CDC Acute Flaccid Myelitis Update
December 5, 2019
Also available as Printable PDF pdf icon[PDF – 901 KB, 24 pages]
Slide 1

CDC Acute Flaccid Myelitis Update
Janell Routh, MD MHS
Measles, Mumps, Rubella, Herpesvirus, and Polio Domestic Epidemiology Team
Division of Viral Diseases
National Center for Immunization and Respiratory Diseases
Board of Scientific Counselors Meeting
December 5, 2019
Slide 2

Background
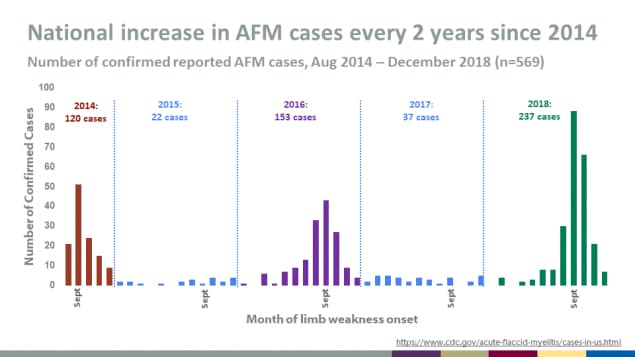
National increase in AFM cases every 2 years since 2014
- Number of confirmed reported AFM cases, Aug 2014 – December 2018 (n=569)
- 2014: 120 cases
- 2015: 22 cases
- 2016: 153 cases
- 2017: 37 cases
- 2018: 237 cases
More information about confirmed AFM cases in the United States
AFM presents with rapid onset of limb weakness and spinal cord grey matter lesions
- Sudden limb weakness
- Difficulty with swallowing or speaking
- Facial droop or weakness
- Ptosis
- Lesions in spinal grey matter, particularly anterior horn cell distribution
- Cervical spinal cord most affected
Slide 5

2019 AFM epidemiology
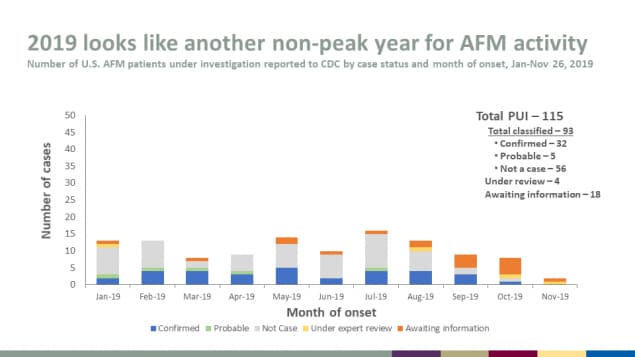
2019 looks like another non-peak year for AFM activity
- Number of U.S. AFM patients under investigation reported to CDC by case status and month of onset, Jan-Nov 26, 2019
Total PUI – 115
Total classified – 93
- Confirmed – 32
- Probable – 5
- Not a case – 56
Under review – 4
Awaiting information – 18
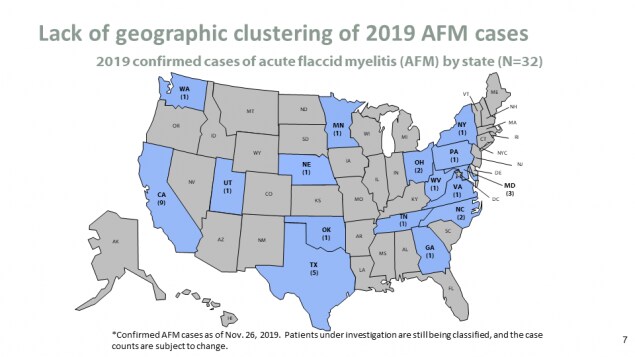
Lack of geographic clustering of 2019 AFM cases
- 2019 confirmed cases of acute flaccid myelitis (AFM) by state (N=32)
*Confirmed AFM cases as of Nov. 26, 2019. Patients under investigation are still being classified, and the case counts are subject to change.
Slide 8

Evidence for a viral etiology
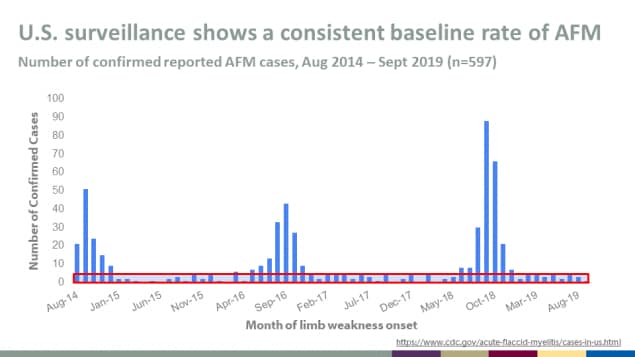
U.S. surveillance shows a consistent baseline rate of AFM
Number of confirmed reported AFM cases, Aug 2014 – Sept 2019 (n=597)
More information about confirmed AFM cases in the United States
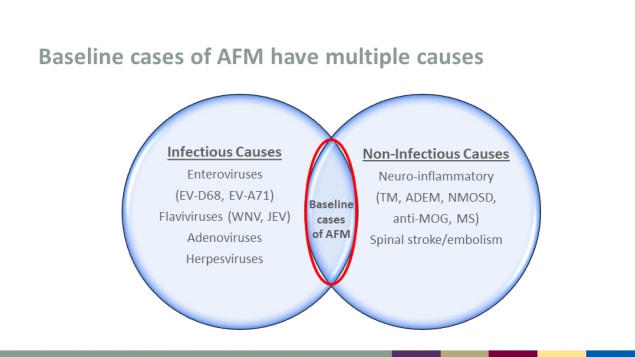
Baseline cases of AFM have multiple causes
Infectious Causes
- Enteroviruses (EV-D68, EV-A71)
- Flaviviruses (WNV, JEV)
- Adenoviruses
- Herpesviruses
Non-Infectious Causes
- Neuro-inflammatory (TM, ADEM, NMOSD, anti-MOG, MS)
- Spinal stroke/embolism
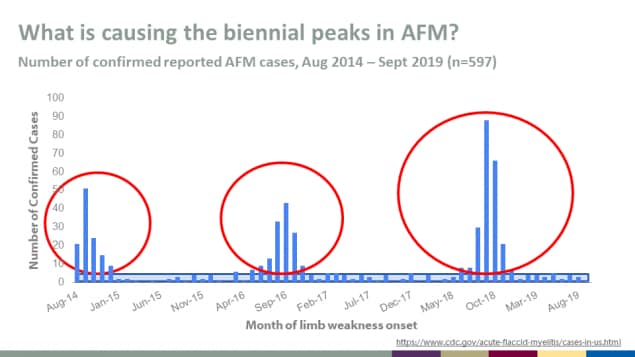
What is causing the biennial peaks in AFM?
Number of confirmed reported AFM cases, Aug 2014 – Sept 2019 (n=597)
More information about confirmed AFM cases in the United States
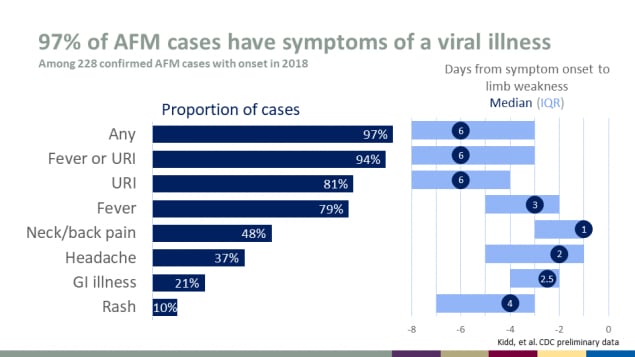
97% of AFM cases have symptoms of a viral illness
Among 228 confirmed AFM cases with onset in 2018
| Symptom | Proportion of cases | Median days from symptom onset to limb weakness |
|---|---|---|
| Any | 97% | 6 |
| Fever or URI | 94% | 6 |
| URI | 81% | 6 |
| Fever | 79% | 3 |
| Neck/back pain | 48% | 1 |
| Headache | 37% | 2 |
| GI illness | 21% | 2.5 |
| Rash | 10% | 4 |
Source: Kidd, et al. CDC preliminary data
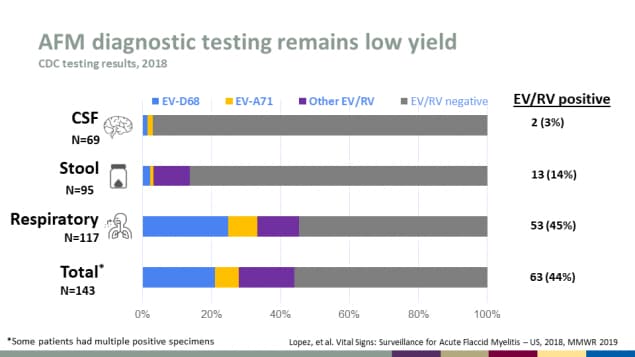
AFM diagnostic testing remains low yield
CDC testing results, 2018
| Specimen Source | Number | EV/RV Positive |
|---|---|---|
| CSF | 69 | 2 (3%) |
| Stool | 95 | 13 (14%) |
| Respiratory | 117 | 53 (45%) |
| Total* | 143 | 63 (44%) |
*Some patients had multiple positive specimens
Source: Lopez, et al. Vital Signs: Surveillance for Acute Flaccid Myelitis – US, 2018, MMWR 2019
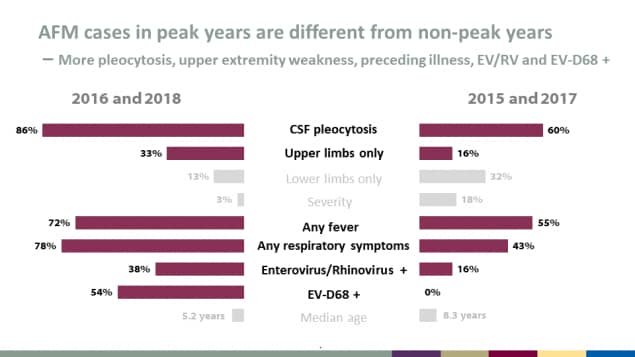
AFM cases in peak years are different from non-peak years
More pleocytosis, upper extremity weakness, preceding illness, EV/RV and EV-D68 +
| Characteristic | 2016 & 2018 (peak years) | 2015 & 2017 (non-peak years) |
|---|---|---|
| CSF pleocytosis | 86% | 60% |
| Upper limbs only | 33% | 16% |
| Lower limbs only | 13% | 32% |
| Severity | 3% | 18% |
| Any fever | 72% | 55% |
| Any respiratory symptoms | 78% | 43% |
| Enterovirus/Rhinovirus + | 38% | 16% |
| EV-D68 + | 54% | 0% |
| Median age | 5.2 years | 8.3 years |
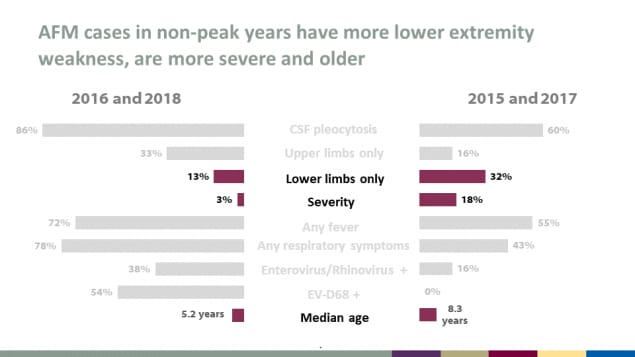
AFM cases in non-peak years have more lower extremity weakness, are more severe and older
| Characteristic | 2016 & 2018 (peak years) | 2015 & 2017 (non-peak years) |
|---|---|---|
| CSF pleocytosis | 86% | 60% |
| Upper limbs only | 33% | 16% |
| Lower limbs only | 13% | 32% |
| Severity | 3% | 18% |
| Any fever | 72% | 55% |
| Any respiratory symptoms | 78% | 43% |
| Enterovirus/Rhinovirus + | 38% | 16% |
| EV-D68 + | 54% | 0% |
| Median age | 5.2 years | 8.3 years |
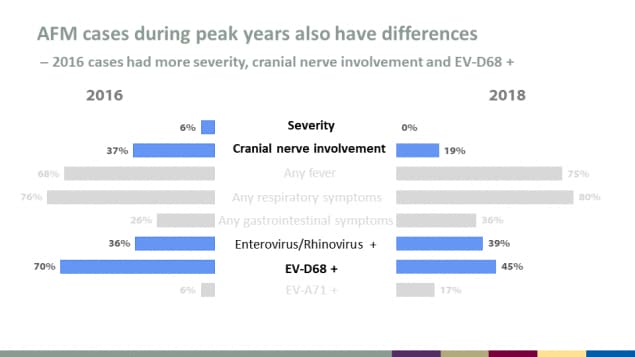
AFM cases during peak years also have differences
2016 cases had more severity, cranial nerve involvement and EV-D68 +
| Characteristic | 2016 | 2018 |
|---|---|---|
| Severity | 6% | 0% |
| Cranial nerve involvement | 37% | 19% |
| Any fever | 68% | 75% |
| Any respiratory symptoms | 76% | 80% |
| Any gastrointestinal symptoms | 26% | 36% |
| Enterovirus/Rhinovirus + | 36% | 39% |
| EV-D68 + | 70% | 45% |
| EV-A71 + | 6% | 17% |
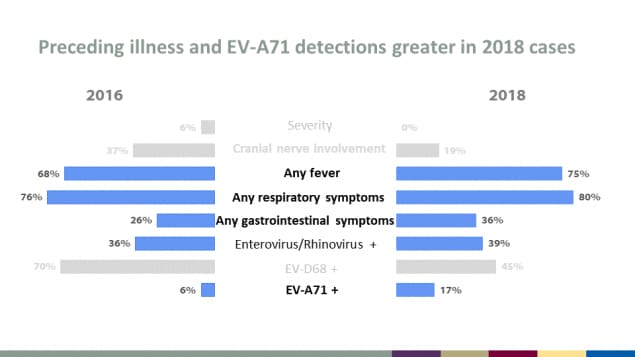
Preceding illness and EV-A71 detections greater in 2018 cases
| Characteristic | 2016 | 2018 |
|---|---|---|
| Severity | 6% | 0% |
| Cranial nerve involvement | 37% | 19% |
| Any fever | 68% | 75% |
| Any respiratory symptoms | 76% | 80% |
| Any gastrointestinal symptoms | 26% | 36% |
| Enterovirus/Rhinovirus + | 36% | 39% |
| EV-D68 + | 70% | 45% |
| EV-A71 + | 6% | 17% |
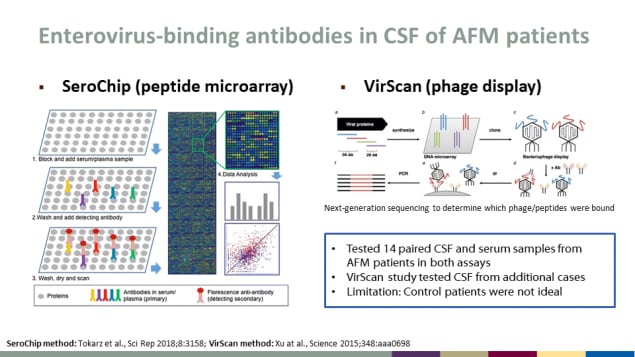
Enterovirus-binding antibodies in CSF of AFM patients
- Tested 14 paired CSF and serum samples from AFM patients in both assays
- VirScan study tested CSF from additional cases
- Limitation: Control patients were not ideal
SeroChip (peptide microarray)
- Block and add serum/plasma sample
- Wash and add detecting antibody
- Wash, dry and scan
- Data analysis
VirScan (phage display)
Next-generation sequencing to determine which phage/peptides were bound
SeroChip method: Tokarz et al., Sci Rep 2018;8:3158; VirScan method: Xu at al., Science 2015;348:aaa0698
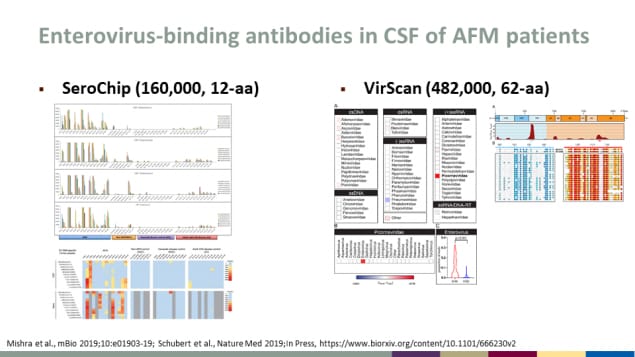
Enterovirus-binding antibodies in CSF of AFM patients
- SeroChip (160,000, 12-aa)
- VirScan (482,000, 62-aa)
Source: Mishra et al., mBio 2019;10:e01903-19; Schubert et al., Nature Med 2019;In Pressexternal icon.
Slide 20

Preparations for AFM Response, 2020
Slide 21
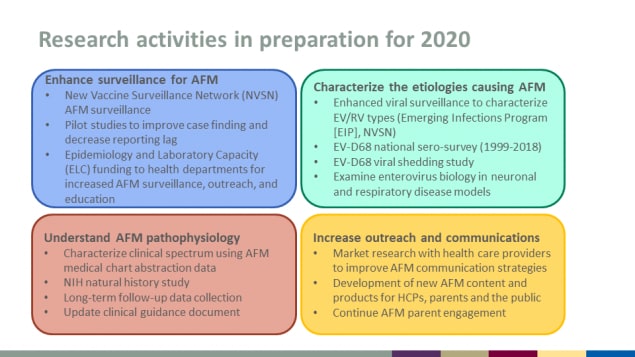
Research activities in preparation for 2020
Enhance surveillance for AFM
- New Vaccine Surveillance Network (NVSN) AFM surveillance
- Pilot studies to improve case finding and decrease reporting lag
- Epidemiology and Laboratory Capacity (ELC) funding to health departments for increased AFM surveillance, outreach, and education
Characterize the etiologies causing AFM
- Enhanced viral surveillance to characterize EV/RV types (Emerging Infections Program [EIP], NVSN)
- EV-D68 national sero-survey (1999-2018)
- EV-D68 viral shedding study
- Examine enterovirus biology in neuronal and respiratory disease models
Understand AFM pathophysiology
- Characterize clinical spectrum using AFM medical chart abstraction data
- NIH natural history study
- Long-term follow-up data collection
- Update clinical guidance document
Increase outreach and communications
- Market research with health care providers to improve AFM communication strategies
- Development of new AFM content and products for HCPs, parents and the public
- Continue AFM parent engagement
Slide 22
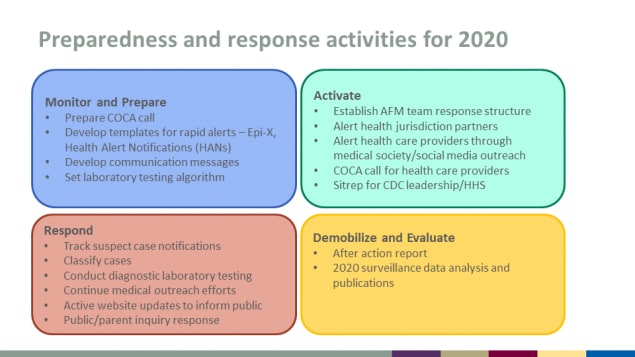
Preparedness and response activities for 2020
Monitor and Prepare
- Prepare COCA call
- Develop templates for rapid alerts – Epi-X, Health Alert Notifications (HANs)
- Develop communication messages
- Set laboratory testing algorithm
Activate
- Establish AFM team response structure
- Alert health jurisdiction partners
- Alert health care providers through medical society/social media outreach
- COCA call for health care providers
- Sitrep for CDC leadership/HHS
Respond
- Track suspect case notifications
- Classify cases
- Conduct diagnostic laboratory testing
- Continue medical outreach efforts
- Active website updates to inform public
- Public/parent inquiry response
Demobilize and Evaluate
- After action report
- 2020 surveillance data analysis and publications
Slide 23

Acknowledgments
CDC: Adriana Lopez, Manisha Patel, Sarah Kidd, Adria Lee, Susannah McKay, Tracy Ayers, Sue Gerber and the EV Team, Nilay McLaren, Steve Oberste, Allan Nix, Will Weldon, Jennifer Anstadt, Shannon Rogers, Brian Emery, Anita Kambhampati
External Collaborators: Sarah Hopkins, Dan Pastula, Cate Otten, Grace Gombolay, State and local health departments, the AFM Task Force, NVSN AFM investigators
Slide 24
Thank you