FIGURE. Investigation timeline after initial report of transmission of hepatitis C virus (HCV) from an organ and tissue donor — Kentucky and Massachusetts, 2011
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Transmission of Hepatitis C Virus Through Transplanted Organs and Tissue — Kentucky and Massachusetts, 2011
Please note: An erratum has been published for this article. To view the erratum, please click here.
On September 29, 2011, the United Network for Organ Sharing notified CDC of two patients who tested positive for hepatitis C virus (HCV) infection approximately 6 months after receiving kidney transplants from a deceased donor. Before transplantation, the donor had tested negative for HCV antibody by the organ procurement organization. Tissue also was procured from the donor for possible transplantation. The tissue bank performed an HCV antibody test on the donor's serum specimen that was negative and nucleic acid testing (NAT) that was positive, but misread as negative. Retesting of the donor specimen during the investigation confirmed the NAT results as positive. Donated tissue included 43 musculoskeletal grafts and one cardiopulmonary patch, which were distributed to health-care facilities in several states. An investigation was initiated to 1) identify potential sources of the donor's infection, 2) document the mode of transmission to the organ recipients, and 3) ensure timely notification of the implanting surgeons and testing of tissue recipients. Implantation of infected HCV tissue occurred after recognition of new HCV infection in the organ transplant recipients, highlighting the need for rapid communication between transplant centers, organ procurement organizations, tissue banks, and public health authorities regarding suspected transplantation transmission events.
Donor Investigation
The donor, a middle-aged man in Kentucky, sustained a traumatic brain injury in March 2011 in an all-terrain vehicular incident and died 2 days later. His medical history was significant for schizophrenia, substance abuse, and a 5-month incarceration approximately 10 years before his death. The donor had no known history of intravenous drug use or other hepatitis risk factors, according to his father at the time of organ procurement; however, further investigation revealed that the donor's father had limited contact with his son during the year before his death and was unfamiliar with recent personal habits or behaviors.
Policies of the Organ Procurement and Transplantation Network (OPTN), the oversight entity for solid organs in the United States, require testing for HCV by antibody only, whereas the Food and Drug Administration (FDA), which regulates human cells, tissues, and cellular and tissue-based products, requires screening of donated tissue for HCV by both antibody and NAT (1). The donor's HCV antibody tested negative on both organ and tissue donor screening, but misreading of the reaction wells on testing led to an incorrectly reported negative HCV NAT result. Once this error was identified, repeat NAT was performed at the tissue bank and confirmed that the donor was HCV-positive at the time of donation. During the donor's final hospital stay in March, he received six units of blood products. Pretransfusion serum from the donor was not available for analysis. Testing of posttransfusion stored serum at CDC on October 28 confirmed by NAT that the donor was HCV-positive with genotype 1a and a viral load of >69,000,000 IU/mL. Blood traceback investigation of the six associated blood donors to the infected donor is ongoing, and all remaining units from these donations have been quarantined.
Organ Transplant Investigation
In March 2011, three organs (two kidneys and the liver) from the donor were transplanted into three recipients at a local hospital in Kentucky (Figure). Both kidney recipients had tested negative for hepatitis C before transplant, whereas the liver recipient had a previous diagnosis of hepatitis C.
First kidney recipient. On July 26, 2011, the recipient of the first kidney, a man aged 41 years, was noted to have elevated liver enzymes (aspartate aminotransferase [AST]: 161 U/L; alanine aminotransaminase [ALT]: 217 U/L). HCV antibody testing conducted August 22 was negative. Liver function tests continued to be elevated, and HCV NAT performed September 19 was positive.
Second kidney recipient. The recipient of the second kidney, a woman aged 46 years, was noted to have elevated liver function tests on August 25 (AST: 206 U/L, ALT: 221 U/L); HCV NAT was positive September 21.
Liver recipient. The liver recipient, a man aged 51 years, had a history of chronic infection with HCV, genotype 1a, before transplant. Liver function testing on September 7 was unchanged from his baseline (AST: 46 U/L, ALT: 55 U/L).
At CDC, serum specimens were tested for HCV RNA. Serum collected after organ transplantation from all three recipients tested positive for HCV by NAT at CDC, and all three HCV strains were confirmed to be genotype 1a.
Tissue Transplant Investigation
On September 29, the organ procurement organization notified the tissue bank of the apparent HCV transmission to the kidney and liver recipients. The tissue bank informed health-care facilities, and a voluntary recall was begun on September 30. The tissue bank had distributed 43 musculoskeletal grafts and one cardiopulmonary patch to health-care facilities, but names and contact information for surgeons who implanted these tissues were not uniformly available at the time of recall. CDC telephone notification of all surgeons and requests for testing of all patients was completed on October 27.
The cardiopulmonary patch, the only nonmusculoskeletal tissue distributed, had been treated with antibiotics by the tissue bank according to protocol and was implanted by a health-care facility in Massachusetts on September 26. After the health-care facility was notified, the recipient underwent testing. Hepatitis C antibody was negative, but NAT was positive at 82,000 IU/mL; the recipient's ALT was normal (12 U/L).
The 43 distributed musculoskeletal grafts were treated chemically and by irradiation at the tissue bank, according to protocol. Fifteen of the musculoskeletal tissues were implanted; the remaining 28 were returned to the tissue bank. The 15 recipients of musculoskeletal tissues were recommended to receive HCV serologic testing and NAT immediately and again 6 months from the time of tissue implantation. As of December 16, initial test results from 14 of the musculoskeletal tissue recipients were known, and all were negative based on HCV NAT.
Molecular Characterization of HCV Strains
To determine the genetic relatedness among the HCV strains obtained from the donor, the two kidney recipients, the liver recipient, and the cardiopulmonary patch recipient, maximum likelihood phylogenetic trees were created (2). These analyses showed that two specimens from the donor and the three specimens from the kidney recipients and cardiopulmonary patch recipient shared identical NS5b sequences; the liver recipient did not share these sequences, indicating previous infection. Quasispecies analysis was performed on the specimens that shared identical NS5b sequences (3). The E1-HVR1 quasispecies sequences from the donor, the two kidney recipients, and the cardiopulmonary patch recipient clustered in a single group, indicating their close genetic relatedness consistent with a common source of HCV transmission. The donor and the two kidney recipients and one cardiopulmonary patch recipient had from two to 10 distinct E1-HVR1 sequences that shared from 99.7% to 100% similarity with each other.
Reported by
Michael R. Marvin, MD, Melissa Steele, Univ of Louisville/Jewish Hospital; Sharon K. Green, Magoffin County Health Dept, Kentucky; Doug Thoroughman, PhD, Tennis J. Sugg, MPH, Kraig E. Humbaugh, MD, Kentucky Dept for Public Health. Louise E. Vaz, MD, Sandra K Burchett, MD, Kristin Moffitt, MD, Children's Hospital, Boston, Massachusetts. Carrie F. Nielsen, PhD, Scott D. Holmberg, MD, Jan Drobeniuc, MD, Yury Khudyakov, PhD, Div of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB; Matthew J. Kuehnert, MD, Susan N. Hocevar, MD, Div of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases; Reena Mahajan, MD, EIS officer, CDC. Corresponding contributor: Reena Mahajan, rmahajan@cdc.gov, 404-718-8563.
Editorial Note
The transmission of HCV associated with transplanted organs and minimally processed tissue has been described previously, but this is the first recognized HCV transmission via a cardiopulmonary patch (4). Although correct reading of tissue donor NAT screening results would have prevented transmission through the tissue patch, the organ recipients still would have become infected because current OPTN policies for organ donor screening only require HCV serologic testing (1). Furthermore, positive organ donor NAT screening likely would have resulted in quarantine of potentially infected tissue. Use of NAT, in addition to anti-HCV serologic testing, has been proposed to decrease the risk for transmitting undetected HCV infection. However, no one test can uniformly detect all infections, either because of false-negative tests resulting from the window period, or assay-related issues, or, as described in this report, because of human error.
Without information regarding a donor's behavioral risk factors, the assay selection and sensitivity of pretransplantation testing is critical. The incidence of HCV infection not detected by serologic screening for anti-HCV antibody varies from 1 in 5,000 for normal-risk patients to 1 in 1,000 for patients at high risk (5). The window period (i.e., the time from exposure to detectable HCV antibody) has a mean of 65–70 days; this period is shortened to 3–5 days with use of NAT (6). A transplant facility's decision to use an organ is based on the organ procurement organization's assessment of the donor's risk status and on test results (5). Multiple factors, including the urgent need for a potentially life-saving transplant and informed consent of the transplant candidate must be considered when determining whether benefits of transplantation outweigh the risk for transmitting HCV. The U.S. Public Health Service recently drafted guidelines recommending testing of all organ donors with NAT for HCV regardless of risk status (7). Even if test results are not available at the time of transplantation, results still can be used afterward to guide recipient evaluation and treatment.
The diagnosis of HCV infection in two recipients of kidneys from the same donor should raise immediate suspicion of donor-derived infection and reporting to OPTN and to local and state health departments as required by policy. Reporting to local and state health departments also should occur because acute HCV infection is a nationally notifiable disease. Reporting of suspected new diagnoses in organ recipients, including to tissue banks, should occur without delay, because such diagnoses might have implications for tissues that have not yet been transplanted.
The events in this report demonstrate the importance of timely communication once a transplant transmission is suspected and the difficulty of tracking tissue to the patient or provider level should a potential transmission be recognized after tissue has been distributed. Although FDA requires that the tissue bank track the distribution of tissues down to the institutional level, no government regulations require tracking tissue to the patient level; hospitals are asked voluntarily to return a record, often a postcard, to the tissue bank to notify them of implantation of the tissue. Many health-care facilities have a mechanism to track tissue to the patient, although approaches are not standardized (8). Systems that facilitate real-time notification of possible disease transmission to tissue banks, organ procurement organizations, and other transplant centers do not exist, and development is hindered by the lack of standardized tissue nomenclature and identification standards (9,10).
This investigation reveals several areas in which current detection and notification might be improved to prevent similar future transplant transmission events, including: 1) consideration of the use of HCV NAT for organ donors; 2) use of algorithms or other procedures to ensure accurate reading of test results and reduce human error; and 3) timely feedback of possible disease transmission in organ or tissue recipients to organ procurement organizations, tissue banks, public health authorities, and regulators.
References
- Health Resources and Services Administration. Minimum procurement standards for an Organ Procurement Organization (OPO). Rockville, MD: Health Resources and Services Administration; 2011. Available at http://optn.transplant.hrsa.gov/policiesandbylaws2/policies/pdfs/policy_2.pdf. Accessed December 19, 2011.
- Felsentein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 1981;17:368–76.
- Ramachandran S, Xia GL, Ganova-Raeva LM, Nainan OV, Khudyakov Y. End-point limiting-dilution real-time PCR assay for evaluation of hepatitis C virus quasispecies in serum: performance under optimal and suboptimal conditions. J Virol Methods 2008;151:217–24.
- Tugwell BD, Patel PR, Williams IT, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med 2005;143:648–54.
- Ellingson K, Seem D, Nowicki M, Strong DM, Kuehnert MJ, Organ Procurement Organization Nucleic Acid Testing Yield Project Team. Estimated risk of human immunodeficiency virus and hepatitis C virus infection among potential organ donors from 17 organ procurement organizations in the United States. Am J Transplant 2011;11:1201–8.
- Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion 2009;49:2454–89.
- Draft PHS guideline for reducing transmission of human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) through solid organ transplantation. Available at http://www.regulations.gov. Enter: ID = CDC-2011-0011. Accessed December 19, 2011.
- Kuehnert MJ, Yorita KL, Holman RC, Strong DM; AABB Tissue Task Force. Human tissue oversight in hospitals: an AABB survey. Transfusion 2007;47:194–200.
- Strong DM, Seem D, Taylor G, Parker J, Stewart D, Kuehnert MJ. Development of a transplantation transmission sentinel network to improve safety and traceability of organ and tissues. Cell Tissue Bank 2010;11:335–43.
- Brubaker S, Wilson D. Coding and traceability: cells and tissues in North America. Cell Tissue Bank 2010;11:379–89.
What is already known on this topic?
Hepatitis C virus (HCV) transmission from antibody-negative organ donors has been documented previously; nucleic acid testing (NAT) is required for tissue donors but not for organ donors.
What is added by this report?
A donor transmitted HCV to two kidney recipients and one tissue recipient because of a negative antibody test (a result of the window period) and an incorrectly read HCV NAT result. Implantation of infected tissue occurred after recognition of the infected organ transplant recipients, highlighting the need for more rapid methods to recognize and communicate information on suspected transplantation transmission.
What are the implications for public health practice?
HCV antibody testing alone might not be adequate to detect disease in organ donors with acute infection or in recipients who are immunosuppressed. A real-time system for notification of disease clusters in transplant recipients is needed to prevent further use of tissue that tests positive for HCV or other infections. Suspected disease transmission through organ and tissue transplantation should be reported by clinicians to appropriate oversight organizations and public health authorities without delay.
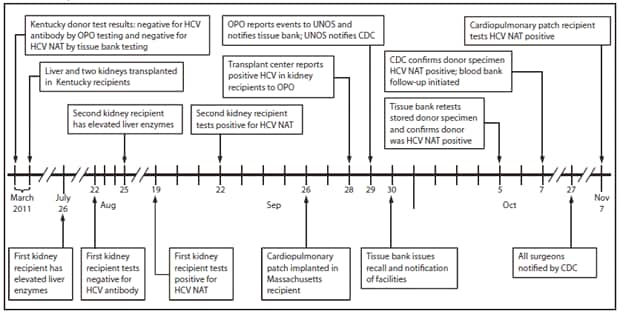
Abbreviations: OPO = organ procurement organization; NAT = nucleic acid testing; UNOS = United Network for Organ Sharing.
Alternate Text: The figure above shows investigation timeline after initial report of transmission of hepatitis C virus (HCV) from an organ and tissue donor in Kentucky and Massachusetts during 2011. In March 2011, three organs (two kidneys and the liver) from the donor were transplanted into three recipients at a local hospital in Kentucky. In September, the two kidney recipients tested positive for HCV by nucleic acid testing (NAT). In November, a cardiopulmonary patch recipient also tested positive for HCV NAT.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


