|
TABLE. Percentage of core data elements* that were complete† in immunization information system (IIS) records for children aged <6 years --- United States, 2008 and 2009 |
|||
|---|---|---|---|
|
Core data element |
2008 (52 grantees) |
2009 (51 grantees) |
Change |
|
(%) |
(%) |
(%) |
|
|
First name |
100 |
100 |
0 |
|
Middle name |
68 |
69 |
+1 |
|
Last name |
100 |
100 |
0 |
|
Birth date |
100 |
100 |
0 |
|
Sex |
97 |
97 |
0 |
|
Birth state |
44 |
46 |
+2 |
|
Birth country |
28 |
28 |
0 |
|
Mother's first name |
67 |
71 |
+4 |
|
Mother's maiden name |
50 |
55 |
+5 |
|
Mother's last name |
59 |
63 |
+4 |
|
Vaccine type |
98 |
100 |
+2 |
|
Vaccine manufacture |
40 |
50 |
+10 |
|
Vaccination date |
98 |
100 |
+2 |
|
Vaccine lot number |
38 |
45 |
+7 |
|
Race§ |
59 |
63 |
+4 |
|
Ethnicity§ |
39 |
43 |
+4 |
|
Patient birth order |
63 |
61 |
-2 |
|
* Recommended by the National Vaccine Advisory Committee. Additional information available at http://www.cdc.gov/vaccines/programs/iis/stds/coredata.htm. † Calculated using the number of data field completions in IIS records and the overall number of IIS records. § Additional core data element recommended by the National Vaccine Advisory Committee in 2007. |
|||
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Progress in Immunization Information Systems --- United States, 2009
An immunization information system (IIS) is a confidential, computerized, population-based system that collects and consolidates vaccination data from vaccine providers and provides tools for designing and sustaining effective immunization strategies at the provider and program levels. Among the capabilities of an IIS are the capacity to inform vaccine providers of upcoming patient vaccination needs; generate vaccination coverage reports, patient reminders, or recalls for past due vaccinations; and interoperate with electronic health record (EHR) systems. In 2010, the Task Force on Community Preventive Services recommended that immunization information systems be used to increase vaccination coverage after showing strong evidence of their effectiveness (1). A Healthy People 2020 objective is to increase to 95% the percentage of children aged <6 years whose immunization records are housed in a fully operational IIS (2). To assess IIS progress toward meeting the Healthy People objective, CDC analyzed data from the 2009 Immunization Information Systems Annual Report (IISAR) survey (completed by 53 of 56 federal grantees with IIS sites), which indicated that 77% of all U.S. children aged <6 years participated in an IIS, an increase from 75% in 2008 (3). In addition, 59% of grantees reported being able to send and receive vaccination data using Health Level Seven (HL7) messaging standards, and 73% reported that some vaccine providers with EHR systems in their geographic area were providing vaccination data directly to an IIS from EHRs. Enhancing IIS and EHR with standards such as HL7 will provide greater consistency in data exchange and likely help to improve the quality and timeliness of IIS data.
To monitor progress toward IIS program objectives, CDC annually surveys 56 IIS grantees (50 states, five cities,* and the District of Columbia) via IISAR. In 2009, 53 (95%) of the 56 grantees completed the IISAR survey (Kentucky and Massachusetts were implementing a new IIS and did not have data to report; New Hampshire elected not to implement an IIS). The self-administered survey asks about vaccination coverage for all age groups, provider participation in IIS, and IIS functionality (e.g., managing vaccine inventory in the vaccine provider office, EHR communication with IIS, and conducting vaccine provider assessments using IIS).
Participation in an IIS
The percentage of children aged <6 years whose immunization records were housed in a fully operational IIS was calculated for each of the 56 grantees. The calculations were made by dividing the number of children participating in an IIS by the 2009 midyear U.S. Census projection of the population of children aged <6 years for that grantee geographic area.
In 2009, of the 53 responding grantees, 23 (43%) reported that >95% of children aged <6 years in their geographic area were participating in an IIS. Ten (19%) of the 53 reported participation ranging from 80% to 94% (Figure) (3). Overall in the United States, approximately 77% of children aged <6 years (18.4 million) participated in an IIS in 2009 (a small but statistically significant increase from 75% in 2008 [3]).
IIS Adherence to Standards
In 2001, the Technical Working Group of the National Immunization Program established 12 standards regarding the minimum technical functions an IIS should implement (4,5). Three of these standards were considered for this report: 1) electronically store data on all 17 core data elements recommended by the National Vaccine Advisory Committee (NVAC), 2) receive and process immunization information within 1 month of vaccine administration, and 3) exchange immunization records using HL7 standards, which allow for efficient transfer of records and data de-duplication within systems (6). To assess adherence to these three standards, data were analyzed from 51 of the 56 grantees (Chicago, Houston, Kentucky, Massachusetts, and New Hampshire were excluded) in 2009 and compared with data from 52 grantees in 2008.
In 2009, six of the 17 NVAC-recommended core data elements (i.e., first name, last name, birth date, sex, vaccine type, and vaccination date) had completion rates of ≥97% for children aged <6 years, a result similar to findings in 2008 (Table). In addition, nine of the remaining 11 core data elements showed increases in completion rates from 2008 to 2009.
Regarding the other standards, 70% of IIS data were received and processed within 1 month of vaccine administration, an increase from 67% in 2008 (3). Also, 30 (59%) of the 51 grantees reported the ability to send and receive HL7 messages, four (8%) grantees reported partial ability to meet HL7 capability by either sending or receiving messages, and 17 (33%) grantees reported having no HL7 functionality.
In 2009, 37 (73%) of 51 grantees reported that at least some vaccine provider--site EHR systems were providing immunization data directly to an IIS. A total of 3,618 provider-site EHR systems provided immunization data directly to a grantee IIS, compared with 1,848 in 2008. Of these 3,618 systems, 2,797 (77%) were among the 33 grantees with >80% child participation.
Reported by
W Brand, MPH, Public Health Informatics Institute, Decatur, Georgia. B Rasulnia, PhD, G Urquhart, MPH, Immunization Services Div, National Center for Immunization and Respiratory Diseases, CDC.
Editorial Note
Despite some progress in increasing the proportion of children aged <6 years whose immunization records are housed in an IIS, challenges remain to successful IIS implementation, such as resource costs to vaccine providers, and quality of data. Some challenges are being addressed through efforts to enhance interoperability of EHR and IIS, increase use of HL7 messaging, and offer vaccine provider incentives. These interventions can help 1) reduce the time from vaccine administration to inclusion of data in an IIS record, 2) reduce dual data entry by vaccine providers because vaccination data will only be entered into the EHR and then exchanged with the IIS using HL7 messaging, and 3) increase completeness of immunization information (core data elements and vaccination data) by adding data not collected previously by an IIS.
Provisions of the Health Information Technology for Clinical and Economic Health (HITECH) Act (7) are intended to accelerate adoption of nationally certified EHR systems, standardize EHR products, support growth in the health information technology workforce, and facilitate secure exchange of health data between disparate partners. A centerpiece of the HITECH Act is the EHR Incentive Program (8), administered by the Centers for Medicare and Medicaid Services (CMS). CMS provides financial incentives to eligible health-care providers, and hospitals must acquire certified products that support standards-based electronic reporting to IIS, including use of the HL7 table of vaccines administered. To receive their incentive payments, eligible professionals (outpatient vaccine providers) have to satisfy at least one of the following public health reporting requirements. They must conduct an HL7 messaging test, IIS reporting (and fulfill reporting requirements as per locality), laboratory reporting, or syndromic surveillance reporting. States can specify as mandatory any of the public health requirements for the Medicaid "meaningful use" program.
In 2010, CDC received HITECH funding for 20 IIS grantees to measurably enhance EHR-IIS interoperability. Over a 24-month project period, the 20 IIS grantees will be developing or enhancing HL7 messaging capacity and increasing the number of interfaces with EHRs. The grantees also will need to ensure adequate programmatic and technical capacity for increased electronic data submission testing, ensuring that electronic files submitted to EHR are complete and accurate. Finally, the grantees will coordinate with state health information technology coordinators and health information exchange organizations to ensure coordination with overall statewide plans, policies, and protocols for secure exchange of data using standards such as HL7 (9).
The findings in this report are subject to at least two limitations. First, although guidance on algorithms to validate their data are provided to IIS grantees by CDC, the data from the 2009 IISAR were self-reported and self-validated. Second, because some of the 56 grantees did not report data during the period studied, the nationwide IIS participation rates for children aged <6 years might be underestimated or overestimated.
Findings from the Taskforce on Community Preventive Services systematic review of the literature have highlighted how the IIS can be effective in increasing vaccination coverage (1). IIS offers capabilities such as patient reminder and recall systems, vaccine provider assessment and feedback, use of data for public health responses to outbreaks of vaccine-preventable disease, facilitation of vaccine management and accountability, and assessment of client vaccination status for decisions made by health-care providers (1). Enhancing IIS and EHR to adopt national standards and interoperability specifications will help provide greater consistency in data exchange and likely reduce interface costs over time. Increased IIS data accuracy, timeliness, and completeness can improve the quality of IIS-based vaccination coverage assessments, better support clinical decisions at the health-care provider level, and improve the data available for other public health functions.
References
- Guide to Community Preventive Services. Universally recommended vaccinations: immunization information systems. Atlanta, GA: Guide to Community Preventive Services; 2010. Available at http://www.thecommunityguide.org/vaccines/universally/imminfosystems.html. Accessed January 10, 2011.
- US Department of Health and Human Services. Healthy people 2020. Washington, DC: US Department of Health and Human Services; 2010. Available at http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23. Accessed January 10, 2011.
- CDC. Progress in immunization information systems---United States, 2008. MMWR 2010;59:133--5.
- National Immunization Program, Technical Working Group. 2001 minimum functional standards for registries. Atlanta, GA: National Immunization Program, Technical Working Group; 2001. Available at http://www.cdc.gov/vaccines/programs/iis/stds/min-funct-std-2001.htm. Accessed January 10, 2011.
- National Vaccine Advisory Committee. Development of community and state-based immunization registries: report of the National Vaccine Advisory Committee (NVAC). Atlanta, GA: National Vaccine Advisory Committee; 1999. Available at http://www.cdc.gov/vaccines/programs/iis/pubs/nvac.htm. Accessed January 10, 2011.
- CDC. HL7 version 2.5.1: implementation guide for immunization messaging. Atlanta, GA: CDC; 2010. Available at http://www.cdc.gov/vaccines/programs/iis/stds/downloads/hl7guide-08-2010.pdf. Accessed January 10, 2011.
- US Department of Health and Human Services, Office of the National Coordinator for Health Information Technology. HITECH programs. Washington, DC: Office of the National Coordinator for Health Information Technology; 2010. Available at http://healthit.hhs.gov. Accessed January 10, 2011.
- Centers for Medicare and Medicare Services. Electronic health records incentive programs. Baltimore, MD: Centers for Medicare and Medicare Services; 2010. Available at https://www.cms.gov/ehrincentiveprograms. Accessed January 10, 2011.
- US Department of Health and Human Services, CDC. American Recovery and Reinvestment Act funding announcement for enhancing the interoperability of electronic health records (EHR) and immunization information systems (IIS). Washington, DC: US Department of Health and Human Services, CDC; 2010. Available at http://www.grants.gov/search/search.do?mode=VIEW&oppId=54435. Accessed January 10, 2011.
* Chicago, Illinois; Houston and San Antonio, Texas; New York, New York; and Philadelphia, Pennsylvania.
What is already known on this topic?
An estimated 75% of all U.S. children aged <6 years (17.7 million children) participated in an immunization information system (IIS) in 2008.
What is added by this report?
In 2009, 77% of all U.S. children aged <6 years (18.4 million children) participated in an IIS. Also, 59% of IIS grantees reported being able to send and receive Health Level Seven (HL7) messages, and another 8% of grantees with IIS were partially able to meet HL7 capability by either sending or receiving messages.
What are the implications for public health practice?
Enhancing the interoperability of IIS and electronic health record systems will help provide greater consistency in data exchange and likely reduce interface costs over time. Increased IIS data accuracy, timeliness, and completeness can improve the quality of IIS-based coverage assessments, better support clinical decisions at the health-care provider level, and increase availability of the data for other public health functions.
FIGURE. Percentage of children aged <6 years participating in a grantee immunization information system --- 50 states, five cities, and District of Columbia, 2009
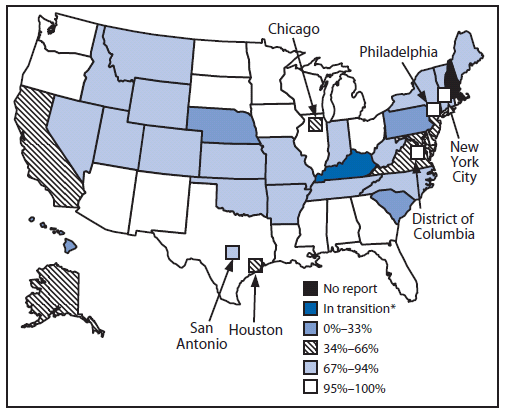
* Grantee is implementing a new IIS project.
Alternate Text: The figure above is U.S.map showing the percentage of children aged <6 years participating in an immunization information system (IIS) in the 50 states, five cities, and District of Columbia in 2009. Of the 53 responding grantees, 23 (43%) reported that >95% of children aged <6 years in their geographic area were participating in an IIS. Ten (19%) of the 53 reported participation ranging from 80% to 94%. Overall in the United States, approximately 77% of children aged <6 years (18.4 million) participated in an IIS in 2009 (a small but statistically significant increase from 75% in 2008.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


