Technical Report 1: Multi-National Mpox Outbreak, United States, 2022
Mpox Archive Content
You are viewing an archived web page, collected from CDC’s Mpox website. The information on this web page may be out of date.
Report reflects data and terms used as of July 25, 2022, prior to the World Health Organization’s decision to adopt the term “mpox.”
This is a technical report intended for scientific audiences. Additional information, including materials targeted to the general public, are available on the mpox site.
The purpose of this report is to provide timely updates regarding CDC’s ongoing response to the mpox outbreak in the United States, and to share preliminary results of new analyses that can improve understanding of the outbreak and inform further scientific inquiry. This report is intended for a technical audience; each report features a combination of standing topics and the results of special analyses.
Executive Summary
Scientists at the U.S. Centers for Disease Control and Prevention (CDC), along with state and local public health partners, are tracking 3,487 cases of monkeypox in the United States as of July 25, 2022. CDC is also tracking multiple clusters of monkeypox that have been reported globally in 69 countries that do not normally report monkeypox.
CDC is urging healthcare providers in the United States to be on alert for patients who have rash illnesses consistent with monkeypox. Distinguishing features include papules, vesicles, pustules, or scabs that are deep-seated, firm or rubbery, and have well-defined round borders. The lesions can sometimes be umbilicated, i.e., have a dent in the middle of them. They may be painful, painless, or itchy. People with monkeypox may develop symptoms including fever, headache, muscle aches, exhaustion, or swollen lymph nodes.
Monkeypox can spread between people through close contact, skin-to-skin contact including sexual contact with a person with monkeypox, or contact with contaminated fomites (e.g., shared linens). While anyone can catch monkeypox if they have close contact with someone who has monkeypox, regardless of gender identity or sexual orientation, many of those affected in the current global outbreaks are gay, bisexual, or other men who have sex with men. Healthcare providers should be on alert for monkeypox regardless of a patient’s travel history, gender identity, or sexual orientation; reported contact with someone who has monkeypox or who has a rash suspicious for monkeypox may assist with clinician decision-making. Additional clinical guidance is available on the CDC website.
Summary: U.S. Case Data
Domestic: On May 17, 2022, the United States confirmed the first monkeypox case in Massachusetts. As of July 25, 2022, there are 3,487 cases in 45 states, the District of Columbia, and Puerto Rico. These case counts include those who tested positive for either monkeypox virus or orthopoxvirus (OPX) as described in the case definition.
The median age of patients is 35 years (range 18 to 76). Of the 1,383 patients with information on sex assigned at birth, 99.1% were assigned male sex (13 assigned female sex). Of the 870 patients with information on gender identity, 1 self-reported as transgender male (July 25, 2022).
Among the male patients with information on sexual activity, 99% (n=624) reported male to male sexual contact. Approximately 38% (n=524) of patients with known race/ethnicity are white/non-Hispanic, 26% (n=358) are Black, and 32% (n=445) are Hispanic (of any race). Data are missing for a large number of cases.
Many of the initial patients reported international travel in the 21 days prior to symptom onset, visiting countries not known to experience endemic monkeypox and participating in large festivals and other activities where close, personal, skin-to-skin contact likely occurred. Recent travel history does not confirm the person acquired their infection while traveling. Since late June, an increasing number of reported cases have been linked to local community transmission.
Case data are voluntarily reported by states.
Epidemiological criteria for cases are one of the following.
Reports of contact with person(s) with a type of rash or who received a diagnosis of confirmed or probable monkeypox within 21 days of the illness onset. The rash can be macular (flat and reddened), papular (raised), vesicular (small, fluid filled), pustular (bulging with fluid inside), generalized or localized, or discrete or confluent.
Had close or intimate in-person contact with individuals in a social network experiencing monkeypox activity.
Traveled outside the United States to a country with confirmed cases of monkeypox or where the virus is endemic.
Had contact with a dead or live wild animal or exotic pet that is an African endemic species or used a product derived from such animals.
Probable cases meet one of the epidemiologic criteria and demonstrate the presence of:
- Orthopoxvirus DNA by polymerase chain reaction of a clinical specimen OR
- Orthopoxvirus using immunohistochemical or electron microscopy testing methods. These cases have no suspicion of other recent Orthopoxvirus exposure and demonstrate detectable levels of anti-orthopoxvirus IgM antibody during the period of 4 to 56 days after rash onset.
Confirmed cases meet the possible case definition above, but also have one of the following components.
- Demonstration of presence of monkeypox virus DNA by polymerase chain reaction testing or Next-Generation sequencing of a clinical specimen.
- Isolation of monkeypox virus in culture from a clinical specimen.
Resource: Case Use Definitions For the 2022 Monkeypox Response
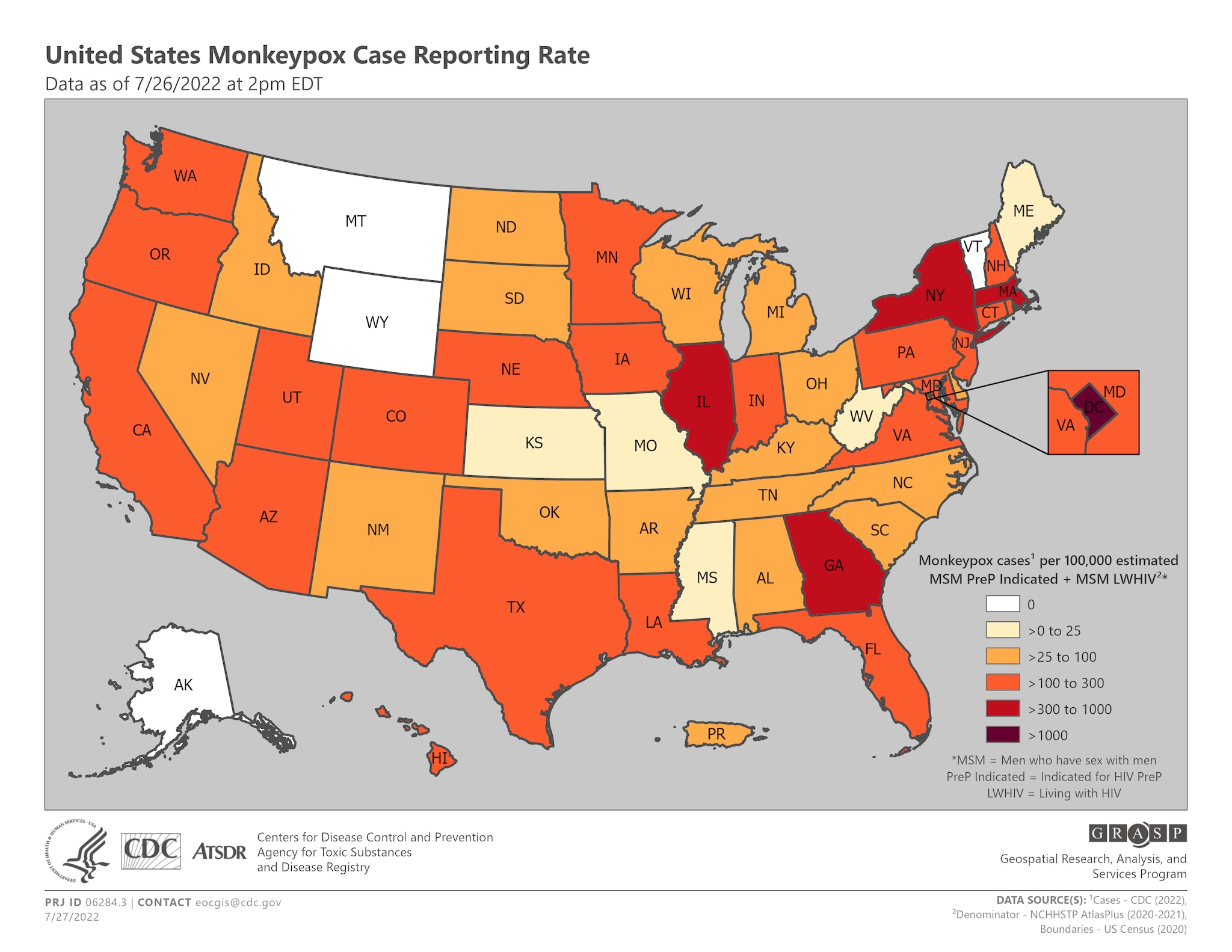
Figure 1. Monkeypox case incidence/diagnosis rate
CDC is tracking 17,492 confirmed cases of monkeypox in 69 non-endemic countries, territories, and areas associated with the 2022 Multi-National outbreak. This count may include confirmed cases not yet reported in World Health Organization (WHO) official counts.
2022 Monkeypox and Orthopoxvirus Global Outbreak Map
Additional data on the global situation is available on the WHO and ECDC
Below is an epidemiological curve of monkeypox cases reported to CDC through July 22, 2022 (for 2,657 cases with available reporting dates)
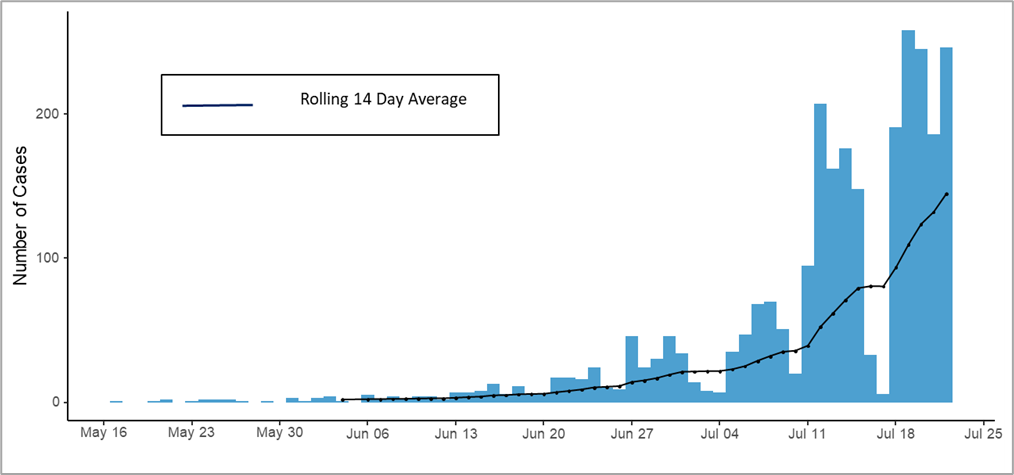
Figure 2. Epidemiological curve of Monkeypox cases, by reporting date (defined as LRN positive date, case reporting to the CDC Call Center, or case entry into Data Collation and Integration for Public Health Event Responses (DCIPHER)). Dates for some cases may be updated when additional LRN test dates are provided to CDC via DCIPHER.
The figure below presents the daily cumulative counts of monkeypox cases, by reporting date to CDC (for 2,657 cases with available reporting date)
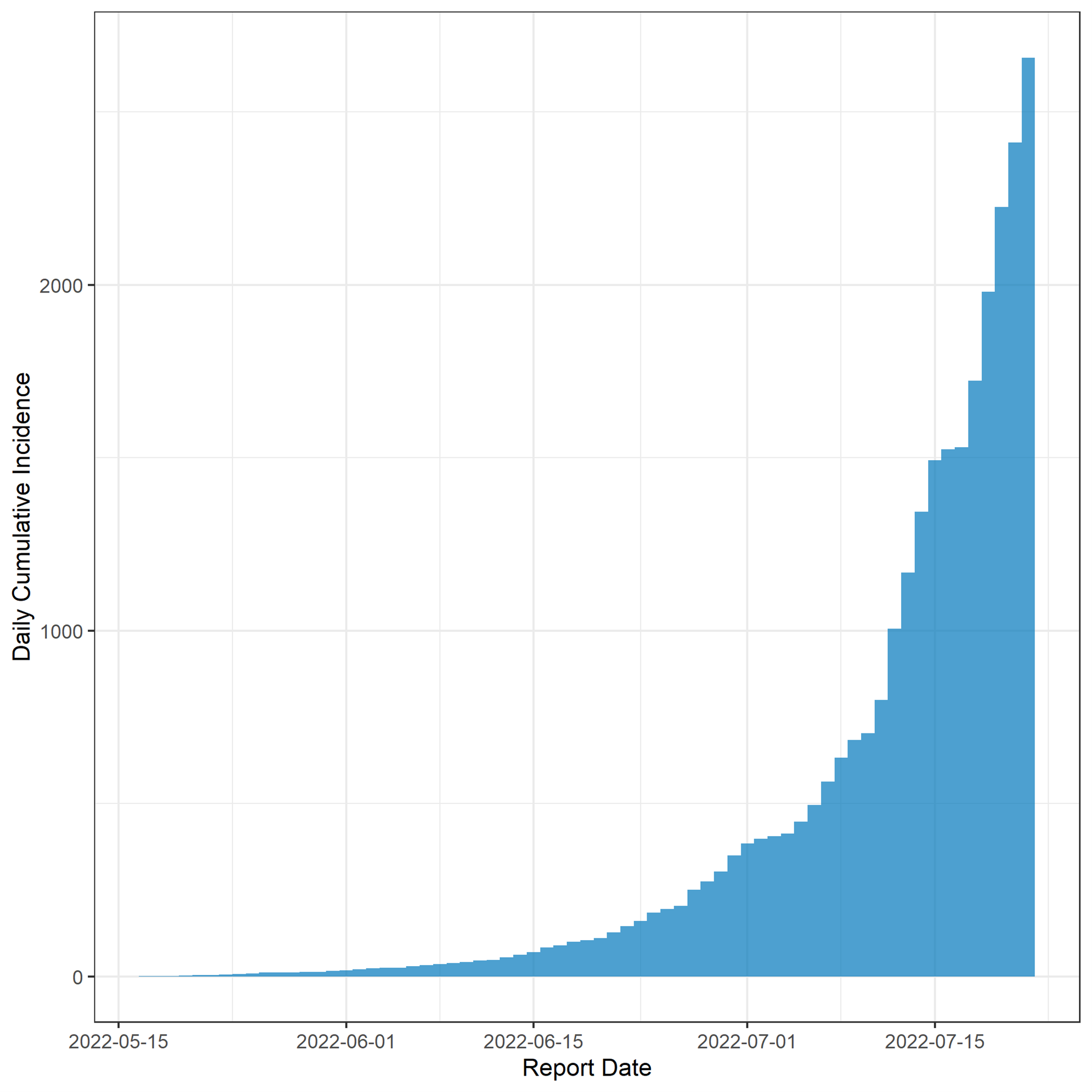
Figure 3. Cumulative incidence of Monkeypox cases, by reporting date (defined as the LRN positive date, case reporting to the CDC Call Center, or case entry into DCIPHER). Dates for some cases may be updated when additional LRN test dates are provided to CDC via DCIPHER.
Epidemiological Parameters
CDC is estimating epidemiological parameters associated with this outbreak.
Due to the small number of identified epidemiologically linked pairs of cases with known symptom onset dates, we have not yet been able to make a confident estimate of the serial interval distribution. Similarly, we do not yet have robust estimates of the reproduction number, R, due to a high degree of missing case data and other sources of uncertainty for the estimates.
Incubation period
We estimated the incubation period of monkeypox virus (MPXV) using information from 22 persons with probable and confirmed monkeypox reported in the United States from May 17, 2022 – June 6, 2022. These persons were among 30 individuals reported in the United States during this time period; however, eight persons were excluded from this analysis due to unknown or uncertain information on window of exposure or symptom onset. From case investigations, we extracted time of exposure to MPXV and time of first symptom onset.
The symptom onset dates of persons with available information range from April 29, 2022 to May 27, 2022. We used the available information to bound the time of exposure by the earliest and latest potential times of exposure.
We included an additional 18 persons with confirmed monkeypox reported in the Netherlands for a total sample size of 40 case-patients. Details about those persons can be found in Miura et al. (The incubation period for monkeypox cases confirmed in the Netherlands, May 2022 eurosurveillance.org).
We constructed a doubly censored dataset for the incubation period and fitted the distribution using the methods described in Lessler et al. (https://apps.who.int/iris/handle/10665/272006). We assumed the incubation period of MPXV followed a log-normal distribution.
As initial symptoms for some persons were non-specific (i.e., fever, diarrhea, headache, sore throat) and may not necessarily be related to their illness, we also estimated the incubation period using the date of rash onset for 21 persons reported in the United States with available information. Dates of rash onset ranged from May 1, 2022 to June 3, 2022.
For time from exposure to first symptom onset, we estimated a mean incubation period of 7.6 days (95% credible interval (CrI): 6.2–9.7) and a standard deviation of 1.8 days (95% CrI: 1.6–2.2). A plot of the incubation period for first symptom onset is shown in Figure 1. Overall, 95% of individuals developed symptoms within 17.1 days (95% CrI: 12.7–24.3).
For time from exposure to rash onset, we estimated a mean incubation period of 8.7 days (95% CrI: 6.9–11.7) days and a standard deviation of 1.6 days (95% CrI: 1.4–2.1). A plot of the incubation period for rash onset is shown in Figure 2. We found 95% of individuals developed rash within 17.7 days (95% CrI 12.4–28.1) after exposure.
Time from Symptom Onset to Test Result
The median number of days between first symptom onset (any monkeypox symptoms) and Laboratory Response Network (LRN) Non-Variola Orthopox (OPX) test result is 8 days, with a mean of 8.6 days (Table 1). We also examined the delay between rash onset and LRN OPX test result: the median number of days between symptom onset and LRN OPX test result is 6 days, with a mean of 7.0 days (Table 2).
Reporting delays decreased after epidemiologic week 20 and have remained stable from epidemiologic week 25 – 29. The number of cases has increased each week; however, recent cases are missing information on symptom onset dates.
We also report the distribution between first symptom onset and rash onset. For cases with available information (n = 545), the mean delay was 1.9 days (range 0 – 33) (Table 3). Note that first symptom can include rash, so the minimum delay is 0. The median time of specimen receipt to test results in an LRN laboratory was 30.7 hours during epidemiologic week 20-26. (footnote: https://www.cdc.gov/mmwr/volumes/71/wr/mm7128e1.htm).
Table 1 – Days between Symptom Onset and LRN Non-Variola Orthopoxvirus (OPX) Test
Table 2 – Days between Rash Onset and LRN Non-Variola Orthopoxvirus (OPX) Test
Table 3 – Days Between Symptom Onset (any Monkeypox Symptoms) and Rash Onset
Response Investigations
CDC has initiated several studies to answer key unknowns related to monkeypox, including seroprevalence studies, clinical studies, and lab studies. Other studies will be included in this report once staff needs are allocated and protocols for investigations are approved.
Type
Type
Investigation
Investigation
Collaborating Institutions
Collaborating Institutions
Status
Status
Serosurvey
Serosurvey
3-week prospective study with certain public health clinics in San Francisco that predominantly care for men who have sex with men (MSM). The study involves collection of serum samples and answers to specific questions about exposures and behaviors among a racially and ethnically diverse population with a broad range of socio-economic status. The goal is to determine the prevalence of monkeypox.
3-week prospective study with certain public health clinics in San Francisco that predominantly care for men who have sex with men (MSM). The study involves collection of serum samples and answers to specific questions about exposures and behaviors among a racially and ethnically diverse population with a broad range of socio-economic status. The goal is to determine the prevalence of monkeypox.
CDC, San Francisco Department of Public Health
CDC, San Francisco Department of Public Health
Ongoing since Monday, June 27.
Ongoing since Monday, June 27.
Testing of residual nucleic acid material
Testing of residual nucleic acid material
Banked nucleic acid and ongoing (prospective) collection from a commercial laboratory. These residual specimens are from clinics across the country and were targeted based on IDC-10 codes relevant to outbreak.
Banked nucleic acid and ongoing (prospective) collection from a commercial laboratory. These residual specimens are from clinics across the country and were targeted based on IDC-10 codes relevant to outbreak.
CDC, HealthTrackRX
CDC, HealthTrackRX
Banked specimens from May 2022. Collection is ongoing.
Banked specimens from May 2022. Collection is ongoing.
Technical Assistance
Technical Assistance
3-4 week technical assistance to improve understanding of monkeypox cases through network analysis and characterizing transmission dynamics, including processing through detailed partner services/contact tracing data to better understand secondary attack rates, delays between exposure and symptom onset, characterization exposure types, and identifying common venues associated with transmission.
3-4 week technical assistance to improve understanding of monkeypox cases through network analysis and characterizing transmission dynamics, including processing through detailed partner services/contact tracing data to better understand secondary attack rates, delays between exposure and symptom onset, characterization exposure types, and identifying common venues associated with transmission.
CDC, DC Department of Health
CDC, DC Department of Health
Ongoing virtually since Tuesday, July 12
Ongoing virtually since Tuesday, July 12
Priority Research Questions
The following is a list of topics that CDC is interested in studying in more depth given adequate resources to do so. This list makes no distinction between the short, medium, and long term, nor any distinction between field work and work for the current outbreak response. However, in one way or another, the CDC believes these topics to be important to detect, prevent, and respond to monkeypox.
Topic
Topic
Gaps and Potential Areas of Focus
Gaps and Potential Areas of Focus
Medical Countermeasure Effectiveness
Medical Countermeasure Effectiveness
- Effectiveness of vaccine to prevent or ameliorate disease when administered as pre- or post-exposure prophylaxis
- Effectiveness of antivirals to prevent disease if given as postexposure prophylaxis or to improve clinical outcomes when given for treatment (e.g., prevention of severe disease/complications, decreased lesion burden, hastening of lesion resolution)
- Decentralized trials, remote case monitoring, and improved access to interventions
- Comparing effectiveness and safety of 1 vs. 2 dose vaccine regiments; possible use of “ring” vaccine strategy
- Novel methods for evaluating medical countermeasures during an outbreak
- Effectiveness of vaccine to prevent or ameliorate disease when administered as pre- or post-exposure prophylaxis
- Effectiveness of antivirals to prevent disease if given as postexposure prophylaxis or to improve clinical outcomes when given for treatment (e.g., prevention of severe disease/complications, decreased lesion burden, hastening of lesion resolution)
- Decentralized trials, remote case monitoring, and improved access to interventions
- Comparing effectiveness and safety of 1 vs. 2 dose vaccine regiments; possible use of “ring” vaccine strategy
- Novel methods for evaluating medical countermeasures during an outbreak
Epidemiology, pathogenesis, and clinical characteristics
Epidemiology, pathogenesis, and clinical characteristics
- Modeling transmission dynamics
- Clinical presentation (particularly of the rash), risk factors, and associated outcomes, as well as similarities and differences in non-US cases
- Disease pathogenesis and relationship to clinical presentation and transmission route
- One Health – transmission to animals, including pets, domestic animals, and wildlife
- Social and behavioral factors associated with transmission
- Modeling transmission dynamics
- Clinical presentation (particularly of the rash), risk factors, and associated outcomes, as well as similarities and differences in non-US cases
- Disease pathogenesis and relationship to clinical presentation and transmission route
- One Health – transmission to animals, including pets, domestic animals, and wildlife
- Social and behavioral factors associated with transmission
Diagnostic tools and surveillance
Diagnostic tools and surveillance
- Validation and strategic deployment of pre-existing and novel diagnostics
- Viral detection across specimen types
- Levels of undetected transmission, especially in populations and regions with less access to MPXV testing
- Serosurveys and improved serologic assays
- Community and wastewater surveillance
- Sequencing to identify changes in circulating viruses
- Sensitivity analysis to test for anti-viral resistant strains
- Validation and strategic deployment of pre-existing and novel diagnostics
- Viral detection across specimen types
- Levels of undetected transmission, especially in populations and regions with less access to MPXV testing
- Serosurveys and improved serologic assays
- Community and wastewater surveillance
- Sequencing to identify changes in circulating viruses
- Sensitivity analysis to test for anti-viral resistant strains
Communications
Communications
- Understand knowledge, attitudes, and practices of key communities and impacted populations.
- Monitor mis/disinformation and implement actions to address
- Understand knowledge, attitudes, and practices of key communities and impacted populations.
- Monitor mis/disinformation and implement actions to address
Health Equity and Stigma Reduction during Public Health Emergency
Health Equity and Stigma Reduction during Public Health Emergency
- Equitably balancing resources for outbreak response in non-endemic countries with control in endemic countries (e.g., access and distribution of vaccines and therapeutics)
- Evaluate the equitable distribution of testing, treatment, prevention measures and other resources; and evaluate measures to improve access for underserved groups
- Equitably balancing resources for outbreak response in non-endemic countries with control in endemic countries (e.g., access and distribution of vaccines and therapeutics)
- Evaluate the equitable distribution of testing, treatment, prevention measures and other resources; and evaluate measures to improve access for underserved groups
Social and Behavioral Science
Social and Behavioral Science
- Gather qualitative and quantitative data directly from affected communities to guide implementation of testing, vaccination, and other prevention strategies.
- Gather qualitative and quantitative data directly from affected communities to guide implementation of testing, vaccination, and other prevention strategies.
Partnerships
CDC’s 2022 Multi-National Monkeypox Outbreak Response is both leveraging existing agency partnerships and building new relationships to extend the reach of CDC’s Monkeypox tools and resources. There are two weekly standing outreach opportunities to disseminate information to partners from 90+ organizations, including state, tribal, local and territorial (STLT) health departments, public health professional organizations, as well as clinical, community-based, LGBTQ+, and professional organizations. CDC hosts a recurring partner webinar which averages around 1000 weekly participants. This session includes a robust question and answer period where CDC SMEs take questions from the audience. CDC also sends a weekly partner email to the partners listed above to share new resources. Ad hoc emails are also sent with new resources or tools. Public health organizations or partners can request an invitation for the webinar or request to be added to the email distribution by emailing eocevent434@cdc.gov with their affiliation. Many of these partners amplify CDC’s messaging by sharing the partner resource email and the webinar invitation with their own members.
CDC continues to engage and work with community and health care partners to raise awareness of monkeypox. Through our partners, CDC has helped craft messages to directly reach populations affected by monkeypox, develop materials to be distributed directly to consumers, and created resources that can be adapted by local and state organizations.
CDC’s outbreak response team is made up of experts from across various disciplines to ensure that a wide range of expertise and perspectives are incorporated into response functions and actions. This includes experts from the poxvirus, HIV/STDs, One Health, Global Migration and Quarantine, Health Quality Protection, and Global Health teams, along with many volunteers across all CDC centers, institutes, and organizations.
Limitations of the Report
Due to monkeypox cases being historically rare in the United States and the limited availability of detailed data from case reports, we have a range of knowledge and data gaps related to monkeypox transmission dynamics, case ascertainment, clinical characteristics, and other key features of this outbreak. CDC is working with state, local, tribal and territorial partners, as well as clinical and laboratory partners, on obtaining improved case and contact tracing data sources to better understand this emerging and rapidly changing outbreak. CDC is also working with other federal partners and academic partners to improve our ability to respond to this outbreak. All data are preliminary and may change as more reports are received.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References to non-CDC sites on the Internet are provided as a service to readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites.
Sources and Acknowledgements
Data comes from information voluntarily reported by states.
Authors and Contributors are members of the 2022 Multi-National Monkeypox Outbreak Response.
References to non-CDC sites are provided as a service and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed were current as of the date of publication.