrupted.
Program improvements during 2012 follow the investment of considerable resources beginning in 2011 by the Chad Ministry of Health and GPEI partners to increase field personnel, training, planning, attention to nomadic and other chronically missed populations, supervision, and political oversight. In addition, innovative, short-interval, additional-dose SIAs have been used to improve population immunity. A prompt investigation of cases reported in Lac during 2012 was followed by timely and aggressive response immunization, including the innovative approach of expanding the SIA target age group to children aged <15 years, and might have substantially limited spread and shortened the outbreak (6). Efforts to improve the implementation of polio immunization activities and to strengthen AFP surveillance are increasingly supported by traditional, religious, and political leaders. The President of Chad launched the National Emergency Action Plan for polio eradication in 2011 (6), emphasizing the key role and responsibility of district and subdistrict authorities. To ensure interruption of reestablished WPV transmission and limit circulation after any WPV importation, Chad authorities will need to continue efforts to strengthen surveillance and enhance routine and campaign immunization planning, management, and supervision.
During 2009 and early 2010, monovalent type 3 OPV primarily was used in SIAs to preferentially raise type 3 immunity while WPV3 was in circulation (1–3,7). After the introduction of WPV1 in 2010, bOPV became the predominant vaccine used in SIAs. A high vulnerability to emergence of cVDPV2 exists in Chad (8), given the continued low levels of routine vaccination coverage and little use of tOPV in campaigns, and therefore low exposure to type 2–containing tOPV; five cVDPV2 cases have been reported to date during 2012. Outbreaks of cVDPV require the same mop-up response campaigns as WPV outbreaks, with use of tOPV.
There have been considerable challenges to achieve and maintain high-quality, sensitive AFP surveillance in Chad. After the provision of additional resources and increased supervisory attention towards stool specimen collection and transport, the overall proportion of AFP cases with collection of adequate stool specimens has increased, and specimen testing has become more timely. However, limitations in the sensitivity of surveillance and adequate specimen collection remain, especially for suspected AFP cases from remote and nomadic populations. As an indication of surveillance limitations, the WPV1 case most closely linked genetically to the Lac outbreak occurred more than 1 year earlier, in Chari-Baguirmi.
With ongoing endemic WPV transmission in Nigeria (1,2) and low routine immunization coverage estimates, Chad remains at risk for new WPV importations and outbreaks. With a recognized risk for failure in reaching the goal of polio eradication (9), the World Health Assembly declared the completion of polio eradication a programmatic emergency for global public health in 2012 (10). WPV circulation during 2012 has continued only in the three remaining endemic countries and in Chad, and the number of cases and of WPV-affected districts globally are at historic lows. In Nigeria, however, the number of cases in 2012 to date has increased from the same period in 2011.†† Until polio is eradicated, all countries remain at risk for WPV importations. The success of GPEI depends on progress in maintaining and improving population immunity and surveillance quality in all countries, while maintaining the commitment of national and international partners.
References
- CDC. Progress toward interruption of wild poliovirus transmission—worldwide, January 2011–March 2012. MMWR 2012;61:353–7.
- CDC. Progress toward global polio eradication—Africa, 2011. MMWR 2012;61;190–4.
- CDC. Progress toward interrupting wild poliovirus circulation in countries with reestablished transmission—Africa, 2009–2010. MMWR 2011;60:306–11.
- World Health Organization/UNICEF. WHO/UNICEF estimates of Pol3 coverage, 2011. Geneva, Switzerland: World Health Organization; 2012. Available at http://apps.who.int/immunization_monitoring/en/globalsummary/timeseries/tswucoveragepol3.htm. Accessed October 19, 2012.
- CDC. Tracking progress towards global polio eradication, 2010–2011. MMWR 2012;61:265–9.
- Global Polio Eradication Initiative/World Health Organization. Global Polio Eradication Initiative emergency action plan 2012–2013. Geneva, Switzerland: World Health Organization; 2012. Available at http://www.polioeradication.org/resourcelibrary/strategyandwork/emergencyactionplan.aspx. Accessed October 19, 2012.
- Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 2010;376:1682–8.
- CDC. Update on vaccine-derived polioviruses—worldwide, April 2011–June 2012. MMWR 2012;61:741–6.
- Global Polio Eradication Initiative. Every missed child: report of the Independent Monitoring Board of the Global Polio Eradication Initiative. Geneva, Switzerland: World Health Organization; 2012. Available at http://www.polioeradication.org/portals/0/document/aboutus/governance/imb/6imbmeeting/imb6_report.pdf. Accessed October 19, 2012.
- Sixty-Fifth World Health Assembly. Poliomyelitis: intensification of the global eradication initiative. Geneva, Switzerland: World Health Organization; 2012. Available at http://apps.who.int/gb/ebwha/pdf_files/wha65/a65_20-en.pdf. Accessed October 19, 2012.
* Mass campaigns conducted for a brief period (days to weeks) in which 1 dose of OPV is administered to all children aged <5 years, regardless of vaccination history. Campaigns often are conducted nationally or in various regions of the country.
† Vaccination histories of children aged 6–23 months with AFP who do not test WPV-positive are used to estimate OPV coverage of the overall target population.
§ Child health days are national campaigns to provide various health interventions simultaneously, including deworming, nutrition-related interventions, and immunization.
¶ Short-interval, additional-dose SIAs are used to enhance access to children and seroconversion per child in which a monovalent OPV or bOPV dose is administered within 1–2 weeks of the previous dose.
** The quality of AFP surveillance is monitored by performance indicators that include 1) detection rate of nonpolio AFP cases and 2) the proportion of AFP cases with adequate stool specimen collection. World Health Organization (WHO) operational targets for countries with endemic polio transmission are a nonpolio AFP detection rate of at least two cases per 100,000 population aged <15 years and adequate stool specimen collection from >80% of AFP cases (in which two specimens are collected at least 24 hours apart, both within 14 days of paralysis onset, and shipped on ice or frozen packs to a WHO-accredited laboratory, arriving in good condition).
†† Additional information available at http://www.polioeradication.org.
What is already known on this topic?
Indigenous wild poliovirus transmission (WPV) has never been interrupted in Afghanistan, Nigeria, or Pakistan. Polio transmission was reestablished (defined as circulation for ≥12 months after importation) in four, previously polio-free countries in Africa, including Chad, in the 2000s.
What is added by this report?
WPV type 3 and WPV type 1 were imported into Chad in 2007 and 2010, and the latest reported cases occurred on March 10, 2011, and June 14, 2012, respectively. In addition, five cases of circulating vaccine-derived poliovirus type 2 occurred during July–August 2012. Current progress suggests that Chad could interrupt WPV transmission in 2012, although challenges remain.
What are the implications for public health practice?
WPV circulation during 2012 has continued only in Chad and the three remaining endemic countries. With ongoing WPV circulation in Nigeria and low routine immunization coverage, Chad remains at risk for new WPV importations and outbreaks. The polio program in Chad has made progress in 2012, and continued efforts will be required.
FIGURE. Reported wild poliovirus (WPV) and circulating vaccine-derived poliovirus (cVDPV) cases, by type and region — Chad, January 2011–August 2012*†
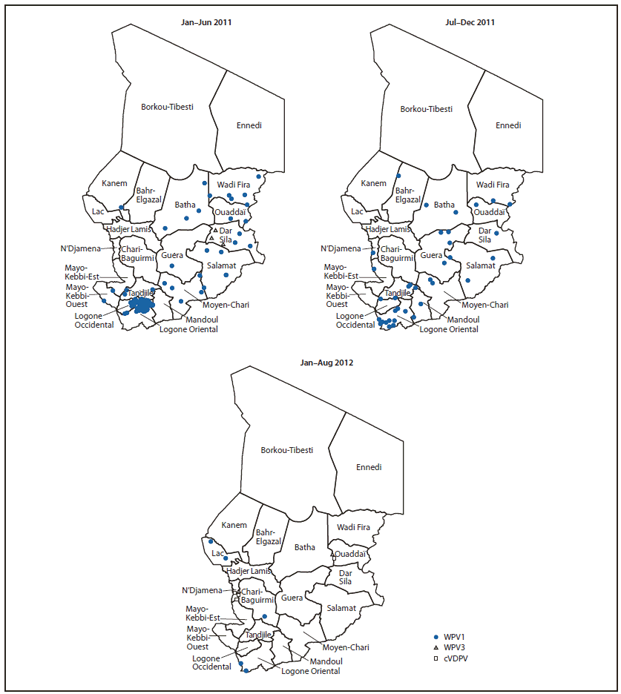
* As of October 2, 2012.
† Each instance of a symbol represents one case of poliovirus and is drawn at random within district boundaries.
Alternate Text: The figure above shows reported wild poliovirus (WPV) and circulating vaccine-derived poliovirus cases, by type and region, in Chad during January 2011-August 2012. In 2011, the most recent year for which complete data are available, a total of 132 WPV cases were reported (129 WPV1 and three WPV3).
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


