Tecovirimat (TPOXX) for Treatment of Mpox
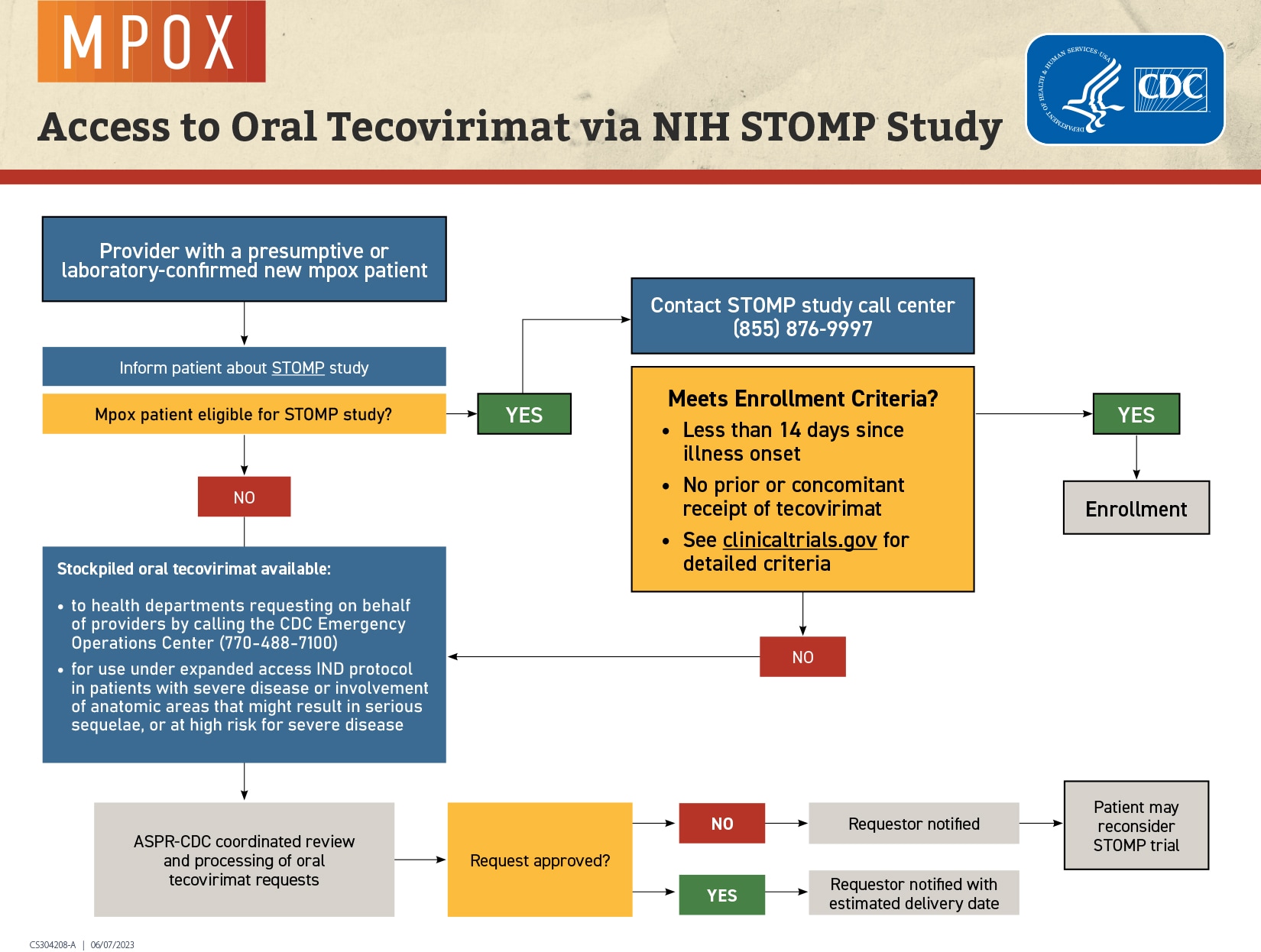
Oral tecovirimat for treatment of mpox is primarily available through Study of Tecovirimat for Human Mpox Virus (STOMP). Healthcare providers should either contact STOMP, or their state/territorial health department.
Summary of Recent Changes
Oral Tecovirimat Access through the STOMP Trial
Oral tecovirimat for treatment of mpox is available through enrollment in the Study of Tecovirimat for Human Mpox Virus (STOMP). Researchers and clinicians do not know whether tecovirimat is safe and effective for the treatment of mpox. The STOMP trial is designed to collect data to help answer these scientific questions.
- Providers are encouraged to inform patients with mpox about STOMP and to recommend they consider seeking enrollment in STOMP, which has randomized and open label treatment arms.
- Patients do not have to have severe disease or be at high risk of severe illness to enroll in the study, and remote enrollment is available.
- The trial includes a placebo-controlled, randomized arm and an open-label option for patients with severe disease, who have severe immunodeficiency, are pregnant or breastfeeding, who are under 18 years of age, or are taking medications that could affect tecovirimat levels.
- Participants will be followed for at least 57 days and will be asked to fill out a symptom diary, do daily skin checks at home, and attend virtual and in-person clinic appointments, where they will undergo physical exams and provide blood and other specimens, including fluid from their lesions or eye if ocular infection is involved. For participants who are enrolled remotely, telehealth visits will be conducted.
- For more information, the STOMP call center can be reached at (855) 876-9997 from 9 a.m. to 10 p.m. Monday through Friday, on Saturday from 9 a.m. to 4 p.m., and on Sunday from 1-6 p.m. (all times U.S. Eastern).
Providers with patients with mpox who are ineligible for STOMP or decline enrollment, or who require intravenous tecovirimat treatment, and meet treatment eligibility under the EA-IND protocol (e.g., have severe disease or involvement of anatomic areas that might result in serious sequelae, are at high risk for severe disease), should contact their state or territorial health departments to inquire about prepositioned oral tecovirimat supply that may be available within their jurisdiction. If there is no local supply of tecovirimat, health departments can request tecovirimat by calling the CDC Emergency Operations Center (EOC) at (770) 488-7100 or poxvirus@cdc.gov. Providers, in conjunction with state health departments, can also request a clinical consultation regarding management of hospitalized patients with mpox by calling the CDC EOC or poxvirus@cdc.gov.
Note: The email box is monitored from 9 a.m. to 4 p.m. U.S. Eastern time on non-holiday weekdays. For urgent issues, contacting your state public health department is preferred. An on-call clinician may be requested via the CDC EOC for hospitalized patients requiring a consultation.
Tecovirimat Expanded Access IND Information
To facilitate access to tecovirimat, CDC holds an expanded access IND (EA-IND) protocol for the treatment of non-variola orthopoxvirus infections, including mpox, in adults and children. See Section 2.1.1 of the EA-IND protocol [836 KB, 25 pages] for details.
The EA-IND provides umbrella regulatory coverage so that clinicians and facilities do not need to request and obtain their own INDs. To be covered under the EA-IND, the providers and affiliated facilities must register online as participating providers/sites.
Tecovirimat use under the EA-IND is also covered under the Public Readiness and Emergency Preparedness (PREP) Act, which provides liability immunity to qualified providers and compensation to eligible patients via the Countermeasures Injury Compensation Program (CICP).
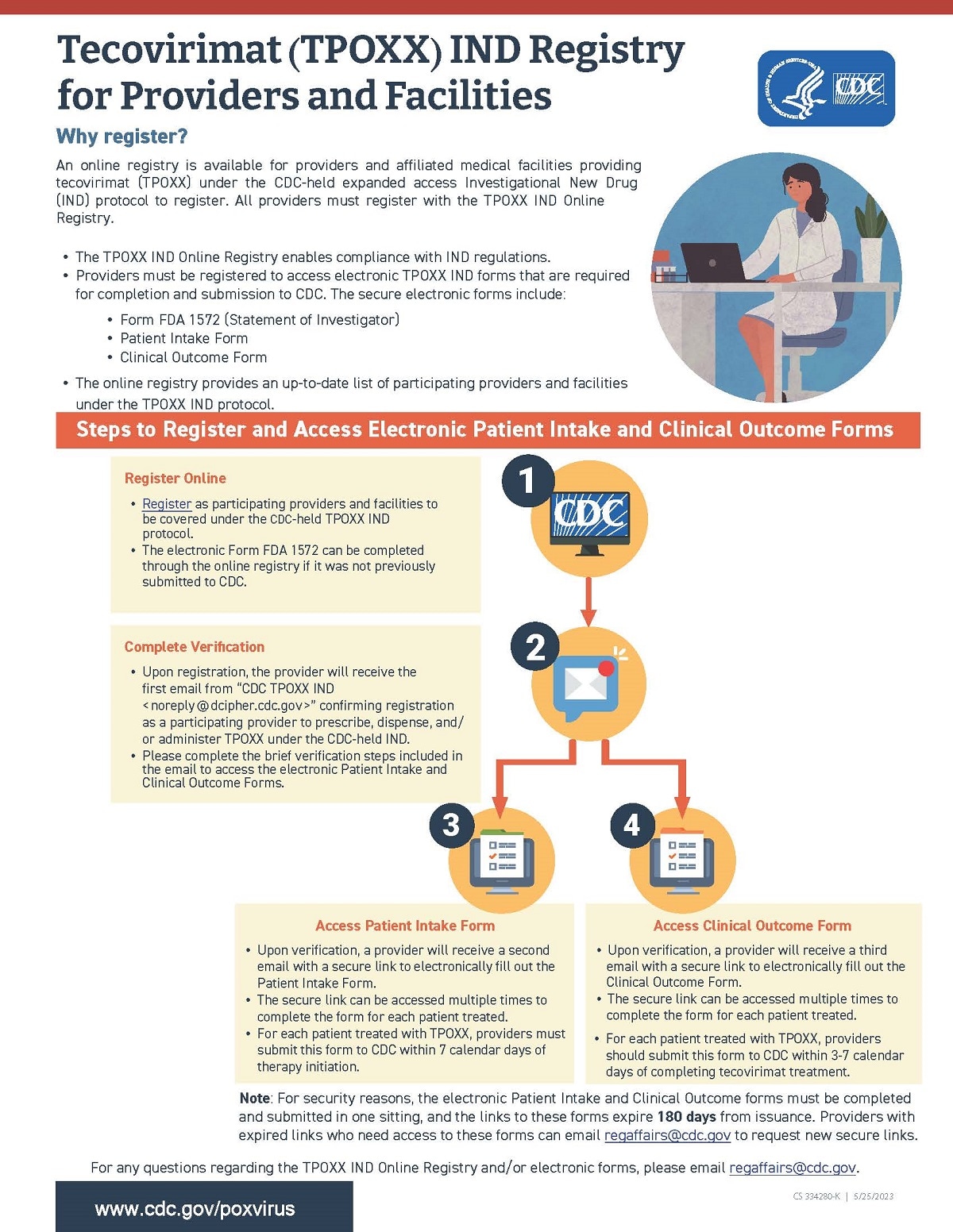
Providers and affiliated facilities must be registered online as participating providers/sites under the CDC-held expanded access IND (EA-IND) for tecovirimat.
The tecovirimat IND Online Registry allows for convenient, time-efficient, and secure completion and return of EA-IND forms to CDC. View this Fact Sheet [540 KB, 1 page] for an overview of the tecovirimat IND online registry process.
Access the Tecovirimat (TPOXX) IND Registry here- Through the registry, providers can submit
- Form FDA 1572
- Patient Intake Form
- Clinical Outcome Form
- Any questions about the registry and accessing electronic tecovirimat IND Patient Intake and Clinical Outcome forms can be directed to regaffairs@cdc.gov.
Tecovirimat EA-IND Protocol
- The current EA-IND protocol [836 KB, 25 pages] is version 6.3 updated December 19, 2023.
- The protocol includes Instructions for mixing tecovirimat capsules with food or water [855 KB, 2 pages]: This patient instruction sheet explains how to open tecovirimat capsules and mix with food or water for infants and children who cannot swallow pills.
- On December 27, 2023, the CDC IRB approved [292 KB, 2 pages] a protocol amendment (version 6.3 updated December 19, 2023); protocol continuation was approved on June 27, 2023 [102 KB, 1 page].
- Clinicians, care facilities, and hospitals providing tecovirimat should immediately transition to the revised protocol (version 6.3 updated December 19, 2023). Healthcare providers will be responsible for completing the following forms:
Required
- Informed Consent Form: English [229 KB, 7 pages] Spanish [250 KB, 7 pages] Obtain prior to treatment.
- Alternative Consent Forms that can be used to obtain informed consent:
- FDA Form 1572: One signed 1572 and treating clinician’s curriculum vitae per facility suffices for all tecovirimat treatments administered under the EA-IND at the same facility. Access the electronic form through the Tecovirimat IND Online Registry.
- Patient Intake Form: Baseline assessment. Access the electronic form through the Tecovirimat IND Online Registry.
- Clinical Outcome Form: Progress and outcome information post treatment. Access the electronic form through the Tecovirimat IND Online Registry.
- Serious Adverse Events: Per FDA requirement, report life-threatening or serious adverse events associated with tecovirimat by completing a PDF MedWatch Form [956 KB, 5 pages] and returning it to CDC via email (regaffairs@cdc.gov) within 72 hours of awareness or sooner, if possible. The PDF MedWatch Form can also be downloaded from the FDA website. Note: The MedWatch Form can only be viewed on the Adobe desktop app. Please save or download the form for viewing.
Optional
- Lesion specimens for resistance testing: Lesion specimens may be sent to CDC for tecovirimat-treated patients with persistent lesions and/or any new lesions that develop during and/or after tecovirimat treatment to assess for development of antiviral resistance mutations. See Optional Lesion Specimens to CDC for Resistance Testing [220 KB, 2 pages] for instructions on collection, storage, and submission of samples.
- Pharmacokinetic samples for testing: During tecovirimat treatment, plasma samples may be collected to monitor tecovirimat levels for adequate drug exposure in patients. Optional Pharmacokinetic Samples for Testing [375 KB, 4 pages] has instructions on collection, storage, and submission of samples.
Institutional Review Board (IRB) Approval of Tecovirimat IND Protocol
- CDC IRB serves as the central IRB for review and approval. Facilities may elect to rely on the CDC IRB for centralized review and approval by submitting a request to the CDC’s Human Research Protection Office within 7 calendar days of tecovirimat treatment at your facility. CDC will promptly document an agreement in writing using the CDC IRB Authorization Agreement (Sample Template) [4 MB, 2 pages], which must be signed by both parties.
- Facilities that elect to obtain their own IRB review must ensure compliance with applicable FDA regulations related to the tecovirimat EA-IND protocol. Note the posted tecovirimat EA-IND protocol and attachments must be used without any changes being made by the IRB.
- Because this tecovirimat EA-IND protocol is solely for treatment use, CDC determined that its use does not constitute research involving human subjects as defined by 45 CFR 46.102, therefore, the federal-wide assurance requirements do not apply.
Facilities that have elected to rely on the CDC IRB for centralized review and approval: If you would like to terminate this reliance agreement, please submit a “Request Action for Closure” in REDCap using your own unique link, which was provided to you via email when the reliance agreement was executed. You may locate it by searching your email inbox for “Fully Executed Agreement to Rely on CDC IRB for Expanded Access IND for TPOXX.” If you are unable to locate your own unique link, please contact huma@cdc.gov.