FIGURE 1. Invasive pneumococcal disease (IPD) resulting from pneumococcal serotypes unique to 13-valent pneumococcal conjugate vaccine (PCV13), exclusion criteria applied, and characteristics of affected children aged ≤59 months --- CDC's PCV13 Vaccine Effectiveness Evaluation,* United States, May 2010--April 2011
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Invasive Pneumococcal Disease and 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Coverage Among Children Aged ≤59 Months --- Selected U.S. Regions, 2010--2011
Please note: An erratum has been published for this article. To view the erratum, please click here.
On March 12, 2010, the Advisory Committee on Immunization Practices (ACIP) published recommendations for use of a newly licensed, 13-valent pneumococcal conjugate vaccine (PCV13) to replace the 7-valent vaccine (PCV7) for all children and for a supplemental dose for those aged 14 through 59 months (1). PCV is given routinely to children at ages 2, 4, and 6 months, and a booster dose is given at 12--15 months (1). PCV13 includes antigens of six pneumococcal serotypes in addition to those in PCV7 (1). Children only vaccinated with PCV7 are susceptible to those six serotypes, which can cause invasive pneumococcal disease (IPD) and death. During 2010 and 2011, CDC evaluated available data to assess the occurrence of PCV13-type IPD cases and PCV13 vaccination coverage among children aged ≤59 months. During May 1, 2010--April 30, 2011, 63 vaccine-eligible children with IPD caused by a serotype that would have been prevented by PCV13 were identified within 12 study regions. Most of those children were aged 24 through 59 months and were vaccinated completely with PCV7 but had not received the recommended supplemental dose of PCV13. Immunization Information System (IIS) sentinel site data from March 2010--June 2011 indicated that the proportion of PCV7-vaccinated children who had received the PCV13 supplemental dose was only 37%. Similarly, among children aged ≤59 months requiring additional primary series doses, PCV13 coverage was only 46%. Given the potential for missed PCV13 vaccination, health-care providers should recommend PCV13 vaccination for all eligible children aged 14 through 59 months during all visits, and continue to ensure receipt of the full PCV13 primary series for younger children.
In June 2011, a girl in California, aged 2 years, died of IPD caused by serotype 19A, one of six serotypes included in PCV13 but not in PCV7. The child had received 3 doses of PCV7 but had not received PCV13. The California Department of Public Health identified an additional 30 PCV13-eligible children who had developed nonfatal IPD caused by the pneumococcal serotypes not covered by PCV7 and who became ill after PCV13 was recommended by ACIP. In August 2011, a health advisory was sent to California health-care providers to remind them of ACIP's recommendation for PCV13 use (2). To determine if other areas of the country were identifying cases of PCV13-type IPD among children eligible to receive PCV13 and to assess PCV13 vaccination coverage among children aged ≤59 months, CDC assessed data from 12 geographic regions participating in its ongoing PCV13 Vaccine Effectiveness Evaluation (3) and from eight IIS sentinel sites (4).
The PCV13 Vaccine Effectiveness Evaluation used data from Active Bacterial Core surveillance (ABCs), an active, population-based and laboratory-based surveillance system for monitoring invasive bacterial pathogens, and the Epidemiology and Laboratory Capacity for Infectious Diseases (ELC) network, a group of U.S. sites supported by CDC to strengthen their capacity to address infectious disease threats (3). Children aged 2 months through 59 months who 1) were identified as having IPD by routine surveillance at ABCs and ELC sites, 2) were recommended by ACIP to receive PCV13, and 3) had a pneumococcal isolate available for serotyping were eligible for inclusion in the PCV13 Vaccine Effectiveness Evaluation. The catchment area for the evaluation comprised the 10 ABCs sites, (Connecticut, Minnesota, and New Mexico, and selected counties in California, Colorado, Georgia, Maryland, New York, Oregon, and Tennessee), plus Utah and Los Angeles County from the ELC sites, with a total population of 3.2 million children aged ≤59 months (5). Cases included in this analysis occurred in children enrolled in the PCV13 Vaccine Effectiveness Evaluation who had IPD caused by one of six serotypes included in PCV13 but not PCV7 and a complete vaccination history available at the time of analysis, and who were eligible to receive PCV13 at least 2 weeks before diagnosis of IPD.
During May 1, 2010--April 30, 2011, 135 cases of IPD caused by serotypes unique to PCV13 were identified among children aged 2 through 59 months. Among those, 81 (58%) had a complete vaccination history; of these, 66 (81%) had no previous PCV13 dose recorded. Three of the 66 cases involved children who were ineligible to receive PCV13 because of dates of PCV7 receipt and timing of disease diagnosis. Of the 63 remaining cases, 43 (68%) involved children aged 24--48 months. Nearly all of these children (94%) had no underlying medical conditions. Among the 63 children, 48 (76%) were hospitalized; no deaths were reported. Thirty-nine of the children (62%) had received a full, 4-dose PCV7 series but had not received a supplemental PCV13 dose, and 11 others (18%) had received 3 doses of PCV7 but had not received a fourth pneumococcal vaccine dose, which should have been PCV13 (Figure 1) (1).
To assess coverage with PCV13, CDC collected data from the eight state and city-based IIS sentinel sites (4). Those collaborating sites include parts of Arizona, Colorado, Michigan, Minnesota, Oregon, and Wisconsin, all of North Dakota, and New York City, and include nearly 2 million children aged <6 years. Data on receipt of PCV13 were collected from March 2010, when PCV13 was first recommended, through June 2011 on children aged 0 through 11 months, 12 through 23 months, and 24 through 59 months as of March 2010, by primary PCV7 series completion status. A complete primary PCV7 series was defined as ≥4 doses, if the fourth dose was administered at age ≥12 months; 3 doses, if the third dose was administered at age ≥12 months; 2 doses, if both doses were administered at age ≥12 months; or 1 dose, if administered at age 24 to <60 months. Overall estimates were calculated by averaging unweighted site-specific estimates. Additionally, to evaluate the overall transition from PCV7 to PCV13, all sites reported the total number of doses administered weekly, by PCV type, during March 7--August 21, 2010.
Among approximately 850,000 children aged 12 through 59 months with a complete PCV7 series as of March 2010, 37% subsequently received the supplemental PCV13 dose as of June 30, 2011. The proportion of children receiving the dose was lower among children aged 24 through 59 months (32%) compared with children aged 12 through 23 months (58%) (Table). Among nearly 700,000 children aged 0 through 59 months with an incomplete primary PCV7 series as of March 2010, PCV13 coverage reached 46% by June 30, 2011. Similarly, children aged 24 through 59 months were less likely (14%) than younger children to receive a PCV13 dose (0 through 11 months: 71%; 12 through 23 months: 45%). Initial coverage with PCV13 occurred quickly after vaccine recommendation (Figure 2). By the week of April 18, the number of weekly PCV13 doses administered exceeded the number of weekly PCV7 doses administered. However, PCV7 continued to be used for a small proportion of children through August 2010.
Reported by
Kathleen Harriman, PhD, California Dept of Health. Tracy N. Thomas, MPH, MSc, Maureen Kolasa, MPH, Karen Cullen, PhD, Laura Pabst, MPH, Abigail Shefer, MD, Immunization Svcs Div; Chad Cox, MD, Matthew Moore, MD, Ruth Link-Gelles, MPH, Div of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC. Corresponding contributor: Tracy N. Thomas, tct5@cdc.gov, 404-639-8542.
Editorial Note
Within 2 months of the ACIP recommendation, PCV13 accounted for more than half of the weekly PCV doses administered. In spite of this rapid transition, not all children eligible for a supplemental dose are receiving it, leaving them more likely to develop IPD secondary to one of six serotypes uniquely covered by PCV13. Current PCV13 vaccination trends suggest that the majority of children not receiving the supplemental dose are aged 24 through 59 months rather than 12 through 23 months.
Health-care providers rapidly transitioned from PCV 7 to PCV13 administration. To facilitate the rapid transition from PCV7 to PCV13, the manufacturer accepted returns of PCV7 from public and private providers. In the public sector, PCV13 was available as of March 18, 2010, and all unused PCV7 had to be returned by May 10, 2010, to receive credit from the manufacturer. By July 2010, Pfizer reported that >90% of its private shipments of pneumococcal conjugate vaccines were for PCV13 (P.L. Alexa, Pfizer Inc., personal communication, July 2010). The underlying factors contributing to PCV13 coverage differences across age groups could not be determined with certainty. Lower coverage among the oldest age group might be related to fewer preventive care visits and vaccination opportunities compared with younger children (6), decreased perceived risk for invasive disease among the older children (7), and health-care provider lack of awareness of or inconsistency in implementing PCV recommendations for older children (8).
Estimates from the National Immunization Survey show coverage with ≥4 doses of PCV increased from 80% in 2009 to 83% in 2010 and remained high at 93% for ≥3 doses of PCV among children aged 19--35 months. Although coverage levels for PCV continue to increase, careful monitoring of coverage levels overall and across subpopulations (i.e., older children) will be important to ensure that all children are protected adequately (9).
The findings in this report are subject to at least three limitations. First, although the ABCs, ELC, and IIS systems are important sources of U.S. population-based data for surveillance of invasive bacterial disease incidence and patterns of vaccine coverage, they represent a select sample of U.S. regions and the findings might not be nationally representative. Second, although IIS sentinel site data are monitored for accuracy, some PCV13 doses might have been misclassified as PCV7 in IIS, thereby underestimating PCV13 coverage. Finally, although no reports of difficulty in obtaining PCV13 have occurred, some vaccination providers might have experienced a delay in receipt of vaccine.
To prevent IPD among children, health-care providers should administer a single supplemental dose of PCV13 to all children aged 14 through 59 months who have received an age-appropriate number of PCV7 doses to provide additional protection against the six serotypes unique to PCV13 (1). Additionally, health-care providers should complete the PCV7 series with PCV13, and continue to ensure that all children receive timely receipt of the full primary series as recommended by ACIP. No PCV7 should be used at this time. To increase PCV13 coverage, health-care providers should take advantage of opportunities to provide the supplemental dose of PCV13 to age-eligible patients during any health-care visit.
Acknowledgments
Principal investigators and surveillance officers at the participating Active Bacterial Core surveillance and Epidemiology and Laboratory Capacity for Infectious Diseases sites, Erin Garcia, Deborah Aragon, Michelle Wilson, Monica Farley, Ramon Guevara, Lee Harrison, Rosemary Hollick, Corinne Holtzman, Karen Scherzinger, Salvatore Currenti, Jennifer Rosen, Jamie Thompson, William Schaffner, Jonathan Anderson. Kathleen Winter, California Dept of Health. Staff members at the eight IIS sites.
References
- CDC. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children---Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59:258--61.
- California Department of Public Health. Health advisory: fatal vaccine-preventable pneumcoccal disease, August 18, 2011. Sacramento, CA: California Department of Public Health; 2011. Available at http://www.cdph.ca.gov/programs/immunize/documents/healthadvisorypneumococcal201108.pdf. Accessed September 9, 2011.
- CDC. Evaluating the effectiveness of a 13-valent pneumococcal conjugate vaccine among children. Protocol 09PRT/8166. Lancet Protocol Reviews. Available at http://www.thelancet.com/protocol-reviews/09PRT-8166. Accessed September 9, 2011.
- CDC. Q&A about sentinel sites. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. Available at http://www.cdc.gov/vaccines/programs/iis/activities/sentinel-sites.htm. Accessed September 15, 2011.
- CDC. Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine---United States, 2007. MMWR 2010;59:253--7.
- Luman ET, Chu SY. When and why children fall behind with vaccinations. Missed visits and missed opportunities at milestone ages. Am J Prev Med 2009;36:105--11.
- CDC. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2000;49(No. RR-9).
- Davis MM, Ndiaye SM, Freed GL, Clark SJ. One-year uptake of pneumococcal conjugate vaccine: a national survey of family physicians and pediatricians. J Am Board Fam Pract 2003;16:363--71.
- CDC. National and state vaccination coverage among children aged 19--35 months---United States, 2010. MMWR 2011;60:1157--63.
What is already known on this topic?
On February 24, 2010, a 13-valent pneumococcal conjugate vaccine (PCV13) was licensed and in March 2010, the Advisory Committee for Immunization Practices (ACIP) published recommendations to use PCV13 exclusively in place of the 7-valent vaccine (PCV7) and to administer a single supplemental dose of PCV13 to all children aged 14 through 59 months who have received an age-appropriate series of PCV7.
What is added by this report?
Children are developing invasive pneumococcal disease (IPD) caused by serotypes that could be prevented by PCV13 but not PCV7. In June 2011, a child, who had received 3 doses of PCV7 died of IPD caused by one of six serotypes to which PCV13 uniquely provides protection. In an evaluation of data from CDC's ongoing PCV13 Vaccine Effectiveness Evaluation, 63 children eligible but not vaccinated with PCV13 developed IPD caused by one those six serotypes. Vaccination trends at eight Immunization Information System sentinel sites indicated that receipt of the supplemental PCV13 dose is <40% among PCV7-vaccinated children.
What are the implications for public health practice?
Immunization programs and vaccination providers should encourage parents of all children aged 14 through 59 months who have received an age-appropriate PCV7 series to have their child receive a single supplemental dose of PCV13. Providers should take advantage of all office visits to vaccinate all eligible children with PCV13 to increase vaccination coverage.
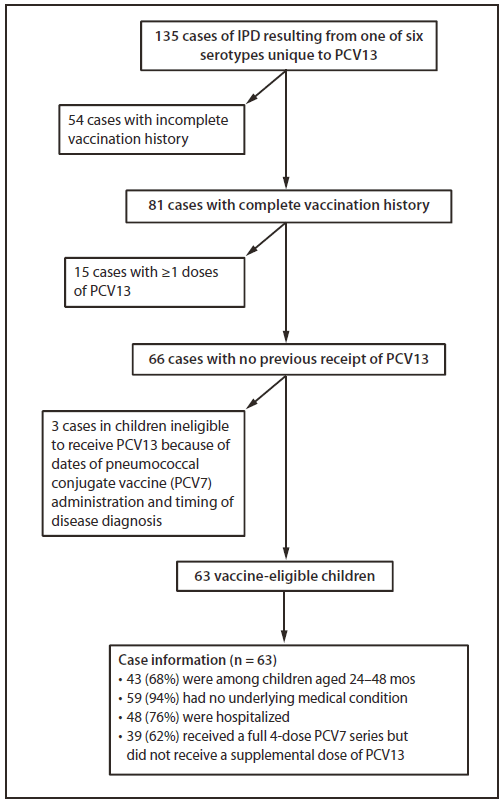
* Twelve areas across the United States participate in CDC's ongoing PCV13 Vaccine Effectiveness Evaluation, representing a population of 3.2 million children aged ≤59 months.
Alternate Text: The figure above shows invasive pneumococcal disease (IPD) resulting from pneumococcal serotypes unique to 13-valet pneumococcal conjugate vaccine (PCV13), exclusion criteria applied, and characteristics of affected U.S. children aged ≤59 months, from CDC's PCV13 Vaccine Effectiveness Evaluation during May 2010 - April 2011. Thirty-nine of the children (62%) had received a full, 4-dose PCV7 series but had not received a supplemental PCV13 dose, and 11 others (18%) had received 3 doses of PCV7 but had not received a fourth pneumococcal vaccine dose, which should have been PCV13.
FIGURE 2. Pneumococcal conjugate vaccine doses administered to children aged 0 through 59 months, by vaccine type and week --- Immunization Information System sentinel sites, United States, March 7--August 21, 2010
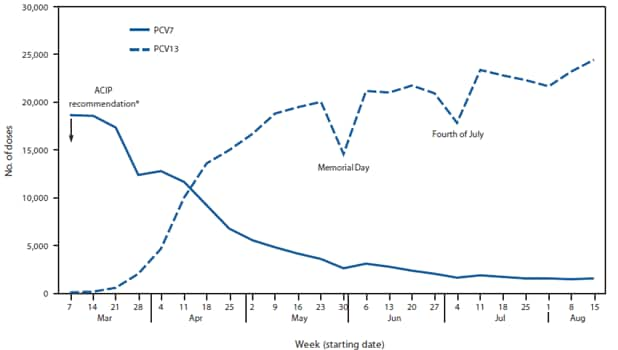
Abbreviations: ACIP = Advisory Committee on Immunization Practices; PCV7 = 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine.
* CDC. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children---Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59:258--61.
Alternate Text: The figure above shows pneumococcal conjugate vaccine doses administered to U.S. children aged 0 through 59 months, by vaccine type and week during March 7-August 21, 2010, according to Immunization Information System sentinel sites. Initial coverage with 13-valet pneumococcal conjugate vaccine (PCV13) occurred quickly after the Advisory Committee on Immunization Practices (ACIP) issued recommendations on used of PCV13.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


