|
What is already known on this topic? Approximately 65% of all U.S. children aged <6 years (15 million children) participated in an immunization information system (IIS) in 2006. What is added by this report? In 2008, 75% of all U.S. children aged <6 years (approximately 18 million children) participated in an IIS. What are the implications for public health practice? Enhanced interoperability between electronic health record systems and IISs can improve 1) the completeness of immunization histories available to clinicians and public health practitioners, 2) the timeliness of immunization data submission to IISs, 3) the quality of IIS coverage assessments, and 4) the data available to other public health systems. |
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Progress in Immunization Information Systems --- United States, 2008
Please note: An erratum has been published for this article. To view the erratum, please click here.
Immunization information systems (IISs) are confidential, computerized information systems that collect and consolidate vaccination data from multiple health-care providers, generate reminder and recall notifications, and assess vaccination coverage within a defined geographic area (1). A CDC program goal for 2010 is to achieve >95% participation in an IIS (defined as having two or more recorded vaccinations) among children aged <6 years. To monitor progress toward this goal, CDC annually surveys immunization grantees in 50 states, five cities, and the District of Columbia, using the Immunization Information Systems Annual Report (IISAR). All 56 grantees were asked to complete the IISAR; 52 did so for 2008. This report highlights results from the 2008 IISAR, which indicated that 75% of all U.S. children aged <6 years (approximately 18 million children) participated in an IIS in 2008, an increase from 65% in 2006 (1). The majority of grantees (82%) reported that their IIS had the capacity to track vaccinations for persons of all ages, compared with 70% in 2006 (1). Data-quality measures of timeliness and completeness indicated that in 2008, 67% of IIS data were received and processed within 30 days of vaccine administration, and data were reported for six of 17 core data elements in >90% of IIS records (both measures are similar to 2006 results). Increased provider use of electronic health record systems can benefit IISs and their users by producing immunization records that are more timely and complete.
The 2008 IISAR, a self-administered, Internet-based questionnaire, was made available to state and local immunization program managers in the 50 states, five cities,* and the District of Columbia that receive funding under section 317 of the Public Health Service Act,† as part of an annual reporting requirement. As in previous years, respondents were asked about the number of children aged <6 years participating in the IIS; the number of health-care--provider sites participating; child, adolescent, and adult vaccination series completion and coverage; and programmatic and technical capabilities (e.g., data linkages with other health information systems, data quality and use, vaccine management, software and hardware capabilities, and report functions). All 56 grantees were asked to complete the IISAR, and 52 did so for 2008. The percentage of all U.S. children aged <6 years participating in each IIS was calculated by dividing the number of children aged <6 years participating in the IIS by the 2008 mid-year U.S. Census projection for all children aged <6 years.
Approximately 18 million U.S. children aged <6 years (75% of all U.S. children in that age group) participated in an IIS in 2008, compared with 15 million (65%) in 2006 (1). Of the 52 responding grantees, 22 grantees (42%) reported that >95% of children aged <6 years participated in the IIS (Figure), compared with 15 of 56 (27%) grantees in 2006. For 2008, a total of 11 (21%) grantees reported child participation ranging from 80% to 94%, compared with 10 of 56 (18%) in 2006.
In 2008, an estimated 23 million adolescents aged 11--18 years (65% of all U.S. adolescents) participated in an IIS, compared with 22.3 million (66%) in 2006. Also in 2008, 54 million adults aged >19 years (24% of all U.S. adults aged >19 years) participated in an IIS, compared with 33.5 million (18%) in 2006. Nationally, 95 million persons of all ages (33% of the U.S. population) were participating in an IIS in 2008. Overall, IIS participation for children aged <6 years accounted for 19% of all participants in IISs, whereas adolescents and adults accounted for 25% and 57%, respectively.
IIS timeliness is measured by the interval between 1) a child's birth and the establishment of an IIS record and 2) administration of a vaccine and submission of its dose-related data to an IIS (2). In 2008, 71% of IISs reported that an IIS record had been established for newborn children within 6 weeks of birth (compared with 69% in 2006) (1). For all vaccine doses administered and recorded in 2008 to children aged <6 years, 67% of all vaccine data were received and processed in the IIS within 30 days of vaccine administration, compared with 69% in 2006.
Completeness of IIS data is measured by 1) assessing IIS records for data on National Vaccine Advisory Committee (NVAC) core data elements (3) and 2) determining the proportion of children aged 19--35 months participating in an IIS who were recorded as having received the complete 4:3:1:3:3:1§ series of recommended vaccine doses. The first completeness measure is assessed by examining required core data elements (4) to determine the proportion that are >90% complete in IIS records. Core data elements are designed to standardize a set of patient demographic and vaccine event elements, which are necessary for identification and removal of duplicate records and data exchange with other health information systems (2,3). In 2008, very little or no change occurred in the percentage of data in IISs for nine of the 17 core data elements (compared with 2006) (1). (Table).
The second completeness measure indicated that of the 4.4 million children aged 19--35 months participating in an IIS, an estimated 2.0 million (47%) had documentation in the IIS of having fully completed the recommended 4:3:1:3:3:1 vaccination series in 2008, compared with 45% in 2006 (1). Completeness of IIS vaccination histories varied substantially by grantee. In 2008, 30% of children aged 19--35 months participating in an IIS who had an incomplete vaccination series were only 1 dose away from series completion.
Reported by
J Kelly, V Heboyan, PhD, B Rasulnia, PhD, G Urquhart, MPH, Immunization Information Systems Support Br, National Center for Immunization and Respiratory Diseases, CDC.
Editorial Note
Grantees have made substantial progress towards CDC's 2010 goal of >95% participation in an IIS by children aged <6 years. From 2006 to 2008, participation increased from 65% to 75%. This upward trend in participation suggests that IISs might be on target to meet the CDC 2010 goal. However, although participation rates have increased, data-quality issues continue to pose challenges.
The push to include more adolescent and adult vaccination data in IISs, in addition to the complexities of receiving administrative data from multiple sources (e.g., vital records, health plans, billing systems, and Medicaid/Medicare), might have contributed to the lack of progress on data-quality measures. CDC's current efforts to improve IIS data quality include collaboration with grantees and the American Immunization Registry Association to develop best practices for IIS functionality (5). CDC IIS sentinel sites¶ will begin to incorporate some of these guidelines within the next year.
Although not directly comparable because of the differences in methodology and sampling techniques, comparison of results from IIS 4:3:1:3:3:1 series completion with results from the National Immunization Survey (NIS) is useful. For 2008, IISAR data indicated that 47% of children participating in an IIS had complete histories for the full 4:3:1:3:3:1 series. This is lower than the estimated proportion (76%) of children reported by NIS to have received the same series (6).
The findings in this report are subject to at least two limitations. First, data from the 2008 IISAR were not validated by independent review. Second, because some grantees did not report data, the nationwide IIS participation rates for children aged <6 years and providers might be underestimated.
An additional CDC initiative is to implement national standards to enhance the interoperability of electronic data exchanges between electronic health record systems in immunization provider offices and IISs. Enhanced interoperability will improve the completeness of immunization histories available to clinicians and public health practitioners, the timeliness of immunization data submission to IISs, the quality of IIS coverage assessments, and the data available to other public health systems (e.g., vaccine-preventable disease surveillance units).
References
- CDC. Immunization information system progress---United States, 2006. MMWR 2008;57:289--91.
- National Vaccine Advisory Committee. 2001 minimum functional standards for registries. Available at http://www.cdc.gov/vaccines/programs/iis/stds/min-funct-std-2001.htm. Accessed February 4, 2010.
- National Vaccine Advisory Committee. Development of community and state-based immunization registries: report of the National Vaccine Advisory Committee (NVAC). Atlanta, GA: US Department of Health and Human Services, CDC; 1999. Available at http://www.cdc.gov/vaccines/programs/iis/pubs/nvac.htm. Accessed February 4, 2010.
- Hinman AR, Urquhart GA, Strikas RA; the National Vaccine Advisory Committee. Immunization information systems: National Vaccine Advisory Committee progress report, 2007. J Public Health Manag Pract 2007;13:553--8.
- American Immunization Registry Association. Modeling of Immunization Registry Operations Workgroup (MIROW). IIS operational best practice guidelines. Available at http://www.immregistries.org/pubs/mirow.phtml. Accessed February 4, 2010.
- CDC. National, state, and local area vaccination coverage among children aged 19--35 months---United States, 2008. MMWR 2009;58:921--6.
* Chicago, Illinois; Houston, Texas; New York, New York; Philadelphia, Pennsylvania; and San Antonio, Texas.
† 42 USC Sect. 247b, project grants for preventive services.
§ Includes 4 doses diphtheria and tetanus toxoids and acellular pertussis vaccines, 3 doses poliovirus vaccine, 1 dose measles, mumps, and rubella vaccine, 3 doses Haemophilus influenzae type B vaccine, 3 doses hepatitis B vaccine, 1 dose varicella.
¶ Additional information available at http://www.cdc.gov/vaccines/programs/iis/activities/sentinel-sites.htm.
FIGURE. Percentage of children aged <6 years participating in the grantee immunization information system (IIS)* --- United States, 50 states, five cities, and the District of Columbia,† 2008
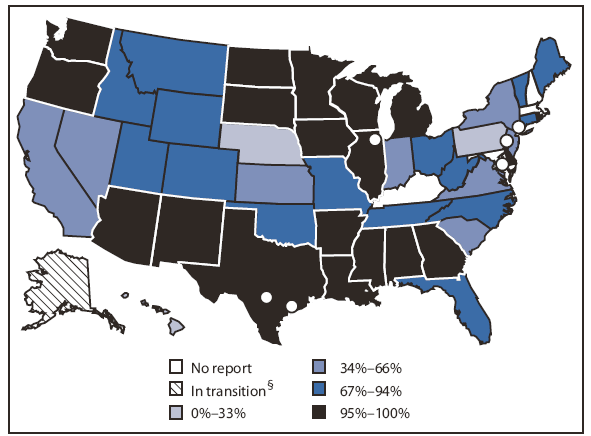
* The percentage of all U.S. children aged <6 years participating in each IIS was calculated by dividing the number of children aged <6 years participating in the IIS by the 2008 mid-year U.S. Census projection for all children aged <6 years.
† Chicago, Illinois (40%); District of Columbia (100%); Houston, Texas (54%); New York, New York (100%); Philadelphia, Pennsylvania (100%); and San Antonio, Texas (72%).
§ In transition is defined as a grantee implementing a new IIS product.
Alternate Text: The figure above shows the percentage of children aged <6 years participating in the grantee immunization information system (IIS) in 50 U.S. states, five cities, and the District of Columbia in 2008. Approximately 18 million U.S. children aged <6 years (75% of all U.S. children in that age group) participated in an IIS in 2008, compared with 15 million (65%) in 2006. Of the 52 responding grantees, 22 grantees (42%) reported that >95% of children aged <6 years participated in the IIS.
|
TABLE. Percentage of core data elements* that were complete† in immunization information system (IIS) records for children aged <6 years --- United States, 2006 and 2008 |
|||
|---|---|---|---|
|
Core data element |
2006 (N = 51) (%) |
2008 (N = 52) (%) |
Change (%) |
|
First name |
100 |
100 |
0 |
|
Middle name |
67 |
68 |
+1 |
|
Last name |
100 |
100 |
0 |
|
Birth date |
100 |
100 |
0 |
|
Sex |
96 |
97 |
+1 |
|
Birth state |
54 |
44 |
-10 |
|
Birth country |
18 |
28 |
+10 |
|
Mother's first name |
71 |
67 |
-4 |
|
Mother's maiden name |
55 |
50 |
-5 |
|
Mother's last name |
66 |
59 |
-7 |
|
Vaccine type |
99 |
98 |
-1 |
|
Vaccine manufacture |
37 |
40 |
+3 |
|
Vaccination date |
99 |
98 |
-1 |
|
Vaccine lot number |
37 |
38 |
+1 |
|
Race§ |
60 |
59 |
-1 |
|
Ethnicity§ |
42 |
39 |
-3 |
|
Patient birth order |
---¶ |
63 |
--- |
|
* Recommended by the National Vaccine Advisory Committee. Additional information available at http://www.cdc.gov/vaccines/programs/iis/stds/coredata.htm. † Calculated by number of data field completions in IIS records and the overall number of IIS records. § Additional required core data elements as recommended by the National Vaccine Advisory Committee in 2007. ¶ Not available. |
|||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


