Dispensing of Oral Antiviral Drugs for Treatment of COVID-19 by Zip Code–Level Social Vulnerability — United States, December 23, 2021–May 21, 2022
Weekly / June 24, 2022 / 71(25);825-829
On June 21, 2022, this report was posted online as an MMWR Early Release.
Jeremy A.W. Gold, MD1; James Kelleher2; Jake Magid; MEng2; Brendan R. Jackson, MD1; Meghan E. Pennini, PhD3; Diana Kushner, MPH3; Emily J. Weston, MPH1,3; Bobby Rasulnia, PhD1; Sachiko Kuwabara, PhD1,3; Kelly Bennett, MPH3; Barbara E. Mahon, MD1; Anita Patel, PharmD1; John Auerbach, MBA1 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Lagevrio and Paxlovid are oral antiviral drugs effective at preventing hospitalization and death in patients with mild to moderate COVID-19 who are at risk for progression to severe disease.
What is added by this report?
During December 23, 2021–May 21, 2022, 1,076,762 oral antiviral prescriptions were dispensed in the United States. The overall number of antivirals dispensed increased; however, by the end of the study period, dispensing rates were lowest in high vulnerability zip codes, despite these zip codes having the largest number of dispensing sites.
What are the implications for public health practice?
Additional public health, regulatory, and policy efforts might help decrease barriers to oral antiviral access, particularly in communities with high social vulnerability.
The COVID-19 pandemic has highlighted and exacerbated long-standing inequities in the social determinants of health (1–3). Ensuring equitable access to effective COVID-19 therapies is essential to reducing health disparities. Molnupiravir (Lagevrio) and nirmatrelvir/ritonavir (Paxlovid) are oral antiviral agents effective at preventing hospitalization and death in patients with mild to moderate COVID-19 who are at high risk* for progression to severe COVID-19 when initiated within 5 days of symptom onset. These medications received Emergency Use Authorization from the Food and Drug Administration (FDA) in December 2021† and were made available at no cost to recipients through the U.S. Department of Health and Human Services (HHS) on December 23, 2021. Beginning March 7, 2022, a series of strategies was implemented to expand COVID-19 oral antiviral access, including the launch of the Test to Treat initiative.§ Data from December 23, 2021–May 21, 2022, were analyzed to describe oral antiviral prescription dispensing overall and by week, stratified by zip code social vulnerability. Zip codes represented areas classified as low, medium, or high social vulnerability; approximately 20% of U.S. residents live in low-, 31% in medium-, and 49% in high-social vulnerability zip codes.¶ During December 23, 2021–May 21, 2022, a total of 1,076,762 oral antiviral prescriptions were dispensed (Lagevrio = 248,838; Paxlovid = 827,924). Most (70.3%) oral antivirals were dispensed during March 7–May 21, 2022. During March 6, 2022–May 21, 2022, the number of oral antivirals dispensed per 100,000 population increased from 3.3 to 77.4 in low-, from 4.5 to 70.0 in medium-, and from 7.8 to 35.7 in high-vulnerability zip codes. The number of oral antivirals dispensed rose substantially during the overall study period, coincident with the onset of initiatives to increase access. However, by the end of the study period, dispensing rates in high-vulnerability zip codes were approximately one half the rates in medium- and low-vulnerability zip codes. Additional public health, regulatory, and policy efforts might help decrease barriers to oral antiviral access, particularly in communities with high social vulnerability.
Nationwide oral antiviral dispensing data are reported to HHS daily through the HHS Health Partner Ordering Portal (HPOP)**; 85%–95% of oral antiviral sites report dispensing data to HHS. Information regarding the location of oral antiviral prescription dispensing and the number of active sites dispensing oral antivirals is geocoded to the zip code level. An active site dispensing oral antivirals was defined as any provider that had ordered oral antiviral courses during the previous 60 days or that reported inventory during the previous 14 days. For this analysis, zip codes were ranked according to the Equitable Distribution Index scale, a proxy for social vulnerability. Based on Equitable Distribution Index score, zip codes were classified as having low (0–0.33), medium (>0.33–0.66), or high (>0.66–1.00) social vulnerability.
Total numbers of Lagevrio and Paxlovid prescriptions dispensed and the number of dispensing sites during December 23, 2021–May 21, 2022, were tabulated and examined by week and zip code–level social vulnerability. Social vulnerability–stratified rates of oral antiviral prescription dispensing (prescriptions dispensed per 100,000 population) were calculated; the population denominators used for rate calculations were obtained from 2018 CDC and Agency for Toxic Substances and Disease Registry social vulnerability index (SVI) data (4). This activity was reviewed by HHS and CDC and was conducted consistent with applicable federal law and CDC policy.††
During December 23, 2021–May 21, 2022, a total of 1,076,762 oral antiviral prescriptions (248,838 Lagevrio; 827,924 Paxlovid) were dispensed (Figure 1); overall, 70.3% (756,858) were dispensed during March 7–May 21, 2022. The weekly number of oral antiviral prescriptions dispensed initially peaked at 56,073 (30,636 Lagevrio; 25,437 Paxlovid) during the week ending February 12, 2022; declined to 14,925 (3,821 Lagevrio; 11,104 Paxlovid) during the week ending March 26, 2022; and increased to 179,728 (19,162 Lagevrio; 160,566 Paxlovid) during the week ending May 21, 2022. The number of dispensing sites increased from 49 during the week ending December 25, 2021, to 39,687 during the week ending May 21, 2022 (Figure 2).
As of May 21, 2022, the largest number of dispensing sites was located in high-vulnerability zip codes (18,844; 47.5%), approximately one third (13,072; 32.9%) were in medium-vulnerability zip codes, and approximately one fifth (7,771; 19.6%) were in low-vulnerability zip codes. Overall, during December 23, 2021–May 21, 2022, the highest rates of oral antiviral prescriptions dispensed were in low-vulnerability zip codes (373.3 per 100,000), followed by medium- (359.5) and high- (287.4) vulnerability zip codes. During December 23, 2021–March 5, 2022, the rates of oral antiviral courses dispensed ranged from 0.2 to 27.0 per 100,000 in high-, 0.2 to 13.4 in medium-, and 0.1 to 8.6 in low-vulnerability zip codes (Figure 3). During March 6, 2022–May 21, 2022, the rates of oral antivirals dispensed increased from 3.3 to 77.4 per 100,000 and from 4.5 to 70.0 in low- and medium-vulnerability zip codes, respectively; rates in high-vulnerability zip codes increased from 7.8 to 35.7, reaching approximately one half the rate in low- and medium-vulnerability zip codes. At the end of the study period, (May 21, 2022), COVID-19 continued to cause an average of 291 deaths and 3,833 new hospitalizations per day.§§
Discussion
This analysis of national oral antiviral dispensing data during December 23, 2021–May 21, 2022, highlights a substantial increase in the number of dispensing sites located throughout the country to 39,687 sites (87% of which were pharmacies) as of May 21, 2022, and in the number of oral antivirals dispensed (1,076,762 total, including 70.3% during March 7–May 21, 2022). These increases were possible because of coordinated efforts among federal, state, local, and pharmacy partners to expand access to COVID-19 therapies, coincident with an increased supply and a rise in the number COVID-19 cases nationwide. Efforts to expand oral antiviral access included the launch of the Test to Treat program, an expansion of the distribution network through federal pharmacy partners, increased access to testing,¶¶ and ongoing implementation of community and clinician outreach efforts.***
Despite the increase in the number of oral antivirals dispensed during the study period, population-adjusted dispensing rates in high-vulnerability zip codes were substantially lower than those in medium- and low-vulnerability zip codes, even though high-vulnerability zip codes had the most dispensing sites. Oral antivirals, particularly Paxlovid, provide an essential tool that can prevent hospitalization and death from COVID-19 (5). The findings in this report highlight an ongoing need to identify and eliminate barriers to oral antiviral access, particularly within socially and economically disadvantaged communities.
Timely administration of oral antivirals depends on multiple factors, including adequate drug supply and distribution; acceptance of the therapy by health care providers and the public; and patient access to testing, prescriptions, and drug dispensing sites (6). To access oral antiviral therapy, a patient must first receive a positive test result for SARS-CoV-2 (the virus that causes COVID-19), followed by a clinical assessment by a health care provider authorized to prescribe the drug (i.e., physicians, advanced practice registered nurses, and physician assistants). Although 47.5% of dispensing sites are located in high-vulnerability zip codes as of May 21, 2022, and approximately 88% of the U.S. population live within 5 miles of a site,††† most pharmacies serving as dispensing sites do not have authorized prescribers available on-site or via telemedicine.§§§ Persons living in high-vulnerability zip codes might face challenges accessing health care providers who are authorized to prescribe oral antivirals (1). In addition, the end of reimbursement for testing, health care provider assessment, and oral antiviral dispensing through the Health Resources and Services Administration Uninsured Program on March 22, 2022, might have contributed to lower oral antiviral dispensing rates for certain populations living within high-vulnerability zip codes.¶¶¶
Several strategies could improve access to oral antivirals in high-vulnerability zip codes. Additional innovative approaches could be considered that facilitate patient access to testing, clinical assessments, and oral antivirals in a single visit (6). As access to prescriptions increases, provider reimbursements for clinical assessment services should be considered, including additional costs that might inadvertently create additional barriers to care.**** Additional needed efforts, with an emphasis on reaching high-vulnerability areas, include increasing access to authorized prescribers; continuing education and outreach for patients, reinforcing the importance of seeking medication early after the onset of COVID-19 symptoms; and continued expansion of oral antiviral dispensing sites nationwide. Health care providers should be aware that Paxlovid is generally well-tolerated, is highly effective at preventing severe disease and hospitalization, and should be prescribed to treat mild to moderate illness in persons who are at high risk for progression to severe COVID-19, including persons aged ≥65 years.††††
The findings in this report are subject to at least four limitations. First, because oral antiviral dispensing data are based on self-reporting by dispensing sites, and 85%–95% of sites reported dispensing data to HHS, the number of prescriptions dispensed is likely underestimated. Second, the calculation of Equitable Distribution Index scores involves the aggregation of U.S. Census Bureau tracts into zip codes, a process that might compound the sampling error already inherent in calculating proxy scores for social vulnerability using U.S. Census Bureau data. Further, individual zip codes might still encompass communities with varying degrees of social vulnerability, and Equitable Distribution Index scores cannot be calculated in areas where U.S. Census Bureau population data are not publicly available. Third, this analysis did not assess correlations between rates of oral antiviral dispensing and measures of COVID-19 prevalence (e.g., percentage of test results that were positive) or associated outcomes (e.g., rates of hospitalization or death); although differences in these factors among zip codes might partially explain disparities in dispensing rates, such differences are unlikely to fully account for the twofold higher rates observed by the end of the study period in low- and medium-vulnerability zip codes compared with those in high-vulnerability zip codes, especially because areas with high social vulnerability have generally had greater COVID-19 disease burden during the pandemic (1,3). Finally, the analysis did not examine person-level data such as age, gender, race and ethnicity, zip code of residence, underlying medical conditions, and indications for oral antiviral medications.
Despite the introduction of highly effective vaccines and medications to treat COVID-19, by the end of the study period, COVID-19 continued to cause substantial morbidity and mortality. Oral antivirals can provide a critical intervention that can mitigate COVID-19–associated morbidity and mortality. Although the overall number of antivirals dispensed has increased, in this analysis, dispensing rates were lowest in high-vulnerability zip codes. Additional public health, regulatory, and policy efforts might help to decrease barriers to oral antiviral access, particularly in communities with high social vulnerability.
Acknowledgments
Kevin Cropper, Steven Griffiths, Alice Jackson, Aaron Jaffe; U.S. jurisdiction and local health departments; Federal Retail Pharmacy Program partners; federal entities participating in the program.
Corresponding author: Jeremy A.W. Gold, jgold@cdc.gov.
1CDC COVID-19 Emergency Response Team; 2Palantir Technologies, Palo Alto, California; 3Office of the Assistant Secretary for Preparedness and Response, U.S. Department of Health and Human Services, Washington, DC.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
* Groups at high risk include persons aged ≥65 years and those with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
† Lagevrio and Paxlovid are oral antiviral therapies indicated for the treatment of patients with mild to moderate COVID-19 who have received positive results of direct SARS-CoV-2 viral testing and are at high risk for progression to severe COVID-19. Lagevrio is indicated for the treatment of adults aged ≥18 years for whom alternative COVID-19 treatment options approved or authorized by FDA are not accessible or clinically indicated. Paxlovid is indicated for persons aged ≥12 years who weigh at least 88 lbs (40 kg). https://www.fda.gov/media/155050/download; https://www.fda.gov/media/155054/download
§ Strategies implemented included the Test to Treat initiative, increased communication to providers and patients, and direct distribution to Federal Retail Pharmacy Therapeutic Partners, enabling expansion of the number of dispensing sites. A program of HHS, Test to Treat is a federal initiative designed to provide rapid access to lifesaving COVID-19 treatments at no cost to recipients. https://aspr.hhs.gov/TestToTreat/Pages/default.aspx The launch of this program garnered media attention and heightened the visibility of oral antivirals to health care providers and the public. At Test to Treat sites, patients can receive COVID-19 testing, obtain assessment by a qualified health care provider who can prescribe antivirals, and receive oral antiviral treatment. Providing these services at a single location ensures rapid and convenient access to treatment. Test to Treat program sites accounted for 6% of all oral antiviral dispensing sites and dispensed 17% of all prescriptions.
¶ Zip code–level social vulnerability was classified according to the equitable distribution index (EDI) score. EDI is used by the federal COVID-19 response because zip code–level data offer a more detailed characterization of population vulnerability than do county level–data, while providing sufficient geographic granularity to accomplish operational goals not achievable using U.S. Census Bureau tract–level data. Similar to the CDC SVI (https://www.atsdr.cdc.gov/placeandhealth/svi/index.html), which produces county-level and U.S. Census Bureau tract–level estimates of social vulnerability, EDI uses 15 indicators categorized into four themes: 1) socioeconomic status, 2) household composition and disability, 3) racial and ethnic minority status and language, and 4) housing type and transportation. EDI includes all 15 indicators as a composite measure, and a final score is ranked from lowest (0) to highest (1) vulnerability. A percent rank function is used, such that an equal number of geographic components are in each percentile of the index. To map U.S. Census Bureau tracts to zip codes, EDI uses a crosswalk file published by the U.S. Department of Housing and Urban Development. https://www.huduser.gov/portal/datasets/usps_crosswalk.html EDI is not generated for zip codes where any of the 15 components are suppressed within the American Community Survey (this represents <1% of all zip codes mapped to U.S. Census Bureau tract data).
** HPOP is used by oral antiviral partners to order oral antivirals cost-free to recipients and to report inventory and product use. HPOP oral antiviral partners include all U.S. states and other jurisdictions, Federal Retail Pharmacy Therapeutics Partners, and federal entities (e.g., Indian Health Service, Bureau of Prisons, and U.S. Department of State). https://aspr.hhs.gov/COVID-19/Therapeutics/Distribution/Pages/process-for-ordering.aspx
†† 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
§§ The number of COVID-19 deaths and new COVID-19 hospitalizations presented are the 7-day moving averages on May 21, 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home (Accessed June 2, 2022).
¶¶ Access to testing for SARS-CoV-2 has been expanded through the postal distribution of home antigen COVID-19 testing kits and through the Increasing Community Access to Testing (ICATT) for COVID-19 program. ICATT has increased the number of pharmacies offering testing in high social vulnerability areas (https://www.cdc.gov/icatt/index.html), augmented testing capacity at Health Resources and Services Administration health clinics, and increased home testing through the launch of Medicare coverage for over-the-counter home tests.
*** Community outreach efforts have included state, local, and jurisdictional public health department efforts to augment direct messaging to communities and partnerships with community-based organizations. Clinician outreach efforts have included collaborations with professional medical associations, dissemination of Health Alert Network communications, and the provision of updated clinical guidance by the National Institutes of Health.
††† This estimate was generated through a geospatial analysis that included zip code–level population density data and therapeutic site locations.
§§§ https://www.scrapehero.com/retail-health-clinic-locations-in-us-location-analysis/
¶¶¶ The Health Resources and Services Administration COVID-19 Uninsured Program was a program through which HHS provided claims reimbursement to health care providers generally at Medicare rates for testing uninsured persons for COVID-19, treating uninsured persons with a COVID-19 diagnosis, and administering COVID-19 vaccines to uninsured persons. The Uninsured Program stopped accepting claims for testing and therapeutic dispensing on March 22, 2022 because of lack of funding. https://www.hrsa.gov/CovidUninsuredClaim
**** Currently, most payors cover the cost of the clinical assessment required to prescribe oral antivirals. However, patients might be required to pay deductibles or copays associated with the service. Financial barriers associated with SARS-CoV-2 testing have been reduced by free at-home test distribution and ongoing access to free testing through the ICATT program. Oral antivirals are provided free of charge to recipients, with no associated dispensing fee to the patient.
References
- CDC. Health equity considerations and racial and ethnic minority groups. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. Accessed May 28, 2022. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html
- Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020;323:2466–7. https://doi.org/10.1001/jama.2020.8598 PMID:32391864
- Bilal U, Jemmott JB, Schnake-Mahl A, Murphy K, Momplaisir F. Racial/ethnic and neighbourhood social vulnerability disparities in COVID-19 testing positivity, hospitalization, and in-hospital mortality in a large hospital system in Pennsylvania: a prospective study of electronic health records. Lancet Reg Health Am 2022;10:100220. https://doi.org/10.1016/j.lana.2022.100220 PMID:35262038
- Agency for Toxic Substances and Disease Registry. CDC/ATSDR social vulnerability index 2018 database: United States. Atlanta, GA: US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; 2018. Accessed June 6, 2022. https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html
- Bhimraj A, Morgan RL, Hirsch Shumaker A, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with coronavirus disease (COVID-19). Arlington, VA: Infectious Diseases Society of America; 2019 Accessed May 30, 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management
- Koonin LM, Patel A. Timely antiviral administration during an influenza pandemic: key components. Am J Public Health 2018;108:S215–20. https://doi.org/10.2105/AJPH.2018.304609 PMID:30192657
 FIGURE 1. Weekly number of courses of oral COVID-19 antiviral therapy (Lagevrio* and Paxlovid†) dispensed — United States, December 23, 2021–May 21, 2022
FIGURE 1. Weekly number of courses of oral COVID-19 antiviral therapy (Lagevrio* and Paxlovid†) dispensed — United States, December 23, 2021–May 21, 2022
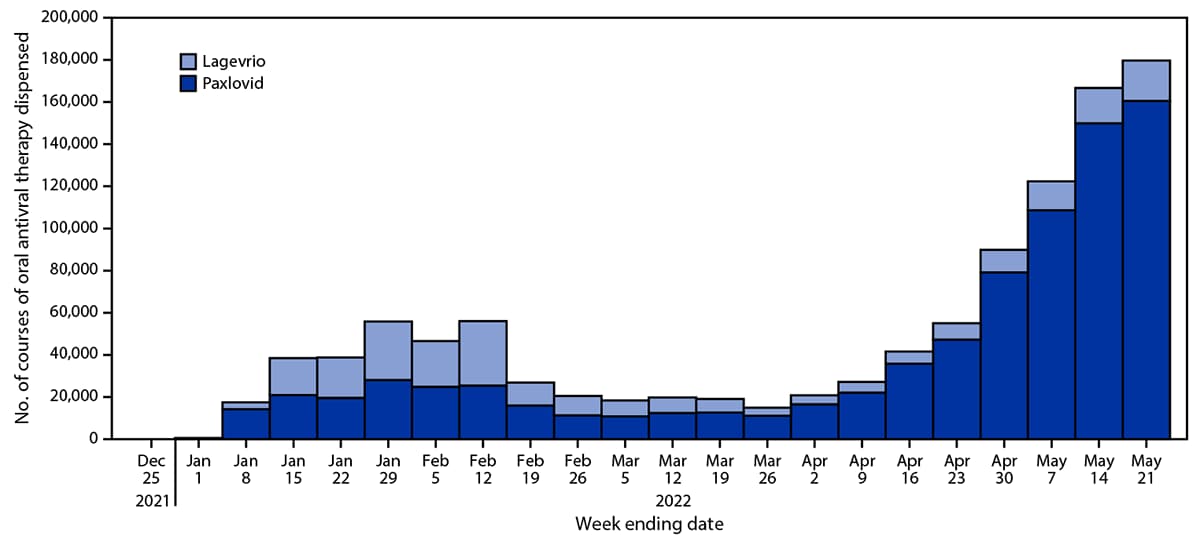
* Molnupiravir.
† Nirmatrelvir/ritonavir.
 FIGURE 2. Number of active provider sites for oral antiviral therapy against COVID-19, by week and zip code social vulnerability score* — United States, December 23, 2021–May 21, 2022
FIGURE 2. Number of active provider sites for oral antiviral therapy against COVID-19, by week and zip code social vulnerability score* — United States, December 23, 2021–May 21, 2022
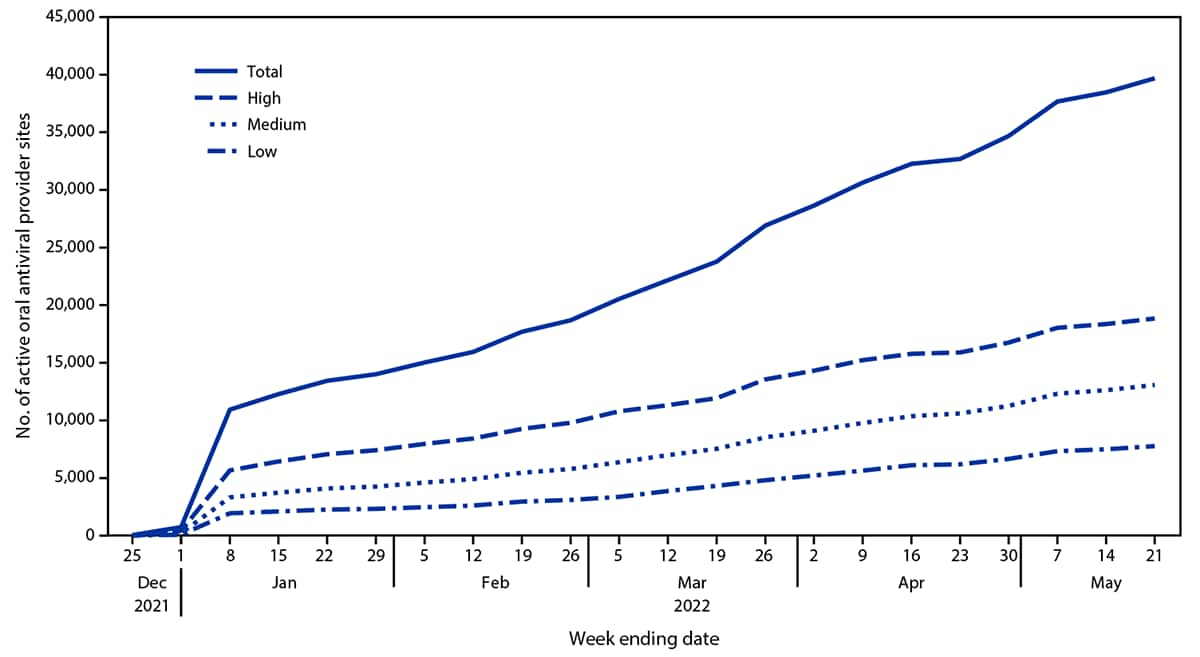
* Zip codes were classified as having low, medium, or high social vulnerability based on ranking within the lower, middle, and upper tertiles of the Equitable Distribution Index score.
 FIGURE 3. Courses of oral COVID-19 antiviral therapy dispensed per 100,000 persons, by week and zip code social vulnerability level — United States, December 26, 2021–May 21, 2022*
FIGURE 3. Courses of oral COVID-19 antiviral therapy dispensed per 100,000 persons, by week and zip code social vulnerability level — United States, December 26, 2021–May 21, 2022*
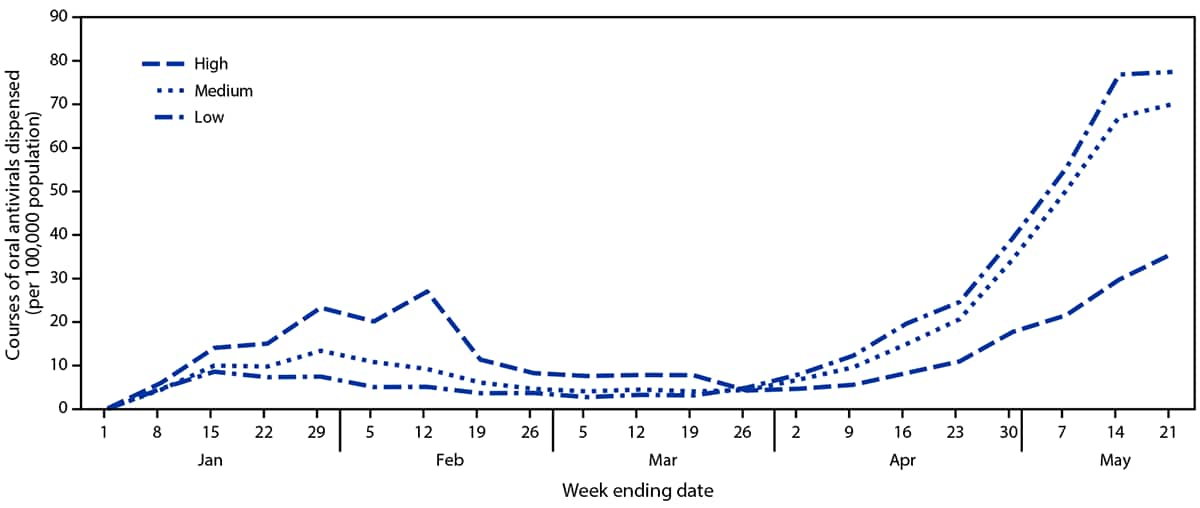
* The week ending December 25, 2021, is not shown because no oral antiviral dispensing was reported during that week. Zip codes were classified as having low, medium, or high social vulnerability based on ranking within the lower, middle, and upper tertiles of the Equitable Distribution Index score.
Suggested citation for this article: Gold JA, Kelleher J, Magid J, et al. Dispensing of Oral Antiviral Drugs for Treatment of COVID-19 by Zip Code–Level Social Vulnerability — United States, December 23, 2021–May 21, 2022. MMWR Morb Mortal Wkly Rep 2022;71:825-829. DOI: http://dx.doi.org/10.15585/mmwr.mm7125e1.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.

