Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Pertussis Epidemic — Washington, 2012
Since mid-2011, a substantial rise in pertussis cases has been reported in the state of Washington. In response to this increase, the Washington State Secretary of Health declared a pertussis epidemic on April 3, 2012. By June 16, the reported number of cases in Washington in 2012 had reached 2,520 (37.5 cases per 100,000 residents), a 1,300% increase compared with the same period in 2011 and the highest number of cases reported in any year since 1942. To assess clinical, epidemiologic, and laboratory factors associated with this increase, all pertussis cases reported during January 1–June 16, 2012, were reviewed. Consistent with national trends, high rates of pertussis were observed among infants aged <1 year and children aged 10 years. However, the incidence in adolescents aged 13–14 years also was increased, despite high rates of vaccination with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine, suggesting early waning of immunity. The focus of prevention and control efforts is the protection of infants and others at greatest risk for severe disease and improving vaccination coverage in adolescents and adults, especially those who are pregnant. Pertussis vaccination remains the single most effective strategy for prevention of infection.
Case Classification and Clinical Characteristics
For this analysis, all cases of pertussis reported to the Washington State Department of Health during January 1–June 16, 2012, were reviewed. Cases were classified according to the Council of State and Territorial Epidemiologists case definition (1). Cumulative incidence was calculated as the number of confirmed and probable cases reported per 100,000 residents using age, race, and county-specific population figures from the U.S. Census Bureau as of June 16, 2012. Confirmed and probable cases reported in Washington were compared with U.S. data in the National Notifiable Diseases Surveillance System from January 1 through June 14, 2012.
During January 1–June 16, 2012, a total of 2,520 pertussis cases were reported in Washington, of which 2,069 were confirmed (83.4% laboratory-confirmed and 16.6% epidemiologically linked) and 451 were probable. In comparison, 180 of 966 total cases for the year had been reported during the same period in 2011 (Figure 1). Cases were reported in 32 of the 39 counties (median: 24 cases; range: 1–485 cases). Statewide incidence was 37.5 cases per 100,000 population, ranging from 4.9 to 414.9 by county. Incidence was highest in infants aged <1 year and children aged 10, 13, and 14 years (Figure 2). Among the 1,867 cases with known race and ethnicity, statewide cumulative incidence was higher in Hispanics than non-Hispanics (53.1 versus 24.6 cases per 100,000 population). Of the 155 reported pertussis cases in infants aged <1 year, 34 (21.9%) were managed in a hospital. Among these hospitalized infants, 14 (41.2%) were aged <2 months. Of the 2,360 cases involving children aged ≥1 year with known outcome, 14 of the children (0.6%) were hospitalized. No fatalities were reported.
Compared with the incidence in Washington, the national incidence for the same period in 2012 was lower overall (4.2 cases per 100,000 population). However, the national incidence was increased among infants and children aged 10, 13, and 14 years, consistent with observations in Washington (Figure 3). Through June 14, 2012, eight deaths have been reported in the United States, with a provisional case-fatality rate of 0.62 per 1,000 for reported cases. In comparison, 0.79 to 2.3 deaths per 1,000 reported cases occurred annually during 2000–2011.
Laboratory Testing
Laboratory confirmation of pertussis cases in Washington was performed by clinical and state health laboratories. Pertussis was laboratory-confirmed in 83.4% of cases: 94.7% by polymerase chain reaction (PCR) alone, 2.4% by culture alone, and 2.9% by both PCR and culture. To further confirm Bordetella pertussis as the etiology and evaluate the contribution of other Bordetella species, multitarget PCR assays were performed on all specimens submitted to the clinical microbiology laboratory at Seattle Children's Hospital and on a subset of specimens submitted to CDC by the Washington State Public Health Laboratories and a commercial laboratory during January 1–June 16, 2012. Among 5,086 specimens tested at Seattle Children's Hospital, 193 had Bordetella DNA detected by PCR. Of these, 175 (90.7%) were positive for B. pertussis, 11 (5.7%) for Bordetella parapertussis, two (1.0%) for Bordetella holmesii, and five (2.6%) were indeterminate. Culture was performed on all 193 PCR-confirmed specimens. Bordetella spp. were isolated from 92 (47.7%) specimens, among which B. pertussis was identified in 85 (92.4%), B. parapertussis in six (6.5%), and B. holmesii in one (1.1%). No discrepancies were detected between culture identification and PCR. At CDC, of the 69 specimens tested by multitarget PCR, 59 (85.5%) were positive for B. pertussis, one (1.5%) was positive for B. holmesii, and nine (13.0%) were indeterminate.
CDC also performed pulsed-field gel electrophoresis (PFGE) testing on 55 isolates and compared those results with a national database of more than 5,000 B. pertussis PFGE profiles compiled by CDC during 1990–2011. Among the 55 isolates, 14 PFGE profiles were identified; 30 (54.5%) of the isolates represented the four most commonly identified profiles in the national database. Of the remaining isolates, 20 demonstrated seven of the less common PFGE profiles, and five had three PFGE profiles not previously seen in the national database.
Vaccination Status
The vaccination status of patients was determined by review of medical records and by patient or parent report. Vaccination was considered up-to-date if the minimum number of doses by age had been received, as recommended by the Advisory Committee on Immunization Practices (2). Patients with invalid dose dates (e.g., date of dose preceding date of birth) were excluded from the vaccination status analysis. Individual doses were excluded if administered <14 days before symptom onset.
Valid vaccination history was available for 1,829 of 2,006 (91.2%) patients aged 3 months–19 years. Overall, 758 of 1,000 (75.8%) patients aged 3 months–10 years were up-to-date with the childhood diphtheria and tetanus toxoids and acellular pertussis (DTaP) doses. Receipt of Tdap was documented in 97 of 225 (43.1%) patients aged 11–12 years and in 466 of 604 (77.2%) patients aged 13–19 years. Estimated DTaP coverage in Washington among children aged 19–35 months was 93.2% for ≥3 doses and 81.9% for ≥4 doses in 2010; Tdap coverage in adolescents aged 13–17 years was estimated at 70.6% (3).
Epidemic Response
In response to this ongoing epidemic, the state health department established an incident command structure to coordinate epidemic response and surveillance activities. State guidance to local health jurisdictions and American Indian tribes for case investigations was modified to prioritize identification of persons at high risk (i.e., infants and pregnant women). Health-care provider education has focused on clinical presentation, appropriate diagnostic testing, and treatment and prevention recommendations, with specific emphasis on preventing transmission to persons at high risk through vaccination and targeted antibiotic chemoprophylaxis. Public awareness efforts have focused on informing residents about the signs and symptoms of pertussis and vaccination recommendations. Recommended vaccines for children aged ≤18 years are provided by Washington's Universal Childhood Vaccine Program. Tdap receipt among adults increased substantially; from March 25 to May 26, 2012, the state immunization registry recorded 82,453 doses of Tdap in adults aged ≥19 years, compared with 34,171 recorded during the same period in 2011, a 140% increase. An additional 27,000 doses of Tdap were allocated for uninsured or underinsured adults.
Reported by
Chas DeBolt, MPH, Azadeh Tasslimi, MPH, Janna Bardi, MPH, Brandon Troy Leader, PhD, Brian Hiatt, Washington State Dept of Health. Xuan Qin, PhD, Microbiology Laboratory, Seattle Children's Hospital. Manisha Patel, MD, Stacey Martin, MSc, Maria Lucia Tondella, PhD, Pam Cassiday, MS, Amanda Faulkner, MPH, Nancy E. Messonnier, MD, Thomas A. Clark, MD, Div of Bacterial Diseases, National Center for Immunization and Respiratory Diseases; Sarah Meyer, EIS Officer, CDC. Corresponding contributor: Sarah Meyer, smeyer@cdc.gov, 404-639-3158.
Editorial Note
Pertussis is endemic in the United States. Although cyclical in nature, a gradual and sustained increase has been observed in the United States after reaching historic lows in the 1970s. In 2010, 27,550 pertussis cases were reported. Year-to-date case counts from 2012 have surpassed those from the previous 5 years for the same period. The high rates of pertussis among adolescents aged 13–14 years in Washington reflect national trends and provide observational data suggesting early waning of immunity from acellular vaccines.
Acellular and whole-cell vaccines both have high efficacy during the first 2 years after vaccination, but recent changes in the epidemiology of pertussis in the United States strongly suggest diminished duration of protection afforded by childhood acellular vaccine (DTaP) compared with that of diphtheria and tetanus toxoids and whole-cell pertussis (DTwP) vaccine (4). In contrast with acellular vaccines, which contain several specific antigens, whole-cell vaccines are suspensions of entire killed B. pertussis organisms. The additional antigenic components in DTwP vaccines might induce immune responses with greater durability. Concerns about adverse events associated with DTwP led to replacement with DTaP for the complete childhood series in 1997. Since the mid-2000s, the incidence of pertussis among children aged 7–10 years has increased. Moreover, the observed increase in risk by year of life from age 7–10 years suggests a cohort effect of increasing susceptibility as those children who exclusively received acellular vaccines continue to age.
In 2006, Tdap was recommended for adults and adolescents, with routine vaccination recommended at age 11–12 years. Although the relative reduction in incidence of pertussis among adolescents aged 11–12 years demonstrates immediate vaccine effectiveness, the increasing number of cases in adolescents aged 13–14 years in both Washington and the United States suggests immunity wanes after Tdap vaccination in those adolescents fully vaccinated with acellular vaccines during childhood (5). In observational studies, Tdap effectiveness was 66%–72% among adolescents who largely received DTwP for some of the childhood doses (5,6). Studies evaluating Tdap effectiveness and duration of protection in adolescents fully vaccinated with DTaP are being conducted in Washington and California.
Investigation of the Washington epidemic demonstrates multiple B. pertussis strains causing infection, primarily in vaccinated persons. Given the high transmissibility of B. pertussis, a proportion of vaccinated persons remains susceptible and can become infected during a pertussis outbreak. Unvaccinated children have at least an eightfold greater risk for pertussis than children fully vaccinated with DTaP (7). However, because in most of the cases the patients were vaccinated, the 4.5% of Washington school children who were exempted from vaccination during 2011–2012 represented only a small proportion of those at risk for pertussis in the state. Although vaccinated children can develop pertussis, they are less infectious, have milder symptoms and shorter illness duration, and are at reduced risk for severe outcomes, including hospitalization (8–10).
The ongoing pertussis epidemic in Washington reflects the evolving epidemiology of pertussis in the United States. Although acellular pertussis vaccines provide excellent short-term protection, early waning of immunity might be contributing to increasing population-level susceptibility. Nevertheless, vaccination continues to be the single most effective strategy to reduce morbidity and mortality caused by pertussis. Vaccination of pregnant women and contacts of infants is recommended to protect infants too young to be vaccinated. In light of the increased incidence of pertussis in Washington and elsewhere, efforts should focus on full implementation of DTaP and Tdap recommendations to prevent infection and protect infants.
Acknowledgments
Thirty-five local public health jurisdictions. Mary Selecky, Maxine Hayes, MD, Allene Mares, MPH, Jennifer Tebaldi, MBA, Wayne Turnberg, PhD, Tim Church, Michele Roberts, MPH, Jan Hicks-Thomson, MSW, Diana McMaster, MHA, Yolanda Houze, Pat DeHart, ScD, Kathy Lofy, MD, Marisa D'Angeli, MD, Tracy Sandifer, MPH, Washington State Dept of Health. Charles Cartwright, PhD, Viromed Laboratories. Gladys Gonzalez, MS, Freda Lyde, Div of Bacterial Diseases, Lin Watson, MSN, Immunization Service Div, National Center for Immunization and Respiratory Diseases, CDC.
References
- Council of State and Territorial Epidemiologists. Public health surveillance, control, and prevention of pertussis. CSTE position statement 1997-id-9. Atlanta, GA: Council of State and Territorial Epidemiologists; 1997. Available at http://www.cste.org/ps/1997/1997-id-09.htm. Accessed July 12, 2012.
- CDC. Recommended immunization schedule for persons aged 0 through 18 years—United States, 2012. MMWR 2012;61(5).
- CDC. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR 2011;60:1117–23.
- Clark TA, Messonnier NE, Hadler SC. Pertussis control: time for something new? Trends Microbiol 2012;20:211–3.
- Skoff TH, Martin K, Cohn A, et al. Tdap vaccine effectiveness among adolescents: a case-control study in Minnesota. Presented at the 9th International Bordetella Symposium; September 30–October 3, 2010; Baltimore, MD.
- Wei SC, Tatti K, Cushing K, et al. Effectiveness of adolescent and adult tetanus, reduced-dose diphtheria, and acellular pertussis vaccine against pertussis. Clin Infect Dis 2010;51:315–21.
- Misegades LK, Winter K, Harriman K, Talarico J, Clark T, Martin SW. DTaP effectiveness: results from the California pertussis vaccine effectiveness assessment. In: Proceedings of the 49th Infectious Diseases Society of America; October 20–23, 2011; Boston, MA. Arlington, VA: Infectious Diseases Society of America; 2011.
- Preziosi MP, Halloran ME. Effects of pertussis vaccination on transmission: vaccine efficacy for infectiousness. Vaccine 2003;21:1853–61.
- Tanaka M, Vitek CR, Pascual FB, Bisgard KM, Tate JE, Murphy TV. Trends in pertussis among infants in the United States, 1980–1999. JAMA 2003;290:2968–75.
- Tozzi AE, Rava L, Ciofi degli Atti ML, Salmosa S, Progetto Pertosse Working Group. Clinical presentation of pertussis in unvaccinated and vaccinated children in the first six years of life. Pediatrics 2003;112:1069–75.
What is already known on this topic?
The incidence of reported pertussis has increased in the United States after reaching historic lows in the 1970s. Since 2007, children aged 7–10 years have accounted for a substantial proportion of pertussis cases in the United States, a finding attributed to waning immunity in persons fully vaccinated with acellular vaccines in childhood.
What is added by this report?
During January 1–June 16, 2012, the number of reported cases of pertussis in Washington reached 2,520 (37.5 cases per 100,000 residents), a 1,300% increase compared with the same period in 2011. In this epidemic, high rates of disease were observed in adolescents aged 13–14 years, despite high vaccination coverage and recent tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine administration. Preliminary national incidence data are consistent with the Washington findings.
What are the implications for public health practice?
Increased rates of pertussis among adolescents aged 13–14 years who were fully vaccinated with acellular vaccines in childhood suggests early waning of immunity after vaccination with Tdap vaccine. Studies are ongoing to evaluate Tdap duration of protection in adolescents. The focus of prevention and control efforts is the protection of infants and others at greatest risk for severe disease and improving vaccination coverage in adolescents and adults, especially those who are pregnant. Pertussis vaccination remains the single most effective strategy for prevention of infection.
FIGURE 1. Number of confirmed and probable pertussis cases reported, by week of onset — Washington, January 1, 2011–June 16, 2012*
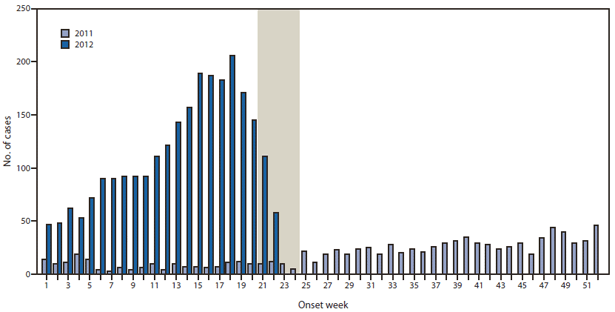
* Reports for 2012 as of June 16, 2012. The shaded area represents a lag during which additional cases likely occurred during 2012, but had not yet been reported to the Washington State Department of Health.
Alternate Text: The figure above shows the number of confirmed and probable pertussis cases reported, by week of onset in Washington, during January 1, 2011-June 16, 2012. During January 1-June 16, 2012, a total of 2,520 pertussis cases were reported in Washington, of which 2,069 were confirmed (83.4% laboratory-confirmed and 16.6% epidemiologically linked) and 451 were probable. In comparison, 180 of 966 total cases for the year were reported during the same period in 2011.
FIGURE 2. Number and incidence of confirmed and probable pertussis cases among persons aged ≤19 years, by patient age and vaccines received* — Washington, January 1–June 16, 2012
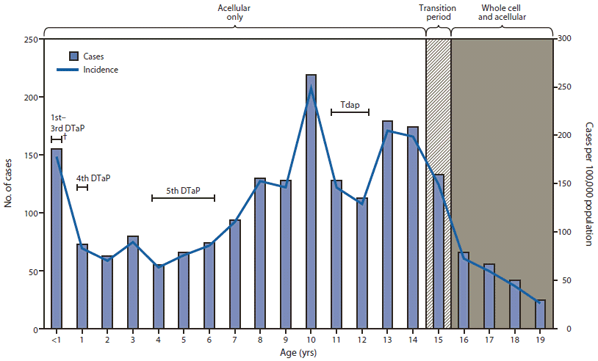
Abbreviations: DTaP = diphtheria and tetanus toxoids and acellular pertussis; DTwP = diphtheria and tetanus toxoids and whole-cell pertussis; Tdap = tetanus and reduced diphtheria toxoids and acellular pertussis.
* Acellular vaccines (DTaP) replaced whole-cell vaccines (DTwP) for the 4th and 5th doses in 1992 and all 5 doses of the childhood series in 1997. Tdap was recommended for adolescents aged 11–12 years in 2006. Thus, all children aged ≤14 years are likely to have received acellular vaccines for the complete childhood series. Adolescents aged 15 years were born during a transition year from whole-cell to acellular vaccines for the childhood series. Adolescents aged ≥16 years received whole-cell vaccines for the first 3 doses, and acellular vaccines for the 4th and 5th doses.
† Ages during which the Advisory Committee on Immunization Practices recommends that specified vaccine doses be administered.
Alternate Text: The figure above shows the number and incidence of confirmed and probable pertussis cases among persons aged ≤19 years, by patient age and vaccines received in Washington, during January 1-June 16, 2012. Statewide incidence was 37.5 cases per 100,000 population, ranging from 4.9 to 414.9 by county. Incidence was highest in infants aged <1 year and children aged 10, 13, and 14 years.
FIGURE 3. Incidence of confirmed and probable pertussis among persons aged ≤19 years, by patient age and vaccines received* — National Notifiable Diseases Surveillance System, United States, January 1–June 14, 2012
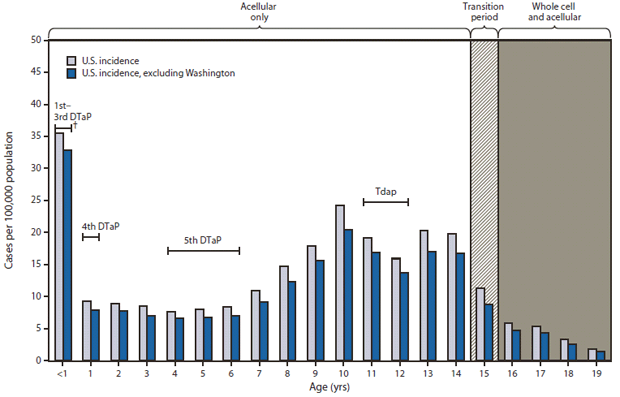
Abbreviations: DTaP = diphtheria and tetanus toxoids and acellular pertussis; DTwP = diphtheria and tetanus toxoids and whole-cell pertussis; Tdap = tetanus and reduced diphtheria toxoids and acellular pertussis.
* Acellular vaccines (DTaP) replaced whole-cell vaccines (DTwP) for the 4th and 5th doses in 1992 and all 5 doses of the childhood series in 1997. Tdap was recommended for adolescents aged 11–12 years in 2006. Thus, all children aged ≤14 years are likely to have received acellular vaccines for the complete childhood series. Adolescents aged 15 years were born during a transition year from whole-cell to acellular vaccines for the childhood series. Adolescents aged ≥16 years received whole-cell vaccines for the first 3 doses, and acellular vaccines for the 4th and 5th doses.
† Ages during which the Advisory Committee on Immunization Practices recommends that specified vaccine doses be administered.
Alternate Text: The figure above shows the incidence of confirmed and probable pertussis among persons aged ≤years, by patient age and vaccines received in the United States, during January 1-June 14, 2012, according to the National Notifiable Diseases Surveillance System. Compared with the incidence in Washington, the national incidence for the same time period in 2012 was lower overall (4.2 cases per 100,000 population). However, the national incidence was increased among infants and children aged 10, 13, and 14 years, consistent with observations in Washington.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


