|
TABLE. Estimated number and rate of emergency department visits for nonmedical use of opioid analgesics and benzodiazepines, by selected characteristics --- United States, 2008 |
||||||
|---|---|---|---|---|---|---|
|
Characteristic |
Opioid analgesics |
Benzodiazepines |
||||
|
No. |
Rate* |
95% CI† |
No. |
Rate |
95% CI |
|
|
Total |
305,900 |
100.6 |
(75.6--125.6) |
271,700 |
89.4 |
(61.6--117.1) |
|
Sex |
||||||
|
Male |
150,800 |
100.6 |
(74.9--126.3) |
119,600 |
79.7 |
(57.1--102.4) |
|
Female |
155,000 |
100.6 |
(75.1--126.1) |
152,100 |
98.7 |
(64.8--132.5) |
|
No. of drugs (including alcohol) |
||||||
|
One drug |
116,800 |
38.4 |
(31.4--45.4) |
56,900 |
18.7 |
(15.1--22.3) |
|
Multidrug |
189,000 |
62.2 |
(42.8--81.6) |
214,800 |
70.6 |
(45.9--95.4) |
|
Alcohol involvement |
46,200 |
15.2 |
(10.9--19.5) |
68,600 |
22.6 |
(14.6--30.6) |
|
Admitted to hospital |
72,700 |
23.9 |
(15.7--32.1) |
81,300 |
26.8 |
(14.5--39.0) |
|
Source: Substance Abuse and Mental Health Services Administration (SAMHSA)'s Drug Abuse Warning Network (DAWN), 2004--2008. Additional information available in appendix C at http://dawninfo.samhsa.gov/files/ed2007/dawn2k7ed.pdf. * Per 100,000 population † Confidence interval. |
||||||
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Emergency Department Visits Involving Nonmedical Use of Selected Prescription Drugs --- United States, 2004--2008
Rates of overdose deaths involving prescription drugs increased rapidly in the United States during 1999--2006 (1). However, such mortality data do not portray the morbidity associated with prescription drug overdoses. Data from emergency department (ED) visits can represent this morbidity and can be accessed more quickly than mortality data. To better understand recent national trends in drug-related morbidity, CDC and the Substance Abuse and Mental Health Services Administration (SAMHSA) reviewed the most recent 5 years of available data (2004--2008) on ED visits involving the nonmedical use of prescription drugs from SAMHSA's Drug Abuse Warning Network (DAWN). This report describes the results of that review, which showed that the estimated number of ED visits for nonmedical use of opioid analgesics increased 111% during 2004--2008 (from 144,600 to 305,900 visits) and increased 29% during 2007--2008. The highest numbers of ED visits were recorded for oxycodone, hydrocodone, and methadone, all of which showed statistically significant increases during the 5-year period. The estimated number of ED visits involving nonmedical use of benzodiazepines increased 89% during 2004--2008 (from 143,500 to 271,700 visits) and 24% during 2007--2008. These findings indicate substantial, increasing morbidity associated with the nonmedical use of prescription drugs in the United States during 2004--2008, despite recent efforts to control the problem. Stronger measures to reduce the diversion of prescription drugs to nonmedical purposes are warranted.
DAWN is a public health information system that tracks the impact of drug use, misuse, and abuse in the United States by monitoring drug-related hospital ED visits. In a manner similar to the National Electronic Injury Surveillance System,* DAWN uses a sample of EDs to estimate national ED visit rates (2). DAWN collects data from a stratified, simple random sample of approximately 220 nonfederal, short-stay, general hospitals that operate 24-hour EDs in the United States. DAWN's sampling frame is based on the American Hospital Association annual survey database and is updated annually to reflect new, closed, merged, and demerged hospitals, and to give new hospitals an opportunity to be selected into the sample.
The DAWN sample is designed to produce estimates and trends for individual metropolitan areas (12 in 2008) and the United States overall (2). To achieve this, the selected metropolitan areas are oversampled. The oversampled hospitals and a supplementary sample of hospitals outside those areas together capture ED visits in all 50 states and the District of Columbia. Trained DAWN reporters review the medical charts of all patients treated in the participating hospital EDs to identify visits for conditions induced by or related to drug use. DAWN reporters record de-identified information from the ED medical records using standard abstraction forms. DAWN does not conduct interviews or follow-up with clinicians, patients, or family members. Rates presented in this report are based on the numbers of ED visits weighted so that they are representative of the U.S. population. Denominators for this report were based on U.S. Census postcensal estimates. Differences between counts and between rates were tested using two-sided t tests.†
DAWN defines nonmedical use of a prescription or over-the-counter drug as taking a higher-than-recommended dose, taking a drug prescribed for another person, drug-facilitated assault, or documented misuse or abuse, all of which must be documented in the medical record. DAWN classifies suicide attempts, patients seeking detoxification, and unintentional ingestions in other categories.
For 2008, a total of 231 hospitals submitted data that were used for estimation. The overall weighted hospital response rate was 32.9% (response rates have been stable from year to year). In 2008, DAWN recorded 351,697 drug-related ED visits. On average, a DAWN member hospital submitted 1,522 DAWN cases.
DAWN estimated 1.6 million ED visits for the misuse and abuse of all drugs in 2004 and 2.0 million in 2008. Among these, illicit drugs such as cocaine and heroin were involved in 1.0 million visits in both 2004 and 2008, whereas prescription or over-the-counter drugs used nonmedically were involved in 0.5 million visits in 2004 and 1.0 million visits in 2008. The estimated number of ED visits involving nonmedical use of opioid analgesics§ increased from 144,600 in 2004 to 305,900 in 2008 (111%, p<0.001), whereas rates increased from 49.4 per 100,000 to 100.6 per 100,000, an increase of 104% (p<0.05).
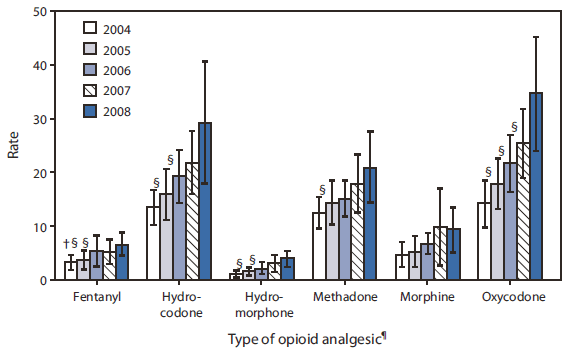
ED visit rates for opioid analgesics were highest for oxycodone, hydrocodone, and methadone during the entire study period (Figure 1). Estimated ED visits involving oxycodone increased from 41,700 to 105,200 (p<0.001), and rates increased from 14.2 per 100,000 to 34.6 per 100,000, an increase of 144% (p<0.05). The estimated number of ED visits involving nonmedical use of benzodiazepines increased from 143,500 in 2004 to 271,700 in 2008 (89%, p=0.01), and rates increased from 49.0 to 89.4 per 100,000, an increase of 82% (p<0.05). The increases in numbers of ED visits during 2004--2008 for individual benzodiazepines were significant: alprazolam (125%, p=0.01), clonazepam (72%, p<0.001), diazepam (70%, p=0.02), and lorazepam (107%, p=0.006), as was the increase for the sleep aid zolpidem (121%, p=0.002). Carisoprodol-related visits also increased significantly (132%, p=0.04). The estimated number of visits for alprazolam in 2008 (104,800) was more than twice the number for the next most common benzodiazepine, clonazepam (48,400).
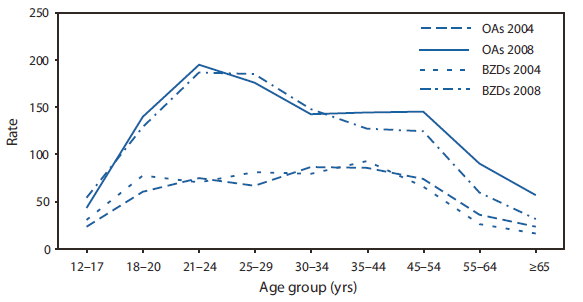
Although women had more benzodiazepine-related visits than men (Table), this difference was not statistically significant. Among opioid analgesic--related visits, 38% did not involve any other drug (including alcohol); the corresponding figure was 21% for benzodiazepine-related visits. Benzodiazepines were involved in 26% of opioid analgesic--related visits. Alcohol was involved in 15% and 25% of visits for opioids and benzodiazepines, respectively. Approximately one in four patients was admitted. For the year 2008, rates for both types of drugs increased sharply after age 17 years, peaked in the 21--24 years age group, and declined after age 54 years (Figure 2). The largest increases during 2004--2008 occurred among persons aged 21--29 years.
Reported by
R Cai, MS, E Crane, PhD, K Poneleit, MPH, Office of Applied Studies, Substance Abuse and Mental Health Services Admin. L Paulozzi, MD, Div of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC.
Editorial Note
The number of ED visits involving nonmedical use of prescription or over-the-counter drugs increased rapidly during 2004--2008, and by 2008 matched the number of ED visits involving illicit drugs. ED visits involving such pharmaceuticals accounted for all of the growth in overall drug misuse/abuse rates during 2004--2008. ED visits involving opioids or benzodiazepines were the largest contributors to the increase in ED visits involving the nonmedical use of prescription or over-the-counter drugs.
Notably, results from 2008 indicate that in addition to the large increase in visits compared with 2004, peak visit rates for both opioids and benzodiazepines appear to have shifted into the 21--24 and 25--29 years age groups and away from the 30--34 and 35--44 years age groups. As late as 2006, the peak mortality rate for fatal drug overdoses involving opioid analgesics had been in the 35--54 years age group (1).
The 5-year increase in ED visit rates reflects, in part, substantial increases in the prescribing of these classes of drugs (3). The increase also might reflect an increase in the rate of nonmedical use of prescription drugs per 1,000 prescriptions, as has been observed for selected opioids (4). In the 2008 National Survey of Drug Use and Health (NSDUH), 4.6% of persons aged ≥18 years reported past-year nonmedical use of prescription pain relievers, and 2.1% reported nonmedical use of tranquilizers, a category that includes benzodiazepines (5).
In contrast to the results of this study, NSDUH results have shown no increase in self-reported rates of nonmedical use of selected pharmaceuticals since 2004 (5). Increasing ED visit rates in the context of stable self-reported nonmedical use rates might indicate that persons seen in EDs are different from typical respondents to NSDUH; a shift toward riskier types of pain relievers and benzodiazepines, riskier modes of use, more frequent or heavier use; and/or an increased tendency to seek emergency care because of greater awareness of the serious consequences of nonmedical use of such drugs. However, changes in health-seeking behavior would not affect changes in drug-related deaths, and DAWN ED visit trends are consistent with medical examiner data from six states also tracked by DAWN (Maine, Maryland, New Hampshire, New Mexico, Utah, and Vermont). In these states, the number of nonsuicidal deaths related to benzodiazepines increased 64.2%, and the number related to opioid analgesics other than methadone increased 47.4% during 2004--2007 (6).
The relative magnitudes of the rates shown generally reflect prescription volumes. For example, the benzodiazepine with the highest number of ED visits, alprazolam, was the most prescribed benzodiazepine in the United States in 2008, with an estimated 44 million prescriptions (7). However, some exceptions exist: hydrocodone was prescribed nearly 124 million times and oxycodone nearly 45 million times in 2008, but hydrocodone ED rates were not higher than oxycodone ED rates. The high frequency of multidrug involvement is a reflection of the tendency of persons who abuse drugs to combine them to moderate or enhance their effects. The lower proportion of single-drug ED visits among benzodiazepine ED visits compared with opioid analgesic visits is consistent with the relative rarity of a benzodiazepine being the sole drug involved in a fatal overdose (6,8).
The findings in this report are subject to at least four limitations. First, the drugs involved in ED visits might not all be identified and documented. The extent to which ED staff members document drug involvement might have increased over time. Second, information on the motivation for use might be incomplete; some of the ED visits might have represented suicide attempts. Third, rates based on population cannot be used to determine risk per patient or per prescription. Finally, distinguishing drugs taken for nonmedical and medical reasons is not always possible, especially when multiple drugs are involved.
These increases in nonmedical use of pharmaceuticals suggest that previous prevention measures, such as provider and patient education and restrictions on use of specific formulations, have not been adequate. Given the societal burden of the problem, additional interventions are urgently needed, such as more systematic provider education, universal use of state prescription drug monitoring programs by providers, the routine monitoring of insurance claims information for signs of inappropriate use, and efforts by providers and insurers to intervene when patients use drugs inappropriately (9,10). This report also reinforces the value of timely, population-based national surveillance for nonmedical use of drugs, which can be used to assess the effect of such interventions.
References
- Warner M, Chen LJ, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999--2006. NCHS data brief, no 22. Hyattsville, MD: National Center for Health Statistics; 2009.
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2007: national estimates of drug-related emergency department visits. Available at http://dawninfo.samhsa.gov/files/ed2007/dawn2k7ed.pdf. Accessed June 10, 2010.
- Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Safety 2006;15:618--27.
- Dormitzer C. Summary of drug abuse "rates" in the United States. Available at http://www.fda.gov/ohrms/dockets/ac/08/slides/2008-4356s1-04-fda-corepresentations.ppt. Accessed June 10, 2010.
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health: national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009. HHS publication no. SMA 09-4434. Available at http://www.oas.samhsa.gov/nsduh/2k8nsduh/2k8results.cfm. Accessed June 10, 2010.
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2007: area profiles of drug-related mortality. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009. HHS publication no. SMA 09-4407. Available at http://dawninfo.samhsa.gov/pubs/mepubs. Accessed June 10, 2010.
- SDI/Verispan. 2008 top 200 generic drugs by total prescriptions. Available at http://drugtopics.modernmedicine.com/top200gen. Accessed June 10, 2010.
- Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA 2008;300:2613--20.
- Kraman P. Drug abuse in America---prescription drug diversion. Lexington, KY: Council of State Governments; 2004. Available at http://www.csg.org/knowledgecenter/docs/ta0404drugdiversion.pdf. Accessed June 10, 2010.
- CDC. CDC's issue brief: unintentional drug poisoning in the United States. Available at: http://www.cdc.gov/homeandrecreationalsafety/poisoning/brief.htm. Accessed June 10, 2010.
* U.S. Consumer Product Safety Commission. NEISS All Injury Program: sample design and implementation. Washington, DC: U.S. Consumer Product Safety Commission; 2001.
† To minimize the effect of nonresponse, the DAWN weighting plan includes nonresponse adjustment factors for within-hospital nonresponse and hospital nonresponse; the weighting plan also includes a poststratification adjustment factor that reconciles the weighted number of total visits for responding hospitals with the number of total visits from the most recent American Hospital Association Annual Survey Database. Estimates for all DAWN-eligible hospitals in the United States are produced by applying poststratified weights to the data received from the sampled hospitals. Estimates (and their associated rates and confidence intervals) are suppressed if based on an unweighted count of fewer than 30 cases, if the estimate is less than 30, or if the relative standard error is greater than 50%. The DAWN data collection protocol aims for 100% chart review but accepts any percentage above 90% as complete. In EDs where chart subsampling has been implemented, reporters review 100% of the charts for sampled days. Chart subsampling is employed at large facilities with more than 3,500 visits per month. In these facilities, charts are typically reviewed every other day. Additional information about DAWN is available in appendix C at http://dawninfo.samhsa.gov/files/ed2007/dawn2k7ed.pdf.
§ An additional 60,900 visits involving "opiates/opioids unspecified" were not included because some might have involved heroin.
What is already known on this topic?
Deaths involving the nonmedical use of prescription drugs increased in the United States through 2006.
What is added by this report?
Emergency department visits involving nonmedical use of two types of prescription drugs, opioid analgesics and benzodiazepines, more than doubled during 2004--2008 in the United States; visits for misused prescription and over-the-counter drugs are now as common as emergency department visits for use of illicit drugs.
What are the implications for public health practice?
Recent public health and law enforcement measures intended to prevent nonmedical use of such drugs have not prevented rate increases, and additional measures are needed urgently.
FIGURE 1. Rates of emergency department (ED) visits* for nonmedical use of selected opioid analgesics, by type --- United States, 2004--2008
Source: Substance Abuse and Mental Health Services Administration (SAMHSA)'s Drug Abuse Warning Network (DAWN), 2004--2008. Additional information available in appendix C at http://dawninfo.samhsa.gov/files/ed2007/dawn2k7ed.pdf.
* Per 100,000 population.
† 95% confidence interval.
§ Rate significantly less than the rate in 2008, by two-sided t test (p<0.05).
¶ Drug types include combination products (e.g., combinations of oxycodone and aspirin).
Alternate Text: The figure above shows rates of emergency department (ED) visits for nonmedical use of selected opioid analgesics, by type, in the United States during 2004-2008. ED visit rates for opioid analgesics were highest for oxycodone, hydrocodone, and methadone during the entire study period.
FIGURE 2. Age-specific rates of emergency department visits* for nonmedical use of opioid analgesics (OAs) and benzodiazepines (BZDs) --- United States, 2004 and 2008
Source: Substance Abuse and Mental Health Services Administration (SAMHSA)'s Drug Abuse Warning Network (DAWN), 2004--2008. Additional information available in appendix C at http://dawninfo.samhsa.gov/files/ed2007/dawn2k7ed.pdf.
* Per 100,000 population.
Alternate Text: The figure above shows age-specific rates of emergency department visits for nonmedical use of opioid analgesics and benzodiazepines in the United States for 2004 and 2008. In 2008, ED visit rates for both types of drugs increased sharply among persons aged >18 years, peaked in the 21-24 years age group, and declined after age 54 years.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


