TB NOTES

TB Notes 1, 2020
Notes from the Director

Dear Colleague,
Many of our TB colleagues across the country and around the world are now engaged in the response to COVID-19. I want to acknowledge and thank all of the public health and medical professionals who are on the frontlines responding to the COVID-19 pandemic. As public health departments shift staff and resources to meet the needs of the response, it is important to take steps to ensure that people who are receiving treatment for TB disease can continue their treatment, even if routine health care services are affected. I encourage you to visit CDC’s TB and Public Health Emergencies and COVID-19 websites for information and resources.
Each year, we recognize World TB Day on March 24th to commemorate the date Dr. Robert Koch announced his discovery of the bacillus that causes tuberculosis (TB). This year, we joined our international partners by adapting the global Stop TB Partnership’s 2020 World TB Day theme, which was once again “It’s Time.”
CDC released new preliminary 2019 TB surveillance data presenting the lowest number of U.S. TB disease cases since 1953, when reporting began. In 2019, 8,920 new cases were reported in the United States. This reduction in new cases and the corresponding TB rate of 2.7 per 100,000 persons is among the lowest in the world thanks to ongoing, national investment in our public health system. But still, too many people in the United States suffer from TB disease.
Eliminating TB in the United States will require a concerted effort to expand testing and treatment for people with latent TB infection. Up to 13 million people in the United States have latent TB infection —and most do not know it. Today, more than 80 percent of U.S. TB disease cases result from untreated latent TB infection. In February 2019, CDC and the National Tuberculosis Controllers Association (NTCA) published “Guidelines for the Treatment of Latent Tuberculosis Infection” in CDC’s Morbidity and Mortality Weekly Report Recommendations and Reports. This is the first comprehensive update to U.S. latent TB infection treatment guidelines since 2000. CDC and NTCA preferentially recommend short-course, rifamycin-based, 3- or 4-month latent TB infection treatment regimens over 6- or 9-month isoniazid monotherapy.
Since late 2019, U.S. TB programs and the National TB Controllers Association (NTCA) have reported difficulties with procuring rifapentine. NTCA recently circulated an email updated regarding information they received from the manufacturer about drug availability. In addition, the U.S. Food & Drug Administration (FDA), as of 03/25/20, lists rifapentine (Priftin) Tablets, 150 mg (NDC 0088-2102-24) on their website as being “on allocation with intermittent supply [due to] demand increase for the drug.” At this time, there is not an estimate related to how long this shortage may last. For future updates on the status of rifapentine availability, visit FDA’s Drug Shortages Database. For more information about selection of treatment for latent TB infection, please see the latent TB infection treatment regimens web page.
Thank you for your hard work in the COVID-19 response, and in all that you do to continue to prevent and control TB.
Philip LoBue, MD, FACP, FCCP
Director
Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention

Dear Colleague Letter from Dr. Redfield, M.D., Director, CDC
This World TB Day, CDC Director, Dr. Robert Redfield, M.D., published a Dear Colleague Letter encouraging stakeholders to celebrate progress towards TB elimination and re-commit to ending suffering and hardship caused by TB. Dr. Redfield recognized the continued work and commitment by CDC and its partners to eliminate TB in the United States and around the world.
World TB Day Personal Stories
Five TB survivors and family of survivors bravely shared their experiences battling latent TB

infection and TB disease in a new World TB Day video. In this multi-language montage, TB survivors and family members of survivors share World TB Day messages to raise awareness of the challenges that hinder our progress towards the elimination of this devastating disease.
CDC would like to express our thanks to “We Are TB” and all of the TB survivors. Visit CDC’s TB Personal Stories webpage for more survivor stories.
CDC US TB Elimination Champions Project
The 2020 CDC U.S. TB Elimination Champions Project marked the 5th year of recognizing the continued hard work and dedication from those working to end TB in their communities.
In keeping with this year’s World TB Day theme “It’s Time!,” CDC highlighted photos and stories from organizations and individuals that represent their work and commitment to the “It’s Time to End TB!” mission.
Congratulations and thank you to the 2020 Champions!
- Jeannette Aldous, California
- Breathe Easy South Texas Project, Texas
- Bruce Chandler, Alaska
- CDC’s Division of TB Elimination Laboratory Branch and Surveillance, Epidemiology, and Outbreak, Investigation Branch/Molecular Epidemiology Activity, Georgia
- Dekalb County Board of Health Refugee and Tuberculosis Program, Georgia
- Genesee and Orleans County Health Departments, New York
- The Hope Clinic and Houston Health Department, Texas
- Jefferson County Department of Health, Alabama
- Karen Landers, Alabama
- Julie Low, California
- Michigan Department of Health and Human Services, Bureau of Laboratories, Michigan
- Marisa Moore, California
- Eric Morgan, Alabama
- Saint Louis County Department of Public Health Chest Clinic, Missouri
- Jacek Skarbinski, California
- Chris Spitters, Washington
- Wadsworth Center, New York State Department of Health, New York
CDC.gov World TB Day Feature Article
CDC.gov published a feature article co-authored by DTBE and the Division of Global HIV and Tuberculosis to highlight progress in the United States and around the world to end TB. CDC highlights the need for better diagnostics, shorter treatment regimens, and strong domestic and global partnerships to accelerate progress toward elimination.

Media Planet Editorial
Dr. Philip LoBue, DTBE Director, authored a Media Planet commentary highlighting the work of CDC and our partners towards TB elimination. The commentary titled, “How the CDC is Helping to Make Tuberculosis a Thing of the Past,” highlights TB prevention and control efforts to eliminate TB in the United States.
World TB Day Social Media Photo Challenge
For the second year in a row, CDC encouraged people to share “It’s Time!” messages on social media. Participants could download a template, write their World TB Day message, take a picture, and post on Twitter, Facebook, and Instagram. Thank you to everyone who participated in this year’s challenge!
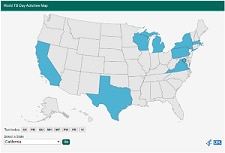
World TB Day Activities Map
To highlight World TB Day activities happening across the country, CDC created a 2020 World TB Day Activities Map. We know many of our partners had to make last minute adjustments to move activities online, or cancel or postpone activities and events due to the COVID-19 response. Thank you to everyone who shared a World TB Day activity!
Electronic DOT (eDOT) for TB Treatment Study Completes Data Collection

Mrs. Sarah Kiskadden-Bechtel (L) and Ms. Charlene Sathi (R)
On January 10, 2020, staff from the Corona, Washington Heights, Morrisania, and Fort Greene Tuberculosis (TB) Chest Clinics in New York City (NYC) gathered with Bureau of TB Control (BTBC) headquarter staff to observe the official end of daily data collection for the eDOT Study. The study, a randomized clinical trial comparing traditional in-person directly observed therapy (ipDOT) with electronic DOT (eDOT) for TB treatment; enrolled the first patients in July 2017. Between 2017 and 2019, study staff worked in tandem with TB Chest Clinic staff to enroll 216 individuals into this program-based study.
The celebratory gathering was organized by two of the study’s coordinators, Mrs. Sarah Kiskadden-Bechtel and Ms. Charlene Sathi. To start, Dr. Joseph Burzynski, Assistant Commissioner and Principal Investigator, shared data related to the historical use of directly observed therapy (DOT) and eDOT in NYC, the rationale for conducting the eDOT study in NYC, and his gratitude to all BTBC staff for their phenomenal support of and assistance with the study.

Clinic Staff and Dr. Joe Burzynski
Dr. Neil Schluger, Chief, Division of Pulmonary, Allergy and Critical Care Medicine, Columbia University Irving Medical Center, and study co-investigator, addressed the group, noting the study’s contributions to the larger TB control community and echoing Dr. Burzynski’s words of thanks. Dr. Joan Mangan, Division of TB Elimination, CDC, and co-principal investigator, discussed trends towards digital health, shared details from CDC’s Antibiotic Resistance Solutions Initiative which funded the study, and delivered a message from Dr. LoBue – expressing sincere appreciation to all BTBC staff for the time and hard work put forth to ensure the study’s success. Dr. Michelle Macaraig, Director of Policy, Planning and Administration, BTBC, and co-principal investigator, presented the immediate impact the eDOT Study has had on the program in NYC. Mr. Marco Salerno, the study’s data manager shared highlights from presentations and scientific posters that had previously reported preliminary data at national and international meetings. Marco also gave kudos to the group for the high quality of the data. Throughout the presentations, the strong collaboration between the NYC BTBC, Columbia University, and CDC was evident.

Clinic Staff and Dr. Joe Burzynski
At the conclusion of these presentations, Dr. Burzynski, Mrs. Kiskadden-Bechtel, and Ms. Sathi presented each clinic with a framed certificate of appreciation for their contributions to the study and recognized four individual BTBC staff as “eDOT Study Champions”. Mr. Nikolaos Mitropoulos and Mr. Gary Henry earned the title of study champion for their invaluable assistance overcoming numerous technical hurdles during the study’s implementation. Ms. Beilei Chen and Dr. Jitendra Shah were recognized as the nurse and physician who helped to recruit the most study participants.
To end the meeting on a fun note, Ms. Kiskadden-Bechtel and Ms. Sathi led the group in a rousing rendition of the word guessing game Taboo that they created for the meeting with a focus on TB and the study.
The study team is now focusing their efforts on data analysis and dissemination of the study’s results. Publication of data from a linked cost study with detailed cost comparisons between ipDOT and eDOT is also forthcoming. Stay tuned!
Submitted by Joan Mangan, PhD, MST
TB ETN-PEN Conference Scheduled for September 2020 is Postponed until 2021
The TB ETN-PEN Conference currently scheduled for September 22-24, 2020 will be postponed until 2021. Many of our TB colleagues across the country and around the world are now engaged in the response to COVID-19. We will share information on the rescheduled TB ETN-PEN conference in 2021 as soon as we determine new dates.
Preliminary TB Surveillance Data for 2018

In recognition of World TB Day, DTBE published preliminary TB surveillance data for 2019. Data from CDC’s national tuberculosis (TB) surveillance system show the lowest number of TB cases in the United States on record, but the pace of decline has slowed in recent years. TB cases declined 1.1% between 2018 and 2019 (from 9,021 to 8,920 cases), while TB rates declined 1.6% (from 2.8 to 2.7 cases/100,000 persons). TB elimination requires maintaining and strengthening current TB control priorities while expanding targeted testing and treatment for high-risk persons with longstanding, untreated latent TB infection. CDC is working with public health partners and primary care providers to encourage testing and treatment for latent TB infection as a routine and integral part of healthcare for patients at-risk for latent TB infection.
Good news from the Surveillance Team!
The deadline for the implementation of the 2020 Report of Verified Case of Tuberculosis (RVCT) has been extended from December 31, 2020 to December 31, 2021. This means reporting areas do not need to make the transition to the new case notification message format until December 31, 2021. Please do continue to implement the new 2020 items to the extent possible. DTBE is prepared to facilitate the onboarding of any reporting area between now and the end of 2021.
In a collaborative effort, DTBE is developing online 2020 RVCT training resources, including:
- 2020 RVCT Instruction Manual
- RVCT 2009 to RVCT 2020 form comparison guide
- Item-by-item review
- 2020 RVCT training PowerPoint slide set with script
- List of Frequently Asked Questions with responses
- Case Studies Manual (with examples)
- Recorded webinars for each RVCT item
For technical assistance related to the 2020 RVCT or any other surveillance data issues, please contact the DTBE Helpdesk at DTBEsupport@cdc.gov.
Submitted by Lauren Lambert, MPH, Epidemiologist, Surveillance Team, Acting Team Lead
Coronavirus (COVID-19): Information for TB Programs

A public health emergency can make efforts to help TB patients continue their treatment difficult. TB programs’ work ensures that people who are receiving treatment for TB disease can continue their treatment, even if routine health care services are affected.
In the event of a public health emergency, TB programs can help to mitigate potential shifts in staff assignments and resources.
- Make sure contact information for patients is current, and staff can reach patients with updates and information.
- Provide contact numbers of TB program personnel to patients.
- Communicate any changes or updates in service to community partners.
- Consider providing additional medications to patients if health department operations are likely to be affected.
- If policies and procedures are in place, consider alternative treatment delivery methods, such as electronic directly observed therapy (eDOT).
Persons with COVID-19 infection may seek care at TB clinics given the primary symptoms are similar to manifestations of TB, e.g., fever, cough, and shortness of breath. Patients with TB can become infected with COVID-19, which could result in an unexpected deterioration of a patient’s condition (e.g., was responding well to treatment and develops new acute cough, fever, or shortness of breath). TB patients who are at least 65 years old; have respiratory compromise from their TB; or other medical conditions, including HIV and other immunocompromising conditions, are at greater risk for severe COVID-19 infection. Therefore, respiratory infection control precautions, with which TB programs are familiar, are of even greater importance now as are general precautions, such as frequent hand washing, disinfecting surfaces, and avoiding touching one’s face.
COVID-19 guidance for healthcare facilities, including outpatient facilities, is available at the CDC COVID-19 website.
Community Partnership to Expand Latent TB Infection Services in Houston, TX
Initiating Partnership

Pictured from left to right: Hanan Noori, MA, Hope Clinic; Maria Galvis, CDC, Public Health Advisor assigned to Houston Health Department; Tonia Ellison, LVN, Houston Health Department; Barbarah Martinez RN, BSN, Hope Clinic; Kara Green MSN, APRN, FNP-BC, Hope Clinic; Nguyen Tran, MA, Hope Clinic
The Houston Health Department Bureau of Tuberculosis Control works to ensure the health of Houstonians by identifying and treating TB cases and their contacts. In addition to the program’s TB core activities, the program launched a new project of partnering with community clinics to expand their latent TB infection services and offering once-weekly isoniazid-rifapentine for 12 weeks (3HP) to high-risk populations. This new initiative is the component needed to steer the program towards TB elimination.
The Hope Clinic was selected for this project since there was already an existing relationship. Also known as the Asian American Health Coalition (AAHC), the Hope Clinic is a federally qualified community clinic serving the uninsured, underinsured multicultural residents in Houston. The Hope Clinic’s Director of Clinical Services has collaborated with the Houston Health Department for several years by reporting, referring, and ensuring optimal TB patient care. This existing relationship facilitated initial conversations regarding implementing latent TB infection treatment in the community.
Overview of the Partnership
The Houston Health Department provided Hope Clinic with a training focused on latent TB infection, specifically the short-course 3HP regimen. The medical assistants at the clinic were observed during the administration of 3HP to ensure the process and documentation was being done according to CDC guidelines. The outlined roles for the Hope Clinic include screening qualified patients for 3HP, providing medical evaluation and performing directly observed therapy (DOT) to the patients on 3HP. The Houston Health Department supports the project by providing medication and patient incentives, in addition to providing training and educational materials as needed. Both parties signed a scope of services agreement, beginning the formal partnership in Fall 2017. The project started as a pilot in early 2018 with a maximum enrollment of 20-25 patients per quarter.
Successes and Challenges
At the beginning of the project, the program experienced very low enrollment in 3HP. Some of the providers were a bit reluctant to prescribe the 3HP regimen. The Houston Health Department conducted a refresher meeting with the medical director to re-emphasize the benefits of offering 3HP to the patients. Incentives were given to Hope Clinic to give to patients who agreed to come for their weekly DOT, and the number of enrollees increased. Clearly, the use of incentives has been instrumental to both increase 3HP enrollment as well as encourage completion of treatment. Since the project started, 22 patients have enrolled, and the treatment completion rate is about 88%.
Conclusion
Although new projects are usually confronted by resistance, the beginning of new projects provide the opportunity for programs to grow and expand their preventive services. In all, the various in-person meetings, education and provider training, incentives, and most importantly the strong communication between both partners have led to a successful latent TB infection project. Future plans for the project include expanding latent TB infection treatment services through the use of video DOT (VDOT).
Submitted by Maria E. Galvis, PHA assigned to Houston Health Department
Acknowledgments:
Hope Clinic Staff: Andrea Caracostis MD, MPH Chief Executive Officer; Kara Green MSN, APRN, FNP-BC; Nguyen Tran, MA; Hanan Noori, MA
Houston Health Department: Richard Stancil, Bureau Chief; Tonia Ellison, LVN
2018 State and City Tuberculosis Report
The 2018 State and City Tuberculosis Report is now available online. This report provides key process and outcomes measures for TB control programs in the United States used to monitor progress towards TB elimination at the state and local level. These indicators were selected by CDC in cooperation with partners in state and local health departments. Data for calculating these indicators are derived from the National Tuberculosis Surveillance System (NTSS) and the Aggregate Reports for Program Evaluation (ARPE). CDC publishes indicator data to assist TB programs in:
- Evaluating progress toward achievement of national objectives through monitoring of TB program performance.
- Assessing the need for education and technical assistance.
- Identifying areas that need improvement.
Learn more about the National TB Program Objectives and Performance Targets for 2025.
Updates from the Division of Tuberculosis Elimination Health Equity Work Group

The goal of the CDC Division of TB Elimination Health Equity Work Group (DTBE-HEWG) is to inform and shape DTBE’s agenda for programs, research, education, and policies towards reducing TB health disparities. The HEWG is made up of representatives from each DTBE branch and the Office of the Director. DTBE strategic priorities include reducing health disparities, congruent with the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB (NCHHSTP) and CDC commitments.
HEWG meets monthly to review and discuss progress made toward reducing TB health disparities, to explore new data sources, to identify gaps in activities based on evidence, and to champion improvements in health equity and efforts to reduce health disparities overall. Activities undertaken by the HEWG include:
- Consistently review social determinants of health data on reported TB cases (e.g., race/ethnicity, country of birth, employment, gender, homelessness, and incarceration) and reviewed data on medical determinants that contribute to TB disparities (e.g., HIV, diabetes, other immunosuppression).
- Explores new cross-cutting data and evidence-based practices to address TB health disparities.
- Contributed to writing the DTBE 2020 notice of funding opportunity (NOFO) that included TB health disparities language and content for applicants to address, including TB prevention activities target populations experiencing persistent TB disparities.
- Represented DTBE on the NCHHSTP Health Equity Work Group and maintained communication between NCHHSTP and other CDC entities and partners on health disparities and health equity issues.
- Developed and maintained a list of contributions by DTBE staff to the literature on TB health disparities and equity issues (presentation, publications, and reports). Published a major article in the American Journal of Public Health (Khan et al., 2018;108: S321-S326) that highlighted persistent disparities and, in some populations, increases in disparities.
- Invited presentations from two DTBE branches (health equity issues in clinical trials, presented by the Clinical Research Branch; health equity related activities by the Communications, Education, and Behavioral Science Branch) and from the NCHHSTP HEWG at DTBE HEWG monthly meetings.
- Developed and shared a DTBE framework to address TB disparities and equity that will serve as guide to identify underlying factors affecting TB disparities and to develop innovative approaches to moving toward health equity.
- Developed a list of potential data sources on latent TB infection diagnosis and treatment to facilitate monitoring of population-specific indicators in the latent TB infection care cascade.
Even though reported TB cases and rates have been decreasing since their height in 1992, TB control and prevention efforts must address the persistent disparities that exist among populations affected by TB.
Submitted by Awal Khan, PhD, and Suzanne Marks, MPH, MA
TB ETN-PEN Conference Scheduled for September 2020 is Postponed until 2021
The TB ETN-PEN Conference currently scheduled for September 22-24, 2020 will be postponed until 2021. Many of our TB colleagues across the country and around the world are now engaged in the response to COVID-19. We will share information on the rescheduled TB ETN-PEN conference in 2021 as soon as we determine new dates.
Coronavirus (COVID-19): Information for TB Laboratories

CDC is providing updates on the current status of national tuberculosis (TB) laboratory services during the ongoing COVID-19 response efforts.
- CDC’s Molecular Detection of Drug Resistance (MDDR) Service is operational, and we are working diligently to ensure sufficient staffing and resources to provide both molecular and growth-based drug-susceptibility testing (DST). Turnaround times for issuance of reports back to submitters could be delayed. If you need additional information or have questions, please feel free to contact TBLab@cdc.gov or 404-639-2455.
- The National DST Reference Center for Mycobacterium tuberculosis at the California Microbial Diseases Laboratory is operational. For enrolled laboratories, please continue to refer specimens and isolates for pyrosequencing or MGIT DST as per your usual procedure. For additional information, please contact CDPHTBDST@cdph.ca.gov or 510-412-3949.
- The National TB Molecular Surveillance Center at the Michigan Department of Health and Human Services Bureau of Laboratories is operational and referral of M. tuberculosis isolates should continue. CDC is continuing to provide conventional genotyping results and conducting analysis of whole genome sequencing data, as applicable. For additional information, please contact Angie Schooley at schooleya@michigan.gov or 517-335-9637.
The response to COVID-19 is an unprecedented event and could impact public health laboratory testing for TB. CDC has received reports of shifting or rotating mycobacteriology staff to support COVID-19 testing and have received notification that some public health laboratories are implementing continuity of operations plans. As always, CDC encourages regular communication between TB programs and public health laboratories to understand any impacts to TB testing capacity.
False Positive Investigation Toolkit: A Resource for Mycobacteriology Laboratories

CDC’s Division of Tuberculosis Elimination, Laboratory Branch recently released the False Positive Investigation Toolkit: A Resource for Mycobacteriology Laboratories False-positive Mycobacterium tuberculosis complex (MTBC) results can be difficult to identify, investigate, and resolve. These results often have serious implications for patient isolation, patient therapy, contact investigations, and unnecessary laboratory testing. The toolkit was developed to provide updated resources to aid recognition of potential false-positive results and to assist staff in conducting false-positive investigations. The false-positive investigation toolkit features a narrative document and job-aids, posters, and templates that can be modified for specific local use.
An accompanying online interactive case study training module, Mycobacteriology False-Positive Case Studies, can be accessed on the Association of Public Health Laboratories (APHL) website. The training module has five different case studies that walks the participant through different scenarios of a potential false-positive investigation, using knowledge checks to guide the participant.
Submitted by Monica Youngblood

TBTC 45th Semi-Annual Meeting is Canceled
Due to issues related to the COVID-19 pandemic, DTBE’s Clinical Research Branch (CRB) has decided to cancel the in-person TBTC 45th Semi-Annual Meeting scheduled for May 6 – 8, 2020. CRB is planning a remote participation alternative to the May meeting that will cover the excellent work done by the consortium over the past six months. CRB looks forward to seeing the consortium group in-person in Atlanta in November 2020 for the 46th Semi-Annual Meeting.

This February, DTBE staff attended the 24th Annual Conference of The Union North America Region in Chicago, IL. This three-day scientific conference brings together national and international experts of national and international to focus on topics of importance to public and respiratory health, with emphasis on TB and lung disease.
Highlights from the conference include:
- Nick Deluca, PhD, gave a talk titled, “Engaging Communities in TB Elimination through Effective Communication”
- Richard Brostrom, MD, MSPH, gave a talk titled, “Active Case Finding in Majuro”
- Sapna B. Morris, MD, served as plenary chair for a session titled, “Taking the Leap – Active Case Finding and TB Elimination Project”
- Joan Mangan, PhD, MST, served as primary chair of the nursing sponsored session, “Compassionate Conversations”
- Neela Goswami, MD, MPH, moderated a session titled, “2020 and Beyond: New Tools in the TB Toolbox for Diagnosis, Infection Control, and Treatment”
- DTBE staff gave several poster presentations with topics related to updated recommendations for TB screening, testing, and treatment of health care personnel, training for the new RVCT form, health message testing findings, using electronic health data to describe the TB care cascade and improve TB infection surveillance, and more
- DTBE staff facilitated poster sessions including Molecular Epi and Outbreaks, Latent TB Infection, Program Evaluation, Training and Education, and Special Populations
- DTBE hosted a booth at the conference to share key resources and connect with participants
Agizew T, Chihota V, Nyirenda S, Tedla Z, Auld AF, Mathebula U, Mathoma A, Boyd R, Date A, Pals SL, Lekone P, Finlay A. Tuberculosis treatment outcomes among people living with HIV diagnosed using Xpert MTB/RIF versus sputum-smear microscopy in Botswana: a stepped-wedge cluster randomised trial. BMC Infect Dis 2019;19:1058.
Ahmed A, Feng PI, Gaensbauer JT, Reves RR, Khurana R, Salcedo K, Punnoose R, Katz DJ; Tuberculosis Epidemiologic Studies Consortium. Interferon-γ release assays in children <15 years of age. Pediatrics 2019:pii:e20191930. Epub ahead of print.
Armstrong GL, MacCannell DR, Taylor J, Carleton HA, Neuhaus EB, Bradbury RS, Posey JE, Gwinn M. Pathogen genomics in public health. N Engl J Med 2019;381:2569–80.
Auld AF, Agizew T, Mathoma A, Boyd R, Date A, Pals SL, Serumola C, Mathebula U, Alexander H, Ellerbrock TV, Rankgoane-Pono G, Pono P, Shepherd JC, Fielding K, Grant AD, Finlay A. Effect of tuberculosis screening and retention interventions on early antiretroviral therapy mortality in Botswana: a stepped-wedge cluster randomized trial. BMC Med 2020;18:19. (Shepherd, also with Yale University School of Medicine).
Chen MP, Miramontes R, Kammerer JS. Multidrug-resistant tuberculosis in the United States, 2011–2016: patient characteristics and risk factors. Int J Tuberc Lung Dis 2020;24:92–9.
Click ES, Kurbatova E, Alexander H, Dalton TL, Chen MP, Posey JE, Ershova JJ, Cegielski P. Isoniazid- and rifampin-resistance mutations associated with resistance to second-line drugs and with sputum culture conversion. J Infect Dis 2020;pii:jiaa042. Epub ahead of print.
Dorman SE, Nahid P, Kurbatova EV, Goldberg SV, Bozeman L, Burman WJ, Chang KC, Chen M, Cotton M, Dooley KE, Engle M, Feng PJ, Fletcher CV, Ha P, Heilig CM, Johnson JL, Lessem E, Metchock B, Miro JM, Nhung NV, Pettit AC, Phillips PPJ, Podany AT, Purfield AE, Robergeau K, Samaneka W, Scott NA, Sizemore E, Vernon A, Weiner M, Swindells S, Chaisson RE; AIDS Clinical Trials Group and the Tuberculosis Trials Consortium. High-dose rifapentine with or without moxifloxacin for shortening treatment of pulmonary tuberculosis: study protocol for TBTC study 31/ACTG A5349 phase 3 clinical trial. Contemp Clin Trials 2020:105938. Epub ahead of print.
Haddad MB, Lash TL, Hill AN, Navin TR, Castro KG, Gandhi NR, Winston CA. Robustness of NHANES estimates of the U.S. prevalence of a positive tuberculin skin test. Epidemiology 2019. Epub ahead of print. (Haddad, Hill, and Winston, also with Emory University).
Klopper M, Heupink TH, Hill-Cawthorne G, Streicher EM, Dippenaar A, de Vos M, Abdallah AM, Limberis J, Merker M, Burns S, Niemann S, Dheda K, Posey J, Pain A, Warren RM. A landscape of genomic alterations at the root of a near-untreatable tuberculosis epidemic. BMC Med 2020;18:24.
Pratt RH, Manangan LP, Cummings CN, Langer AJ. Noncountable tuberculosis case reporting, National Tuberculosis Surveillance System, United States, 2010–2014. Public Health Rep 2019:33354919884302. Epub ahead of print.
Reichler MR, Bruden D, Thomas H, Erickson BR, Knust B, Duffy N, Klena J, Hennessy T; Household Transmission Investigative Team. Ebola patient virus cycle threshold and risk of household transmission of Ebola virus. J Infect Dis 2019;pii:jiz511. Epub ahead of print.
Vernon A, Bishai W. Modelling treatment of latent TB: shortening the leap of faith? Am J Respir Crit Care Med 2019. Epub ahead of print.
Vernon A, Fielding K, Savic R, Dodd L, Nahid P. The importance of adherence in tuberculosis treatment clinical trials and its relevance in explanatory and pragmatic trials. PLoS Med. 2019;16):e1002884.
To receive the TB Notes Newsletter, enter your email address at the bottom of the TB Notes webpage. If you would like to submit an article or update in TB Notes, please email Annie Rossetti at wuw0@cdc.gov.
You can follow us on Twitter @CDC_TB and Facebook @CDCTB and sign up for email updates through Adobe Campaign.