Model Performance Evaluation Program Report of Results: March 2023
The purpose of this report is to present results of the U.S. Centers for Disease Control and Prevention (CDC) Model Performance Evaluation Program (MPEP) for Mycobacterium tuberculosis complex (MTBC) drug susceptibility testing survey sent to participants in March 2023.
Primary Classification
This report contains DST results submitted to CDC by survey participants at 60 laboratories in 32 states, all of whom have participated in previous MPEP panels.
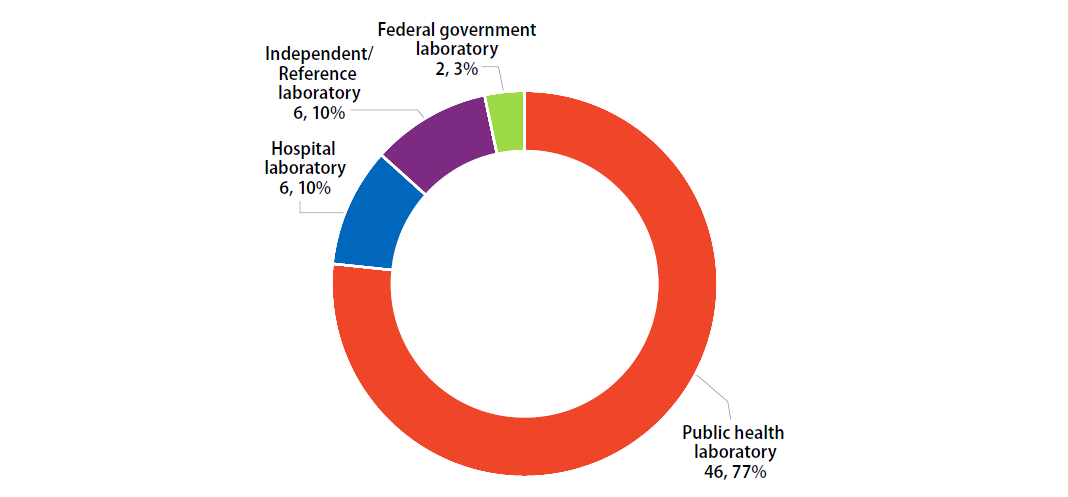
Participants were asked to indicate the primary classification of their laboratory (Figure 1).
Annual Number of MTBC Drug Susceptibility Tests Performed
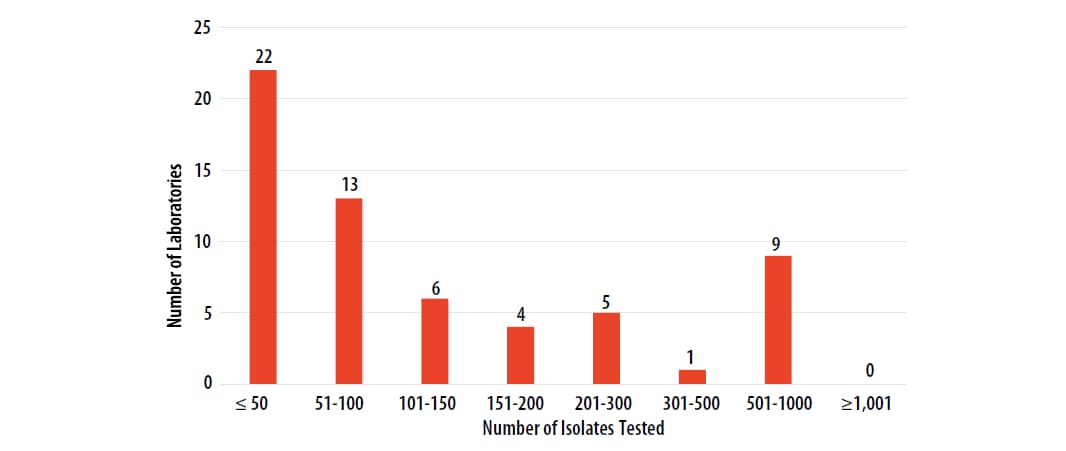
The number of MTBC isolates tested for drug susceptibility by the 60 participants in 2022 (excluding isolates used for quality control) is shown in Figure 2. In 2022, the counts ranged from 0 to 922 tests. Participants at 22 (37%) laboratories reported testing 50 or fewer DST isolates per year. Laboratories with low MTBC DST volumes are encouraged to consider referral of testing because of concerns about maintaining proficiency [11].
MTBC Drug Susceptibility Test Methods Performed by Participants
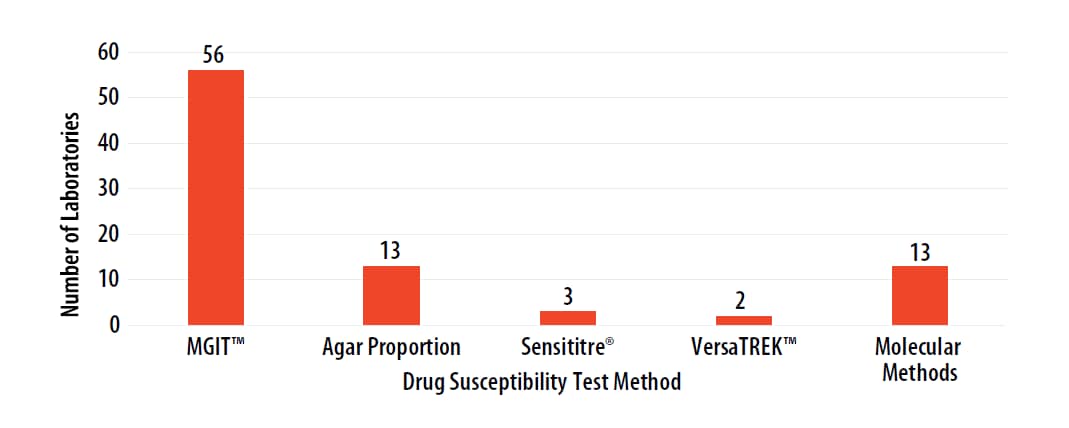
The DST methods that were performed by participating laboratories for this panel of MTBC isolates are displayed in Figure 3. Of participating laboratories, 37 (62%) reported results for only one method, 19 (32%) reported two methods, and 4 (7%) noted three susceptibility methods. Fifty-six (93%) participating laboratories indicated use of MGIT.
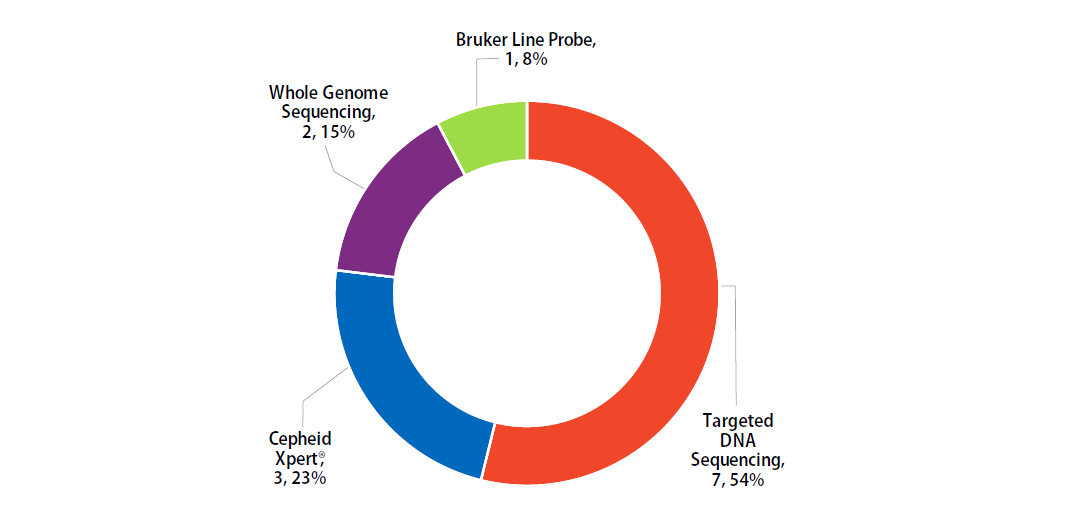
Molecular methods reported by participants are shown in Figure 4. The method performed most frequently (54%) was targeted DNA sequencing.
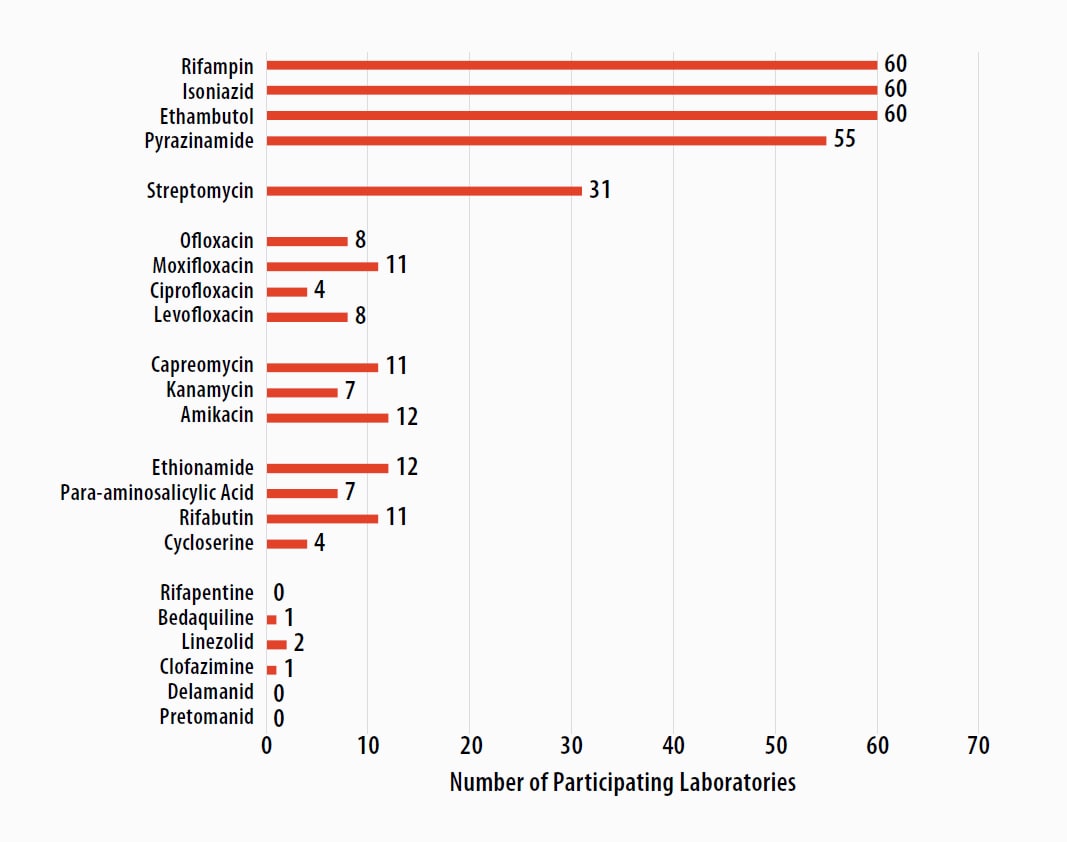
Antituberculosis Drugs Tested by Participants
The number of participating laboratories that reported testing each antituberculosis drug in the March 2023 survey is presented in Figure 5. CLSI recommends testing a full panel of first-line drugs (rifampin [RIF], isoniazid [INH], ethambutol [EMB], and pyrazinamide [PZA])[1] because it represents a combination of tests that provides the clinician with comprehensive information related to the 6- or 9-month four-drug RIPE TB treatment regimen used for many patients. Laboratories should consider the addition of fluoroquinolones to their testing panel as CDC recommends susceptibility testing for fluoroquinolones (e.g., moxifloxacin) with use of the alternate 4-month rifapentine-moxifloxacin treatment regimen; RIF may be used as a proxy for rifapentine [12].
Anticipated growth-based and molecular results for the panel of MTBC isolates sent to participants in March 2023 are shown in the tables below. Although CDC recommends broth-based methods for routine first-line DST of MTBC isolates, the results obtained by the reference agar proportion method (except for pyrazinamide, in which MGIT™ was performed) are shown in Table 1. Molecular results obtained by whole genome sequencing are listed in Table 2 [6].
Table 1. Expected Growth-based Results for March 2023 Survey
| Isolate | RIF | INH | EMB | PZA | Second-line Drug Resistances: |
|---|---|---|---|---|---|
| 2023A | S | R (high-level*) | R | S | STR†, ETA† |
| 2023B | R | S | S | S | |
| 2023C | R | S | S | S | |
| 2023D | R | S | S | S | |
| 2023E | S | S | S | S | AMK, KAN, CAP |
Note—S=susceptible, R=resistant
*Resistant at 0.2 µg/ml and 1.0 µg/ml by agar proportion. See Equivalent Critical Concentration table on page 8 for more information.
†Resistance to STR and ETA was not included on Expected Results report.
Table 2. Expected Molecular Results (Mutations Detected in Loci Associated with Resistance) for March 2023 Survey
| Isolate | rpoB* | katG | embB | pncA | rrs | ethA |
|---|---|---|---|---|---|---|
| 2023A | Ser315Thr Arg463Leu† |
Met306Val | Ser65Ser† | Partial deletion |
||
| 2023B | His445Tyr | |||||
| 2023C | Ser450Leu | |||||
| 2023D | Val170Phe | Thr135Ala⟡ | ||||
| 2023E | A1401G |
Note—Empty cell=No mutation detected
*M. tuberculosis numbering system used [7, 8]
†Mutation not associated with resistance [9]
⟡Effect of mutation is unknown.
- CLSI, Susceptibility Testing of Mycobacteria, Nocardiae spp., and Other Aerobic Actinomycetes, in 3rd Ed. CLSI Standard M24. 2018, Clinical and Laboratory Standards Institute: Wayne, PA.
- CLSI, Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, in 1st Ed. CLSI supplement M62. 2018, Clinical and Laboratory Standards Institute: Wayne, PA.
- CLSI, Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, in 2nd edition. CLSI supplement M24S. 2023, Clinical and Laboratory Standards Institute: Wayne, PA.
- World Health Organization, Technical Report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. 2018: Geneva.
- World Health Organization, Technical report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). 2021, Geneva: World Health Organization.
- Campbell, P.J., et al., Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother, 2011. 55(5): p. 2032-41.
- Andre, E., et al., Consensus numbering system for the rifampicin resistance-associated rpoB gene mutations in pathogenic mycobacteria. Clin Microbiol Infect, 2017. 23(3): p. 167-172.
- APHL, Issues in Mycobacterium tuberculosis complex (MTBC) Drug Susceptibility Testing: Rifampin (RIF), in APHL Issues in Brief: Infectious Diseases. 2019, Association of Public Health Laboratories: Washington, D.C.
- World Health Organization, Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance. 2021, World Health Organization: Geneva.
- World Health Organization, Technical Report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). 2021: Geneva.
- APHL, TB Drug Susceptibility Testing Expert Panel Meeting Summary Report. 2007, Association of Public Health Laboratories: Washington, D.C.
- Carr W, K.E., Starks A, Goswami N, Allen L, Winston C., Interim Guidance: 4-Month Rifapentine-Moxifloxacin Regimen for the Treatment of Drug-Susceptible Pulmonary Tuberculosis — United States, 2022. MMWR Morb Mortal Wkly Rep, 2022(71): p. 285–289.
- Zhang, Y. and W.W. Yew, Mechanisms of drug resistance in Mycobacterium tuberculosis: update 2015. Int J Tuberc Lung Dis, 2015. 19(11): p. 1276-89.
- Rigouts, L., et al., Rifampin resistance missed in automated liquid culture system for Mycobacterium tuberculosis isolates with specific rpoB mutations. J Clin Microbiol, 2013. 51(8): p. 2641-5.
- Van Deun, A., et al., Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol, 2009. 47(11): p. 3501-6.
- Ramirez-Busby, S.M. and F. Valafar, Systematic Review of Mutations in Pyrazinamidase Associated with Pyrazinamide Resistance in Mycobacterium tuberculosis Clinical Isolates. Antimicrob Agents Chemother, 2015. 59(9): p. 5267-77.
- Chedore, P., et al., Potential for erroneous results indicating resistance when using the Bactec MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Clin Microbiol, 2010. 48(1): p. 300-1.
- Siu, G.K., et al., Mutations outside the rifampicin resistance-determining region associated with rifampicin resistance in Mycobacterium tuberculosis. J Antimicrob Chemother, 2011. 66(4): p. 730-3.
- Maus, C.E., B.B. Plikaytis, and T.M. Shinnick, Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother, 2005. 49(8): p. 3192-7.