Biological Risk Management for Point-of-Care Testing Sites
As someone who delivers point-of-care (POC) testing, you help people understand their health status. You collect specimens that contain biological material, such as blood or saliva, from people to test and determine what is making them sick. The results from these tests can help the people you serve make informed decisions about what to do next.
From the time you start each testing process until you finish, there are risks involved. Use this guidance to help make sure you reduce those risks as much as possible to keep you and your coworkers, patients, customers, family, and community safe and healthy while you perform POC tests. Learn how to evaluate and reduce risks using the information below; learn why risk assessment is important in your role here.
Risk management is a five-step process of identifying and analyzing risks and taking steps to reduce or eliminate them.
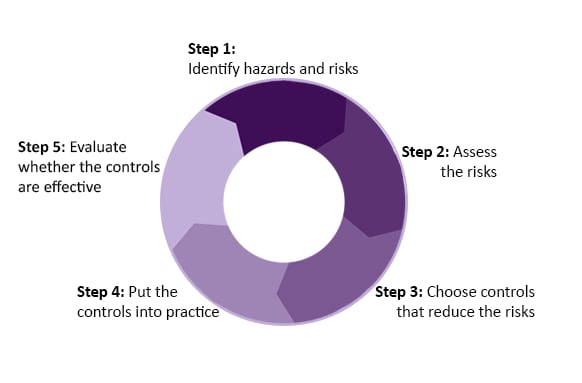
See ISO 35001 for the complete risk management process.
A biological hazard is something from biological material that can cause harm, such as viruses or bacteria (living biological organisms). A biological hazard can reside in a human specimen, like mucus, that you can collect from inside the nose, or that someone can cough up from their lungs. A human specimen can carry viruses or bacteria that might harm you or make you sick. For example, if someone is sick with tuberculosis (TB), the infectious bacteria that they may put into the air when they sneeze is a biological hazard.
A biological risk is the combination of the likelihood of an undesirable incident occurring and the severity of the harm if the incident happens. One example might be what can go wrong while testing blood from someone with hepatitis or HIV, such as potential exposure that leads to an accidental infection.
The guidance presented here focuses on hazards and risks that come with working with specimens that contain harmful biological material while performing POC testing. We explain how to conduct a risk management (steps 1-2) to help you reduce risk when you perform POC tests.
You can also find out where to get more information about steps 3–5 of the risk management process on this page.
For more details on the complete risk management process, see the World Health Organization (WHO) Laboratory Biosafety Manual, 4th Edition. Read section 2, pages 5-26. You can also refer to Section II of the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories, 6th Edition.
Create a Risk Assessment Team
You and the people who work at your POC site should form a risk assessment team. When you include multiple viewpoints, you can reduce the possibility that the input from one or two people will strongly influence the process. This team could be made up of managers, testing staff, safety professionals, facility staff, and other employees familiar with the site-specific and activity-specific testing activities.
Choose the Right Time for Risk Assessment
Plan ahead and do a risk assessment before testing begins. Repeat the risk assessment when any change is introduced into your process, such as changes in practices, personnel, equipment, or testing location.
Select the Right Risk Assessment Type
This guidance focuses on informal risk assessments, which can be performed in many POC testing settings. This usually involves short discussions among staff about potential risks and the controls to reduce those risks. They should occur weekly, daily, or even throughout the day, depending on how much testing is happening at your site. Formal risk assessment guidance can be found here.
Your risk assessment team should consider hazards, the specific testing steps, existing controls, the competency and experience of POC testing staff, and the testing site.
Step 1: Identify the Hazards and Risks
To identify the hazards in each testing task that must be completed, ask what, where, and how the testing is occurring, and who is doing the work. Then, determine what could go wrong in every step of the procedure and the risks, such as an injury or an exposure to biological material (a pathogen) that could make you sick. One way to accomplish this is to perform a job hazard analysis (JHA). The federal Occupational Health and Safety Administration (OSHA) published an example of this tool; look on pages 4–12. A JHA is a technique you can use to break down specific tasks to identify hazards and risks before they occur.
Examples of Hazards vs. Risks

It is important to remember the difference between hazard and risk. Hazard is a source or situation with the potential for causing harm, while risk is the combination of the likelihood of something bad occurring and the harm if that event occurs.
Step 2: Assess the Risks
2a. Characterize the risks
In this step, you start by considering how likely it is that the undesirable event will occur, and what the consequences would be if it occurs.
Likelihood addresses everything leading up to an event happening. Consequence addresses everything that results from that event happening.
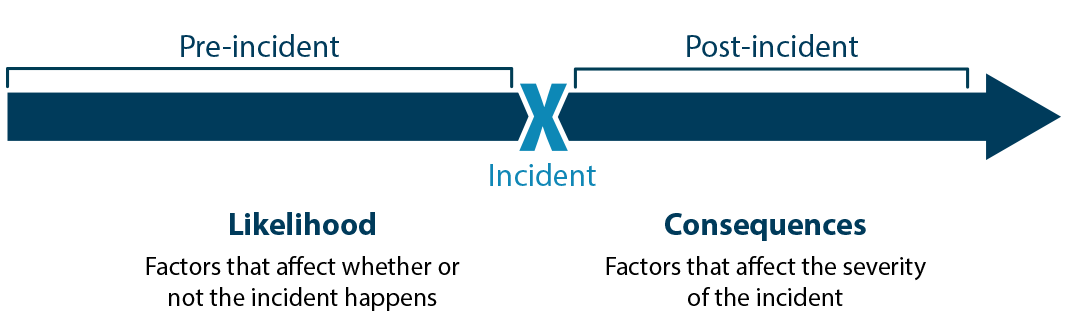
Source: Sandia National Laboratories Biosafety and Biosecurity Risk Assessment Technical Guidance Document, 2014.
Likelihood
Some factors to consider that can affect how likely an undesirable event may be include:
- Biological agent factors
- Ways that biological organisms can enter your body by splashing small liquid droplets into your eyes or nose, inhaling tiny particles suspended in air, getting harmful organisms into your mouth, or by penetration through the skin from a needlestick
- Testing site factors
- The place where testing happens, whether in a building or outside (physical infrastructure), the type of facility, presence of safety controls, type of equipment used, functioning and reliability of ventilation systems and electrical power
- Procedural: existence of policies and training, availability of appropriate personal protective equipment (PPE), work procedures that may produce aerosols or cause cuts by using sharps, the types and complexity of procedures being conducted, and the competency and experience of those performing the tests
Consequences
Some factors to consider that can affect the consequences of an undesirable event include:
- Biological agent factors
- How easily the infection can spread from person to person (contagiousness)
- How severe the disease could be
- Human factors
- Willingness of the testing site staff to receive medical treatment that could reduce how sick they might get if they are exposed to hazards
- Understanding the procedures to follow if something goes wrong and how they can limit the consequences of an adverse event
2b. Prioritize the risks and determine if risks are acceptable
The risk assessment team determines how it defines “likelihood” and “consequence.” One example of how likelihood and consequence are defined is:
Likelihood
- Low: Unlikely to occur. Highly trained and experienced staff, comprehensive safety procedures and controls in place
- Moderate: Might occur. Moderately trained staff, some procedures and controls in place
- High: Likely to occur. Staff lack sufficient training, few safety procedures and controls in place
Consequence
- Negligible: Trivial incident or near miss that requires reporting and follow-up
- Moderate: Incident that causes harm and requires medical intervention and treatment or has environmental consequences
- Severe: Potential fatality, serious illness, secondary transmission, or significant environmental impact
Once likelihood and consequences are defined, POC testing risk assessment teams should determine what level of risk is acceptable. If the risks for a particular activity are not acceptable to leadership, then the activity should not occur.
Determining acceptable risk is a subjective process, meaning that each person or organization will use their own judgment. At your site, your team will determine what risk levels are considered acceptable and what risk levels are considered unacceptable. If you determine the risks are acceptable, proceed in your work with the existing controls. For the risks considered unacceptable, stop work until you can put in place additional risk-reducing controls that bring risks to an acceptable level.
Steps 3-4: Determine and Implement Controls
If your risk assessment team finds some risks unacceptable, develop a mitigation control plan (see an example from the Occupational Safety and Health Administration – OSHA). Additional controls can reduce the likelihood or consequences of specific risks and bring them to a level that is acceptable. For example, additional PPE and additional training and monitoring could significantly reduce the risk of accidental exposure and infection during POC testing.
Step 5: Review Effectiveness of Controls
Your risk assessment team should review and evaluate the effectiveness of implementing additional controls, such as the use of PPE. For more information on mitigation and evaluation of the performance of controls, refer to this document from the World Health Organization: WHO Risk Assessment Monograph.
At your testing site, identify a team to perform a risk assessment using a job hazard analysis (JHA) prior to initiating testing activities. A simple table may be developed to perform and document the risk assessment prior to testing. The risk assessment team should review the JHA table once it is completed, with testing personnel, ideally daily. Additionally, the risk assessment team should discuss any changes to testing activities to ensure that potential hazards are identified before testing begins. A sample JHA table is shown below; download or print this template of the JHA table [PDF] [Word].
| Testing Location: Site A | Analyst: Jane Doe | Date: 06/08/2021 |
| Testing Activity | Risks | Risk Mitigations |
| Specimen collection | Exposure to harmful biological material during specimen collection | Change gloves between individuals when collecting specimens |
| Reagent mixing | Exposure to harmful biological material by aerosol generation | 1) Place a cap on reagent bottles before mixing and tighten cap
2) Mix reagents gently while ensuring proper mixing |
| Discarding materials from completed tests | Exposure to harmful biological material through spread of biological material across testing surfaces | 1) Carefully pick up and dispose completed test materials in appropriate biohazardous waste containers located near the last step of the testing process
2) Disinfect all testing surfaces after completion of testing |
Aerosol-generating procedure: Any procedure that intentionally or inadvertently results in the creation of tiny liquid or solid particles, which become suspended in the air as aerosols or droplets.
Droplets: Tiny amounts of liquids that can come from a person (through breathing, sneezing, or coughing) or from collecting or processing samples. Droplets can contaminate surfaces or be ingested (breathed in or swallowed) by testing staff, patients, or others.
Hazard: Something that is intrinsically dangerous such as an object, a chemical, an infectious organism, or a situation.
Personal protective equipment (PPE): Equipment and/or clothing worn by personnel to provide a barrier against biological organisms, thereby minimizing the likelihood of exposure. PPE includes, but is not limited to, laboratory coats, gowns, gloves, safety glasses, safety goggles, masks, and respirators.
Point-of-care (POC) testing involves performing a test outside of a laboratory that produces a rapid and reliable result, aiding in identifying or managing chronic diseases and acute infections.
Risk: Combination of the likelihood of an incident occurring and the consequences if that incident occurs.
Risk Assessment: Process of evaluating the risks arising from a hazard(s), taking into account the adequacy of any existing controls, prioritizing those risks, and deciding whether the risks are acceptable. Risk assessment is one part of risk management.
Risk Management: Overall process to identify, evaluate, control, and monitor risks.
Risk Mitigation: Actions and control measures that are put into place to reduce the risks to an acceptable level.
- ABSA International Risk Group Database
- Association of Public Health Laboratories:
- Biosafety in Microbiological and Biomedical Laboratories (BMBL) (6th Edition)
- CDC Guidance for Business and Employers
- Clinical and Laboratory Standards Institute (CLSI)
- EP23 Laboratory Quality Control Based on Risk Management, 1st Edition
- Clinical Laboratory Improvement Amendments (CLIA) Standards:
- Community Mitigation Framework | CDC
- Community, Work, and School | COVID-19 | CDC
- CWA 15793 Laboratory biorisk management
- Guidance for SARS-CoV-2 Point-of-Care and Rapid Testing | CDC
- Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories, MMWR 61(01)
- Introduction to Laboratory Risk Management (LRM)
- ISO 35001 Laboratory biorisk management system for laboratories and other related organizations
- Material Safety Data Sheets
- Occupational Safety and Health Administration (OSHA): Find information about hazard assessments and mitigation plans
- OSHA Job Hazard Analysis
- WHO Laboratory Biosafety Manual, 4th Edition