Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices
Recommendations and Reports / January 12, 2018 / 67(1);1–31
Sarah Schillie, MD1; Claudia Vellozzi, MD1; Arthur Reingold, MD2; Aaron Harris, MD1; Penina Haber, MPH3; John W. Ward, MD1; Noele P. Nelson, MD1 (View author affiliations)
View suggested citationSummary
Hepatitis B virus (HBV) is transmitted via blood or sexual contact. Persons with chronic HBV infection are at increased risk for cirrhosis and liver cancer and require medical care. This report updates and summarizes previously published recommendations from the Advisory Committee on Immunization Practices (ACIP) and CDC regarding the prevention of HBV infection in the United States. ACIP recommends testing all pregnant women for hepatitis B surface antigen (HBsAg), and testing HBsAg-positive pregnant women for hepatitis B virus deoxyribonucleic acid (HBV DNA); administration of HepB vaccine and hepatitis B immune globulin (HBIG) for infants born to HBV-infected women within 12 hours of birth, followed by completion of the vaccine series and postvaccination serologic testing; universal hepatitis B vaccination within 24 hours of birth, followed by completion of the vaccine series; and vaccination of children and adolescents aged <19 years who have not been vaccinated previously. ACIP recommends vaccination of adults at risk for HBV infection, including universal vaccination of adults in settings in which a high proportion have risk factors for HBV infection and vaccination of adults requesting protection from HBV without acknowledgment of a specific risk factor. These recommendations also provide CDC guidance for postexposure prophylaxis following occupational and other exposures. This report also briefly summarizes previously published American Association for the Study of Liver Diseasest guidelines for maternal antiviral therapy to reduce perinatal HBV transmission.
Introduction
Hepatitis B virus (HBV) is transmitted through percutaneous (i.e., puncture through the skin) or mucosal (i.e., direct contact with mucous membranes) exposure to infectious blood or body fluids. HBV is highly infectious, can be transmitted in the absence of visible blood (1,2), and remains viable on environmental surfaces for at least seven days (3). Persons with chronic infection (e.g., those with persistent hepatitis B surface antigen [HBsAg] in the serum for at least 6 months following acute infection) serve as the main reservoir for HBV transmission (4).
This report summarizes and consolidates previously published recommendations from the Advisory Committee on Immunization Practices (ACIP) and CDC. It also contains updates to recommendations for the prevention of HBV infection in the United States. A list of frequently used abbreviations is provided (Box 1).
New or Updated Recommendations
The following recommendations are new or updated:
- universal hepatitis B (HepB) vaccination within 24 hours of birth for medically stable infants weighing ≥2,000 grams;
- testing HBsAg-positive pregnant women for hepatitis B virus deoxyribonucleic acid (HBV DNA);
- postvaccination serologic testing for infants whose mother’s HBsAg status remains unknown indefinitely (e.g., when a parent or person with lawful custody surrenders an infant confidentially shortly after birth);
- single-dose revaccination for infants born to HBsAg-positive women not responding to the initial vaccine series;
- vaccination for persons with chronic liver disease (including, but not limited to, those with hepatitis C virus [HCV] infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase [ALT] or aspartate aminotransferase [AST] level greater than twice the upper limit of normal); and
- removal of permissive language for delaying the birth dose until after hospital discharge.
This report also briefly summarizes American Association for the Study of Liver Diseases (AASLD) guidelines for maternal antiviral therapy to reduce perinatal HBV transmission, published previously (5). Recommendations from the Infectious Diseases Society of America (IDSA) regarding vaccination of the immunocompromised host are published separately (6).
Methods
ACIP’s Hepatitis Work Group comprises professionals from academic medicine (pediatrics, family medicine, internal medicine, infectious disease, occupational health, and preventive medicine specialists), federal and state public health agencies, and medical societies.* The Work Group reviewed epidemiology and literature, directed an economic analysis, and deliberated upon recommendations. The Work Group considered existing published ACIP and CDC vaccine recommendations in summarizing recommendations contained herein for the prevention of HBV infection.
This report updates and supplants ACIP recommendations for HepB vaccination of children and adults published previously (7,8). This report incorporates ACIP and CDC recommendations published previously (9–11).
Guidelines from AASLD inform the use of antiviral therapy among pregnant women with elevated HBV DNA for the purpose of preventing perinatal HBV transmission. Surveillance data were obtained from the National Notifiable Diseases Surveillance System (NNDSS) (https://wwwn.cdc.gov/nndss/).
Data informing clarifications to the recommendations were summarized on the basis of findings from literature searches that were completed on May 11, 2016. Two search terms were used to ascertain data regarding maximum number of doses for dialysis patients and minimum intervals for dialysis dosing: “Hepatitis b vacc* dialysis boost*” and “Dialysis hepatitis b vacc* schedule.” Epidemiologic and vaccine coverage data were reviewed, as well as publicly available data on the number of infant abandonments and safely surrendered infants. The literature searches included clinical trials and comparative studies conducted worldwide and published in English since 2000. All studies yielding pertinent information were eligible for inclusion. Search results were supplemented by additional relevant papers identified by subject matter experts on the Work Group. Per the ACIP process, it was predetermined that Grading of Recommendations Assessment, Development and Evaluation (GRADE) was not required for these updates of existing recommendations.
To assess vaccine safety, the Work Group searched two postlicensure surveillance systems for adverse events from 2005 through 2015: the Vaccine Adverse Events Reporting System (VAERS) (https://vaers.hhs.gov) and the Vaccine Safety Datalink (VSD) (https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd). VAERS is a national passive surveillance system, and VSD conducts population-based vaccine safety studies. VAERS can generate vaccine safety hypotheses but cannot assess causality and is subject to several limitations, including reporting biases and inconsistent data quality (12,13). VSD can be used to assess hypotheses that arise from reviews of medical literature, reports to VAERS, changes in immunization schedules, or the introduction of new vaccines (14).
During February–September 2016, the Work Group held five teleconference meetings. Work Group and ACIP members also reviewed and commented on a draft of the statement prior to the ACIP’s October 2016 meeting. A summary of Work Group discussions was presented to ACIP on October 19, 2016. At that time, ACIP members voted to approve a draft HepB vaccine recommendations statement, including recommending universal HepB vaccination within 24 hours of birth for medically stable infants weighing ≥2,000 grams. In January 2017, the Work Group held a teleconference meeting to review results of an economic analysis of single-dose revaccination for infants born to HBsAg-positive women. Results from that analysis were presented to ACIP on February 22, 2017. Recommendations were not evaluated using GRADE, but expert opinion was used to shape the recommendations. At that time, ACIP members voted to approve language for single-dose revaccination for infants (regardless of birth weight) born to HBsAg-positive women. Modifications were made to the ACIP statement during the subsequent review process at CDC to update and clarify wording in the report.
HBV Background
Epidemiology
In 2015, a total of 3,370 cases of acute HBV infection were reported to CDC. The actual number of acute cases is believed to be 6.5 times the number of reported cases in any year. It is estimated that 21,900 new cases of HBV occurred in 2015 after under-ascertainment and under-reporting were considered (4). The rate of reported acute HBV infections declined 88.5% since recommendations for HepB vaccination were first issued, from 9.6 cases per 100,000 population in 1982 to 1.1 cases per 100,000 population in 2015 (15), although the rate of acute HBV infections remained fairly stable during 2010–2015 (4) (Figure 1). The 2015 incidence is greatest for persons aged 30–39 years (2.6 per 100,000 population). In 2015, persons aged ≤19 years had the lowest incidence (0.02 cases per 100,000 population), likely a result of routine infant vaccination. Although the incidence of acute HBV infection is greater for males than for females, the gap has narrowed; in 2015, the rate for males was approximately 1.6 times higher than that for females (1.3 cases and 0.8 cases per 100,000 population, respectively) (4). During 2009–2013, the combined incidence of acute HBV infection in three states (Kentucky, Tennessee, and West Virginia) increased 114% and was associated with increasing injection-drug use (16).
On the basis of national health survey data, it is estimated that approximately 850,000 persons are living with HBV infection (prevalence) in the United States (17,18). Studies based on data from countries of persons migrating to the United States and census data indicate that the total prevalence of chronic hepatitis B might be as high as 2.2 million persons (19), suggesting that the national health survey-based estimate might be conservative. Foreign-born persons account for approximately 95% of newly reported chronic infections in the United States (20); the prevalence of chronic HBV infection is approximately 3.5% among foreign-born persons (19), and the majority of chronic HBV infections in the United States are among Asians/Pacific Islanders.
Strategy to Eliminate HBV
In 1991, the United States adopted a strategy for universal HepB vaccination of infants (21). A comprehensive strategy to eliminate HBV transmission evolved over the ensuing 3 decades and encompasses 1) routine testing of all pregnant women for HBsAg and prophylaxis for infants born to HBsAg-positive mothers, 2) universal vaccination of infants beginning at birth, 3) routine vaccination of previously unvaccinated children and adolescents, and 4) vaccination of adults at risk for HBV infection (7–11,21–26). Preventing perinatal transmission relies upon testing all pregnant women for HBsAg and administering timely prophylaxis (HepB vaccine and hepatitis B immune globulin [HBIG]) to infants born to infected mothers. Universal HepB vaccination of all infants beginning at birth provides a critical safeguard and prevents infection among infants born to HBsAg-positive mothers not identified prenatally (e.g., in situations where the mother was not tested or when testing, interpretation, or transcription errors occurred). Vaccination of children and adolescents not previously vaccinated and vaccination of adults at risk for HBV infection (e.g., by sexual or percutaneous exposure and international travelers to certain countries) is recommended to prevent HBV transmission outside of the perinatal setting (Box 2).
HBV prevention strategies have been implemented successfully in the United States, but challenges remain. Approximately 88% of commercially insured women and 84% of Medicaid-enrolled women are tested for HBsAg during pregnancy (27). In one study of a large health system in northern California, 93% of HBsAg-positive pregnant women were tested for HBV DNA (28). Most (94.9%) infants born to infected women receive recommended prophylaxis within 12 hours of birth (29). Universal HepB vaccine birth dose coverage, defined as 1 dose of vaccine administered by 3 days of life, is 71.1% (30), an increase from 50.1% during 2003–2005 prior to revised ACIP recommendations for the birth dose before hospital discharge (31), but below the Healthy People 2020 target of 85% (32). HepB vaccine coverage (≥3 doses) among children aged 19–35 months and 13–17 years is 90.5% (30) and 91.4% (33), respectively. Vaccine coverage (≥3 doses) is lower among adults: 27.4% among adults who report chronic liver conditions; 31.6% among adults who traveled outside the United States to countries other than Europe, Japan, Australia, New Zealand, or Canada since 1995; and 24.4% among adults with diabetes aged 19–59 years and 12.6% of adults with diabetes aged ≥60 years (34). Among health care personnel (HCP), ≥3-dose coverage was 64.7%, an increase from 51% in 1992 shortly after implementation of the Needlestick Safety and Prevention Act (35), but well below the Healthy People 2020 target of 90% (32,34).
New strategies for further reducing HBV transmission in this report include testing HBsAg-positive pregnant women for HBV DNA to identify infants at greatest risk for infection and guide the use of maternal antiviral therapy (36,37). Published evidence indicates that maternal antiviral therapy during pregnancy further reduces perinatal HBV transmission; hence, AASLD suggests antiviral therapy when maternal HBV DNA is >200,000 IU/mL (5,38,39).
Virus Description and Transmission
HBV is a 40–42-nm enveloped virus classified in the Hepadnaviridae family. HBV contains a circular, partially double-stranded DNA genome that is 3.2 kb in length. After a susceptible person is exposed, the virus enters the liver via the bloodstream. The liver is the primary site of HBV replication (40–43).
HBV has been classified by two separate systems: serologic subtype and genotype. Nine serologic subtypes initially were described based on the heterogeneity of HBsAg: adrq+, adrq–, ayr, ayw1, ayw2, ayw3, ayw4, adw2, and adw4 (44,45). Ten HBV genotypes, designated A–J, have been described. HBV serotypes and genotypes vary geographically. Infection or immunization with one genotype generally confers immunity to all genotypes (7,44,46,47).
HBV is highly infectious, can be transmitted in the absence of visible blood (22), and remains infectious on environmental surfaces for at least 7 days (2,3). All HBsAg-positive persons are infectious, but those with elevated HBV DNA or those with hepatitis B e antigen (HBeAg), a protein from the hepatitis B virus that circulates in the blood and is a marker of infectivity, are most infectious. Persons with occult HBV infection (i.e., those who test negative for HBsAg but have detectable HBV DNA) also might transmit infection (48).
HBV is transmitted through percutaneous, mucosal, or nonintact skin exposure to infectious blood or body fluids. HBV is concentrated most highly in blood, and percutaneous exposure is an efficient mode of transmission. Semen and vaginal secretions are infectious, and HBV also can be detected in saliva, tears, and bile. Cerebrospinal fluid, synovial fluid, pleural fluid, peritoneal fluid, pericardial fluid, and amniotic fluid are also considered potentially infectious. Urine, feces, vomitus, nasopharyngeal washings, sputum, and sweat are not efficient vehicles of transmission unless they contain blood because they contain low quantities of infectious HBV. HBsAg found in breast milk is also unlikely to lead to transmission, and hence HBV infection is not a contraindication to breastfeeding (2,7,22).
Among adults, HBV is transmitted primarily by percutaneous exposure to blood (e.g., by injection-drug use) and sexual contact. HBV is transmitted efficiently by sexual contact both among heterosexuals and among men who have sex with men (MSM). Risk factors for sexual transmission among heterosexuals include having unprotected sex with an infected partner, having unprotected sex with more than one partner, and a history of another sexually transmitted infection (STI). Risk factors associated with sexual transmission among MSM include having multiple sex partners, history of another STI, and anal intercourse. Transmission can occur from interpersonal contact (e.g., sharing a toothbrush or razor, contact with exudates from dermatologic lesions, or contact with HBsAg-contaminated surfaces) and in settings such as schools, child care centers, and facilities for developmentally disabled persons. Transmission of HBV from transfusion of blood or blood products is rare because of donor screening and viral inactivation procedures. Other possible sources of infection include contaminated medical or dental instruments, unsafe injections, needle-stick injuries, organ transplantation, and dialysis (49).
Clinical Features and Natural History
Clinical manifestations of HBV infection range from asymptomatic infection to fulminant hepatitis. The average incubation period is 60 days (range: 40–90 days) from exposure to onset of abnormal serum ALT levels and 90 days (range: 60–150 days) from exposure to onset of jaundice (8,42,43). Infants, children aged <5 years, and immunosuppressed adults with newly acquired HBV infection typically are asymptomatic, whereas symptomatic illness is noted in 30%–50% of older children, adolescents, and adults (7,8,44,50). When present, signs and symptoms include nausea, vomiting, abdominal pain, fever, dark urine, changes in stool color, hepatomegaly, splenomegaly, and jaundice. Malaise and anorexia might precede jaundice by 1–2 weeks. Fulminant HBV infection is uncommon (<1%) but often results in death or liver failure necessitating liver transplantation. Extrahepatic manifestations of disease (e.g., skin rash, arthralgias, and arthritis) also might occur (51). The fatality rate among persons with reported cases of acute HBV infection is <1.5%, with the highest rates in adults aged ≥55 years. Because a substantial number of infections are asymptomatic and therefore are not reported, the overall fatality rate among all persons with HBV infection is likely lower (8).
Chronic infection occurs among 80%–90% of persons infected during infancy, 30% of persons infected before age 6 years, and <1%–12% of persons infected as an older child or adult (7,52–54). Approximately 95% of primary infections in immunocompetent adults are self-limited, with elimination of the virus from blood and generally immunity to reinfection. Chronic infection develops more frequently in immunosuppressed persons (e.g., hemodialysis patients and persons with human immunodeficiency virus [HIV] infection) (54,55) and persons with diabetes (54). Chronic HBV infection can result in cirrhosis of the liver, liver cancer, liver failure, and death. Approximately 25% of persons who become chronically infected during childhood and 15% of those who become chronically infected after childhood will die prematurely from cirrhosis or liver cancer (8,56–58).
There are four phases of chronic HBV infection: immune tolerant, immune active, immune inactive, and reactivation. Chronically infected persons do not necessarily pass through these phases in a linear fashion. Persons in the immune tolerant phase have no or minimal hepatic inflammation or fibrosis; most chronically infected children will remain in the immune tolerant phase until late childhood or adolescence. The immune active phase is characterized by an active immune response resulting in hepatic inflammation, with or without fibrosis. Persons who remain in the immune active phase for prolonged periods of time are at high risk for developing cirrhosis and hepatocellular carcinoma. Persons in the immune inactive phase have improvement of hepatic inflammation and fibrosis. Risk for progression to hepatocellular carcinoma is lower among persons in the immune inactive phase compared with the active phase. Persons in the reactivation phase have active liver inflammation with or without fibrosis (44,59–61). HBV reactivation might occur with immunosuppressive therapy or treatment for HCV (62).
No specific treatment exists for acute HBV infection; supportive care is the mainstay of therapy. Guidelines for management of chronic HBV infection in children and adults, including disease monitoring and antiviral therapy, are available (5). Antiviral therapy generally should be initiated in patients with chronic HBV infection who are likely to respond to treatment and who are at high risk for liver-related morbidity (5). Maternal antiviral therapy to reduce perinatal transmission is suggested for HBsAg-positive pregnant women whose HBV DNA level is >200,000 IU/mL (5).
In areas in which HBV is highly endemic, HBV frequently is transmitted perinatally from HBV-infected pregnant women to their newborns. The majority of cases of perinatal HBV transmission occur during delivery, with rare instances of in utero transmission (63). HBV transmission might occur in germ cell lines, as the virus has been detected in sperm, oocytes, and embryos. Available data do not support the need for a cesarean delivery among HBV-infected pregnant women with low HBV DNA (63). Prior to the widespread availability of postexposure prophylaxis, the proportion of infants born to HBsAg-positive women acquiring HBV infection was approximately 30% for those born to HBeAg-negative mothers and 85% for those born to HBeAg-positive mothers. With postexposure prophylaxis, comprised of HepB vaccine and HBIG at birth, followed by completion of the HepB vaccine series, 0.7%–1.1% of infants develop infection (28,29,64); infants born to mothers with high viral loads are at greatest risk for infection despite receipt of HepB vaccine and HBIG (29). Unvaccinated infants and children are also at risk for horizontal transmission from infected household and other contacts.
Interpretation of Serologic Markers
Serologic markers for HBV infection include HBsAg, antibody to HBsAg (anti-HBs), immunoglobulin class M (IgM) antibodies to hepatitis B core antigen (IgM anti-HBc), and immunoglobulin class G (IgG) anti-HBc (IgG anti-HBc) (49,65,66). At least one serologic marker is present during the different phases of infection. HBV DNA is a measure of viral load and reflects viral replication (49) (Table 1). Hepatitis B e antigen (HBeAg) can be detected in persons with acute or chronic HBV infection; the presence of HBeAg correlates with viral replication and high infectivity; antibody to HBeAg (anti-HBe) correlates with the loss of replicating virus, although reversion to HBeAg positivity can occur (7).
A confirmed positive HBsAg result indicates current HBV infection, either acute or chronic. All HBsAg-positive persons are infectious. If HBsAg persists for >6 months, spontaneous clearance is unlikely, and the infection is deemed chronic. HBV DNA can be detected prior to the detection of HBsAg in an infected person. Occult infection occurs when HBsAg is undetectable despite the presence of HBV DNA (66–68). Transient HBsAg positivity can occur up to 18 days following vaccination (up to 52 days among hemodialysis patients) and is clinically insignificant (69).
In acute HBV infection, anti-HBc (initially both IgM and IgG) appears 1–2 weeks after the appearance of HBsAg (49) (Figure 2). IgM anti-HBc often becomes undetectable within 6 months, and IgG anti-HBc predominates and remains detectable for a lengthy period of time, often life-long (65,66). The presence of IgM anti-HBc is indicative of acute infection, while IgG anti-HBc indicates past infection (65,66). In persons who recover from HBV infection, HBsAg is eliminated from the blood and anti-HBs develops, typically within 3–4 months. The presence of anti-HBs is generally indicative of immunity to HBV infection (8). Anti-HBs also can be detected for 4–6 months following HBIG administration (10). Persons who recover from natural HBV infection are typically positive for both anti-HBs and anti-HBc, whereas persons who respond to HepB vaccine are positive only for anti-HBs. Approximately 0.5%–2% of persons with chronic infection spontaneously clear HBsAg yearly; anti-HBs will develop in the majority of these persons (8).
In certain persons, anti-HBc is the only serologic marker detected. Isolated anti-HBc-positivity can be detected following HBV infection in persons who have recovered but whose anti-HBs levels have waned; in populations with a high prevalence of HBV infection, isolated anti-HBc likely indicates previous infection with loss of anti-HBs. Some chronically infected persons with isolated anti-HBc-positivity have circulating HBsAg that is not detectable by a laboratory assay. HBV DNA has been detected in <10% of persons with isolated anti-HBc (70,71), although the presence of detectable HBV DNA might fluctuate (72). These persons are unlikely to transmit infection except under circumstances in which they are the source of a large exposure, such as a blood transfusion (8,73). Persons who are HBsAg-negative and anti-HBc-positive can experience reactivation of infection during chemotherapy or immunosuppressive therapy, with reappearance of HBsAg (49). Infection with a mutant HBV strain can result in positive laboratory tests for HBsAg, total anti-HBc, anti-HBs, and HBV DNA, with a negative IgM anti-HBc.
Perinatal HBV infection in a child aged ≤24 months is typically asymptomatic although fulminant hepatitis can occur; a positive HBsAg test, positive HBeAg test, or detectable HBV DNA may be considered laboratory evidence of perinatal HBV in an infant born to an HBV-infected mother if timing criteria are met (74). Infants who are born to HBsAg-positive mothers and who do not become infected might have detectable anti-HBc for up to 24 months after birth from passively acquired maternal antibody (7).
Adults at Risk for HBV Infection
In 2015, CDC received 3,370 surveillance case-reports of acute HBV infection. Of 2,207 case-reports with risk information, 1,151 (52.2%) indicated no risk for HBV during the 6 weeks to 6 months prior to illness onset, and the remainder indicated at least one risk factor. Injection-drug use and multiple sex partners were the most common reported sources of HBV transmission (4).
Injection-drug use. Injection-drug use was reported by 30.3% of 1,657 new reported HBV cases that included information about injection-drug use (4). Since 2009, there has been an increase in acute HBV infection among non-Hispanic whites aged 30–39 years residing in nonurban areas reporting injection-drug use as a risk factor (16). Chronic HBV infection has been identified in 3.5%-20.0% (midpoint estimate: 11.8%) of persons who inject drugs (PWID) in a variety of settings (75) and 22.6% of PWID have evidence of past infection (75). The proportion of HBV cases reporting injection-drug use in three states (Kentucky, Tennessee, and West Virginia) increased significantly, from 53% during 2006–2009 to 75% during 2010–2013 (p<0.001, chi-square) (16).
Sexual (heterosexual and MSM) exposure. Among persons with case-reports of HBV infection with information about sexual exposure, 26.4% reported having two or more sexual partners, 3.3% reported sexual contact with an HBV-infected person, and 11.8% of males reported having had sex with another male (4). As many as 10%–40% of adults seeking treatment in STI clinics have evidence of current or past HBV infection. Among adults with acute HBV infection, 39% were screened or sought care for an STI prior to becoming infected with HBV (76).
Household contacts. An estimated 45% of persons living in households with others with chronic HBV infection have serologic evidence of past HBV infection, and 16% have evidence of current infection (CDC, unpublished data, 2017. Prior to universal infant vaccination, the risk for infection was greatest among unvaccinated children living with a person with chronic HBV infection in a household or in an extended family setting (67,77,78).
Developmentally disabled persons in long-term-care facilities. Developmentally disabled persons in residential and nonresidential facilities historically have had a chronic HBV infection prevalence as high as 20%. The prevalence of infection has declined substantially since the implementation of routine HepB vaccination in these settings (79–82).
Correctional facilities. The prevalence of chronic HBV infection has been higher among prison inmates (1.0%–3.7%) than among the general population (83,84), reflecting an overrepresentation of persons entering correctional facilities with risks for HBV infection (e.g., injection-drug use and histories of multiple sex partners).
Persons at risk for occupational exposure to HBV. Before HepB vaccination was widely implemented, HBV infection was recognized as a common occupational risk among HCP (85,86). Routine HepB vaccination of HCP and the use of standard precautions have resulted in a 98% decline in HBV infections from 1983 through 2010 among HCP (10). The Occupational Safety and Health Administration mandates that employers offer HepB vaccination to all employees who have occupational risk and that postexposure prophylaxis be available following an exposure (10,87).
Hemodialysis patients. Since the initiation of HepB vaccination and additional infection control precautions for hepatitis B in dialysis centers, the incidence of HBV infection among hemodialysis patients has declined approximately 95% (88,89). Since 1995, the annual incidence has been stable and HBsAg seroprevalence has remained at 1% (90). Receipt of dialysis was reported in <1% of acute HBV surveillance cases with information reported to CDC (4).
Persons with HCV infection. The number of reported HCV cases in four Appalachian states (Kentucky, Tennessee, Virginia, and West Virginia) increased 364% during 2006–2012 among persons aged ≤30 years, with injection-drug use as the most common reported risk factor (91). The increase in HCV infections occurred concomitantly with an increase in HBV infections among young adults in rural communities in Appalachian states.
Persons with chronic liver disease. Persons with chronic liver disease (e.g., cirrhosis, fatty liver disease, alcoholic liver disease, and autoimmune hepatitis) are not at increased risk for HBV infection unless they have percutaneous or mucosal exposure to blood or body fluids. However, concurrent chronic HBV infection might increase the risk for progressive chronic liver disease in these persons (92).
Travelers to countries where HBV is endemic. Short-term travelers to countries in which HBV infection is of high or intermediate endemicity (Box 3) typically are at risk for infection only through exposure to blood in medical or disaster-relief activities, receipt of medical care that involves parenteral exposures, sexual activity, or drug use. Monthly incidence of 25–420 per 100,000 travelers has been reported among long-term travelers to countries where the disease is endemic (93).
Persons with HIV. Approximately 10% of HIV-positive persons are coinfected with HBV (94–97). Chronic HBV infection has been identified in 6%–14% of HIV-positive persons, including in 9%–17% of MSM and in 7%–10% of PWID (98). Coinfected persons have increased rates of cirrhosis and liver-related mortality (99).
Persons with diabetes. Compared with adults without diabetes, adults with diabetes have a 60% higher prevalence of past or present HBV infection and twice the odds of acquiring acute HBV. Repeated outbreaks of HBV infection associated with assisted blood glucose monitoring underscore the continued risk for this population (100–102). Data also suggest the possibility of a higher case-fatality proportion among persons with diabetes acutely infected with HBV compared with those without diabetes (9).
Prophylaxis Against HBV Infection
Hepatitis B Vaccines and Hepatitis B Immune Globulins
HepB vaccination is the mainstay of HBV prevention efforts; HBIG is generally used as an adjunct to HepB vaccine in infants born to HBsAg-positive mothers and in certain other postexposure prophylaxis situations. The first HepB vaccines consisted of plasma-derived HBsAg. Recombinant HepB vaccines containing yeast-derived HBsAg purified by biochemical and biophysical separation techniques replaced the plasma-derived vaccines in the United States by the late 1980s (64,103,104). HepB vaccines recommended for use in the United States are formulated to contain 10–40 µg of HBsAg protein/mL and do not contain thimerosal as a preservative (105). HBIG can augment protection until a response to vaccination is attained. For those who do not respond to HepB vaccination, HBIG administered alone is the primary means of protection after an HBV exposure. HBIG provides passively acquired anti-HBs and temporary protection (i.e., 3–6 months). Passively acquired anti-HBs can be detected for 4–6 months after administration of HBIG (10).
HepB vaccines are available as a single-antigen formulation and in combination with other vaccines. The two single-antigen vaccines recommended for use in the United States, Engerix-B (GlaxoSmithKline Biologicals, Rixensart, Belgium) and Recombivax HB (Merck & Co., Inc., Whitehouse Station, New Jersey), are used for the vaccination of persons starting at birth. Of the two combination vaccines, Pediarix (GlaxoSmithKline Biologicals, Rixensart, Belgium) is used for the vaccination of persons aged 6 weeks–6 years and contains recombinant HBsAg, diphtheria and tetanus toxoids and acellular pertussis adsorbed, and inactivated poliovirus and Twinrix (GlaxoSmithKline Biologicals, Rixensart, Belgium) is used for the vaccination of persons aged ≥18 years and contains recombinant HBsAg and inactivated hepatitis A virus (Table 2). Comvax (Merck & Co., Inc., Whitehouse Station, New Jersey), which was used previously for the vaccination of persons aged 6 weeks–15 months and contained recombinant HBsAg and Haemophilus b conjugate vaccine, has not been available for purchase directly from Merck since January 1, 2015. Discontinuation of Comvax was not related to any product safety or manufacturing issues. Aluminum salts generally are used as adjuvants to enhance the immune response of vaccinated persons.
Two HBIG products are licensed for use in the United States: HepaGam B (Cangene Corporation, Winnipeg, Canada) and Nabi-HB (Biotest Pharmaceuticals Corporation, Boca Raton, Florida). HBIG is prepared from the plasma of donors with high concentrations of anti-HBs. Source plasma tests negative for evidence of HIV, HBV, and HCV. Investigational nucleic acid testing for hepatitis A virus and parvovirus B19 also is performed on pooled samples of source plasma. The manufacturing process contains two steps to inactivate viruses in the final product: the solvent and detergent step inactivates enveloped viruses, and the virus filtration step removes viruses based on their size. HBIG products licensed for use in the United States contain no preservative and are intended for single use only (106).
Vaccine-Induced Seroprotection
The presence of anti-HBs typically indicates immunity against HBV infection. Immunocompetent children and adults who have vaccine-induced anti-HBs levels of ≥10 mIU/mL 1–2 months after having received a complete HepB vaccine series are considered seroprotected and deemed vaccine responders (107). Vaccine-induced seroprotection is considered a surrogate of clinical protection. Anti-HBs levels wane over time following vaccination related in part to the age at vaccination. Approximately 16% of persons vaccinated at age <1 year have antibody levels of ≥10 mIU/mL 18 years following vaccination, compared with 74% for those vaccinated at age ≥1 year (10). However, persons initially responding to the full 3-dose HepB vaccine series and who are later found to have anti-HBs <10 mIU/mL remain protected. Most persons (88%) who receive a challenge dose of HepB vaccine 30 years after HepB vaccination as children or adults develop an antibody response of ≥10 mIU/mL indicating persistent immunity to HBV infection (108). Data from this and other studies suggests protection against acute symptomatic and chronic HBV infection persists for 30 years or more among immunocompetent persons who originally responded to HepB vaccine (108–110).
The 3-dose HepB vaccine series produces a protective antibody response (anti-HBs ≥10 mIU/mL) in approximately 95% of healthy infants overall (response is lower for infants with lower birth weights) (64) and >90% of healthy adults aged <40 years (111,112). Among healthy infants, 25% and 63% achieve anti-HBs levels ≥10 mIU/mL after the first and second dose, respectively. Among healthy adults aged <40 years, 30%–55% and 75% achieve anti-HBs levels ≥10 mIU/mL after the first and second dose, respectively (7,8,64). Vaccine response is decreased among infants weighing <2000 grams and older adults. Other factors (e.g., smoking, obesity, aging, chronic medical conditions, drug use, diabetes, male sex, genetic factors, and immune suppression) contribute to a decreased response to vaccine (113–116). Although immunogenicity is lower among immunocompromised persons, those who achieve and maintain seroprotective antibody levels before exposure to HBV have a high level of protection (8).
Birth dose. A birth dose of HepB vaccine serves as postexposure prophylaxis to prevent perinatal HBV infection among infants born to HBV-infected mothers. Although infants requiring postexposure prophylaxis should be identified by maternal HBsAg testing, administration of a birth dose to all infants (even without HBIG) serves as a safeguard to prevent perinatal transmission among infants born to HBsAg-positive mothers not identified prenatally because of lack of maternal HBsAg testing or failures in reporting test results. HepB vaccine or HBIG given alone are 75% and 71% effective in preventing perinatal HBV transmission, respectively; their combined efficacy is 94% (29,52,117). The birth dose also provides protection to infants at risk from household exposure after the perinatal period (29,64).
Vaccination produces seroprotection in 98% of healthy term infants. Vaccine response is lower among infants with birth weights <2000 grams (64). A study among low birth weight infants demonstrated that more infants achieved seroprotective anti-HBs levels when vaccine was initiated at 1 month of age versus within the first 3 days of life (96% vs. 68%, p<0.02) (118). Vaccine response among infants does not vary appreciably by maternal HBsAg status or HBIG administration (64).
Adolescents. Approximately 95% of adolescents achieve seroprotection following HepB vaccination with a complete series (7). The adult (10 µg) dose of Recombivax HB administered using a 2-dose compressed schedule at 0 and 4 months or 0 and 6 months for persons aged 11–15 years produces seroprotection proportions nearly equivalent to those obtained with the standard regimen of 5 µg administered on a 3-dose schedule at 0, 1, and 6 months (99.2% vs. 98.3%) (119,120). Data on long-term antibody persistence or protection among adolescents for 2-dose schedules are lacking.
Adults. Vaccination with a complete series results in seroprotection in >90% of healthy adults aged <40 years. Response decreases with age, and seroprotection is achieved in 75% of persons aged 60 years (8).
Diabetes. A review of studies assessing HepB vaccine response among persons with diabetes mellitus demonstrated seroprotection in 93.9% for children with diabetes mellitus compared with 100% for children without diabetes mellitus (112,121).
Among adults, 88.2% of those with diabetes mellitus, compared with 93.6% of those without diabetes mellitus, achieved seroprotection (112). Among hemodialysis/chronic kidney disease patients, the median proportion protected was 60.1% for those with diabetes mellitus, compared with 75.1% for those without diabetes mellitus (112).
Immunocompromising conditions. The humoral response to HepB vaccine is reduced in children and adults who are immunocompromised (e.g., hematopoietic stem cell transplant recipients, patients undergoing chemotherapy, and HIV-infected persons) (122,123). Modified dosing regimens, including a doubling of the standard antigen dose or administration of additional doses, might increase response rates. However, data on response to these alternative vaccination schedules are limited (6).
Vaccine Safety
In prelicensure trials, adverse events following HepB vaccination were most commonly injection site reactions and mild systemic reactions (106). Commonly reported mild adverse events from postmarketing data include pain (3%–29%), erythema (3%), swelling (3%), fever (1%–6%), and headache (3%) (124). The estimated incidence of anaphylaxis among HepB vaccine recipients is 1.1 per million vaccine doses (125). In 2011, the Institute of Medicine concluded that the evidence convincingly supports a causal relationship between HepB vaccine and anaphylaxis in yeast-sensitive persons, and that the evidence is inadequate to accept or reject a causal relation between HepB vaccine and several neurologic, chronic, and autoimmune diseases (126).
During early postlicensure surveillance, several adverse events following HepB vaccination have been described in the scientific literature, including Guillain-Barré Syndrome (GBS), chronic fatigue syndrome, optic neuritis, multiple sclerosis, and diabetes mellitus; however, multiple studies have demonstrated no association between receipt of HepB vaccine and these conditions (126–129). In addition, no evidence of a causal association between rheumatoid arthritis (130), Bell’s palsy (131), autoimmune thyroid disease (132), hemolytic anemia in children (133), anaphylaxis (134), optic neuritis (135), Guillain-Barré Syndrome (136), sudden-onset sensorineural hearing loss (137), or other chronic illnesses and receipt of HepB vaccine has been demonstrated through analysis of VSD data.
During 2005–2015, a total of 20,231 reports of adverse events following HepB vaccination among all ages were submitted to VAERS. The majority of primary U.S. reports (15,787 of 20,231, 78%) were following HepB vaccine administered with other vaccines on the same visit. Among these, the percentage classified as serious (i.e., if one or more of the following is reported: death, life-threatening illness, hospitalization or prolongation of existing hospitalization, or permanent disability)† was 16.7%, including 402 deaths, of which 388 were among infants aged 6 weeks–23 months (138). The most frequently reported adverse events for vaccines given in combination were fever (23%), injection site erythema (11%), and vomiting (10%) (138). Among the 4,444 single-antigen HepB reports, 6.5% were classified as serious, including 43 deaths, of which 27 were among infants aged ≤4 weeks. The most frequently reported adverse events for single-antigen HepB vaccine were nausea/dizziness (8%) and fever/headache (7%).
Vaccination Schedules
Vaccine schedules are determined on the basis of immunogenicity data, and, for infants and children, the need to integrate HepB vaccine into a harmonized immunization schedule (Tables 3 and 4). Primary vaccination generally consists of three intramuscular doses administered on a 0-, 1-, and 6-month schedule (Table 4). Recombivax HB may be administered in a 2-dose schedule at 0 and 4–6 months for persons aged 11–15 years using the adult formulation. Pediarix is administered at ages 2, 4, and 6 months; it is not used for the birth dose. Twinrix may be administered before travel or any other potential exposure on an accelerated schedule at 0, 7, and 21–30 days, followed by a dose at 12 months. HepB vaccination of adult hemodialysis patients consists of high-dose (40 µg) Recombivax HB administered on a 0-, 1-, and 6-month schedule or high-dose (40 µg) Engerix-B administered on a 0-, 1-, 2-, and 6-month schedule (106).
Alternative vaccination schedules (e.g., 0, 1, and 4 months or 0, 2, and 4 months) have been demonstrated to elicit dose-specific and final rates of seroprotection similar to those obtained on a 0-, 1-, and 6-month schedule. Increasing the interval between the first 2 doses has little effect on immunogenicity or the final antibody concentration (139–141). The third dose confers the maximum level of seroprotection and provides long-term protection (142). Longer intervals between the last 2 doses (e.g., 11 months) result in higher final antibody levels (142) but might increase the risk for acquisition of HBV infection among persons who have a delayed response to vaccination. Higher geometric mean titers are associated with longer persistence of measurable anti-HBs.
Response to Revaccination
A challenge dose of HepB vaccine may be used to determine the presence of vaccine-induced immunologic memory through generation of an anamnestic response. The term “booster dose” has been used to refer to a dose of HepB vaccine administered after a primary vaccination series to provide rapid protective immunity against significant infection (i.e., infection resulting in serologic test results positive for HBV and/or clinically significant disease). Among persons who were vaccinated prior to age 1 year and found to have anti-HBs levels <10 mIU/mL 6–18 years later, a single challenge dose of HepB vaccine resulted in anti-HBs levels ≥10 mIU/mL in 60%–97% of those tested. Similar results were found among persons initially vaccinated at age ≥1 year (10). Immunocompetent persons with a response ≥10 mIU/mL following a challenge dose are considered protected, regardless of subsequent declines in anti-HBs (10,109).
One study found that of infants born to HBsAg-positive women who were not infected at birth and who did not respond to a primary vaccine series, all developed seroprotective levels of anti-HBs after receipt of 3 additional doses (143). No data exist that suggest that children who have no detectable antibody after 6 doses of vaccine benefit from additional doses.
Maternal Antiviral Therapy for Preventing Perinatal HBV Transmission
Antiviral therapy (i.e., lamivudine, telbivudine, and tenofovir) has been studied as an intervention to reduce perinatal HBV transmission among pregnant women with high HBV DNA levels (e.g., average HBV DNA levels of 7.6 log10 IU/mL) (144). Maternal antiviral therapy started at 28–32 weeks’ gestation, as an adjunct to HepB vaccine and HBIG administered to the infant shortly after delivery, has been associated with significantly reduced rates of perinatal HBV transmission (5). The use of lamivudine and telbivudine is limited by viral resistance and mutations. Tenofovir is not associated with resistance and is the preferred agent (5). Available data support the safety of tenofovir during pregnancy, although its use might be associated with reduced bone mineral content in infants with in utero exposure (5,39,63,144–146). AASLD suggests antiviral therapy to reduce perinatal HBV transmission when maternal HBV DNA is >200,000 IU/mL. Maternal therapy is generally discontinued at birth to 3 months postpartum (5).
Cost-Effectiveness Considerations
HBV prevention strategies targeting perinatal transmission are considered very cost-effective (i.e., an incremental cost-effectiveness ratio <$25,000). The current strategy of administering HepB vaccine and HBIG within 12 hours of birth for infants born to HBsAg-positive mothers and universal infant vaccination prior to hospital discharge has an incremental cost-effectiveness ratio of $6,957 per quality-adjusted life year (QALY) saved when compared with a strategy of universal infant HepB vaccination prior to hospital discharge alone (147). CDC’s U.S. Perinatal Hepatitis B Prevention Program (https://www.cdc.gov/hepatitis/partners/perihepbcoord.htm), which provides case management services to infants born to HBsAg-positive women, also has been demonstrated to decrease infections, increase QALYs saved, and be a cost-effective use of resources (148). A strategy of testing HBsAg-positive pregnant women for HBV DNA, followed by maternal antiviral prophylaxis for women with high HBV DNA, would cost an additional $3 million but would save 2,080 QALYs and prevent 324 chronic HBV infections, and therefore would be considered cost-effective, with an incremental cost-effectiveness ratio of $1,583 per QALY saved (36).
Cost-effectiveness also has been assessed for HBV prevention strategies outside of the perinatal setting. Vaccinating adults aged 20–59 years with diabetes mellitus costs $75,094 per QALY saved; cost-effectiveness ratios increase with age at vaccination (149). Among previously vaccinated current HCP (including those in training), pre-exposure anti-HBs testing followed by revaccination and retesting (if necessary, based on anti-HBs levels), compared with no intervention, was not considered cost-effective with an incremental cost per QALY saved of $3–$4 million at year one and approximately $800,000 over 10 years (150).
Recommendations
This section contains guidance for the prevention of HBV infection, including ACIP recommendations for HepB vaccination of infants, children, adolescents, and adults (Box 4) and CDC and ACIP recommendations for HBV prophylaxis following occupational and nonoccupational exposures, respectively.
Prevention of Perinatal HBV Transmission
Identification and Management of HBV-Infected Pregnant Women
- All pregnant women should be tested for HBsAg during an early prenatal visit (e.g., first trimester) in each pregnancy, even if they have been vaccinated or tested previously. Testing those pregnant women known to be chronically infected with HBV provides documentation of the positive HBsAg test result obtained during pregnancy and helps to ensure that their infants will be identified for timely prophylaxis.
- All HBsAg-positive pregnant women should be tested for HBV DNA to guide the use of maternal antiviral therapy during pregnancy for the prevention of perinatal HBV transmission (new recommendation).
- AASLD suggests maternal antiviral therapy when the maternal HBV DNA is >200,000 IU/mL (new recommendation).
- All HBsAg-positive pregnant women should be referred to their jurisdiction’s Perinatal Hepatitis B Prevention Program (PHBPP) for case management to ensure that their infants receive timely prophylaxis and follow-up. A copy of the original laboratory report indicating the pregnant woman’s HBsAg-positive status should be provided to the hospital or birthing facility where the delivery is planned and to the HCP who will care for the newborn infant.
- All HBsAg-positive pregnant women should receive information concerning HBV that discusses the potential use of antiviral therapy, the importance of prophylaxis for their infant (HepB vaccine and HBIG within 12 hours of birth), completion of the vaccine series, and postvaccination serologic testing.
- Women not tested prenatally, those with clinical hepatitis, and those whose behaviors place them at high risk for HBV infection (e.g., recent or current injection-drug use, having had more than one sex partner in the previous 6 months or an HBsAg-positive sex partner, having been evaluated or treated for a STI) should be tested at the time of admission to the hospital or birthing facility for delivery.
- All laboratories that provide HBsAg testing of pregnant women should use a Food and Drug Administration–licensed or approved HBsAg test and should perform testing according to the manufacturer’s labeling, including testing of initially reactive specimens with a licensed neutralizing confirmatory test. When pregnant women are tested for HBsAg at the time of admission for delivery, shortened testing protocols may be used and initially reactive results reported to expedite administration of postexposure prophylaxis of infants. Commercial laboratories should be encouraged to capture pregnancy status for women tested for HBsAg to aid in identification of HBV-infected pregnant women.
Management of Infants Born to Women Who Are HBsAg-Positive
- All infants born to HBsAg-positive women should receive HepB vaccine and HBIG within 12 hours of birth, administered at different injection sites (e.g., separate limbs). Only single-antigen HepB vaccine should be used for the birth dose (Table 3).
- Infants born to women for whom HBsAg testing results during pregnancy are not available but other evidence suggestive of maternal HBV infection exists (e.g., presence of HBV DNA, HBeAg-positive, or mother known to be chronically infected with HBV) should be managed as if born to an HBsAg-positive mother (new recommendation).
- The HepB vaccine series should be completed according to the recommended schedule for infants born to HBsAg-positive mothers. The final dose in the series should not be administered before age 24 weeks (164 days). Although not indicated in the manufacturers’ package labeling, Pediarix may be used for infants aged ≥6 weeks born to HBsAg-positive mothers to complete the vaccine series after receipt of a birth dose of single-antigen HepB vaccine and HBIG.
- For infants weighing <2,000 grams, the birth dose (i.e., the initial HepB vaccine dose) should not be counted as part of the vaccine series because of the potentially reduced immunogenicity of HepB vaccine in these infants; 3 additional doses of vaccine (for a total of 4 doses) should be administered beginning when the infant reaches age 1 month. The final dose in the series should not be administered before age 24 weeks (164 days).
- Postvaccination serologic testing for anti-HBs and HBsAg should be performed after completion of the vaccine series at age 9–12 months (generally at the next well-child visit following completion of the HepB vaccine series). Anti-HBs testing should be performed using a method that allows detection of the protective concentration of anti-HBs (≥10 mIU/mL). Testing should not be performed before age nine months to avoid detection of passive anti-HBs from HBIG administered at birth and to maximize the likelihood of detecting late HBV infection. Anti-HBc testing of infants is not recommended because passively acquired maternal anti-HBc might be detected in infants born to HBsAg-positive mothers up to age 24 months.
- HBsAg-negative infants with anti-HBs levels ≥10 mIU/mL are protected and need no further medical management.
- HBsAg-negative infants with anti-HBs <10 mIU/mL should be revaccinated with a single dose of HepB vaccine and receive postvaccination serologic testing 1–2 months later (new recommendation). Infants whose anti-HBs remains <10 mIU/mL following single dose revaccination should receive two additional doses of HepB vaccine to complete the second series, followed by postvaccination serologic testing 1–2 months after the final dose.
- Based on clinical circumstances or family preference, HBsAg-negative infants with anti-HBs <10 mIU/mL may instead be revaccinated with a second, complete 3-dose series, followed by postvaccination serologic testing performed 1–2 months after the final dose of vaccine.
- Available data do not suggest a benefit from administering additional HepB vaccine doses to infants who have not attained anti-HBs ≥10 mIU/mL following receipt of two complete HepB vaccine series.
- HBsAg-positive infants should be referred for appropriate follow-up.
- Infants who are born to HBsAg-positive mothers and receive postexposure prophylaxis may be breastfed beginning immediately after birth.
- For infants transferred to a different facility after birth (e.g., hospital with higher level of neonatal care), staff at the transferring and receiving facilities should communicate regarding the infant’s HepB vaccination and HBIG receipt status to ensure prophylaxis is administered in a timely manner (new recommendation).
Management of Infants Born to Women with Unknown HBsAg Status
- Infants born to women for whom HBsAg testing results during pregnancy are not available but other evidence suggestive of maternal HBV infection exists (e.g., presence of HBV DNA, HBeAg-positive, or mother known to be chronically infected with HBV) should be managed as if born to an HBsAg-positive mother (new recommendation). The infant should receive both HepB vaccine and HBIG within 12 hours of birth.
- Women admitted for delivery without documentation of HBsAg test results should have blood drawn and tested as soon as possible.
- While maternal HBsAg test results are pending, infants with birth weights ≥2,000 grams born to women with an unknown HBsAg status should receive the first dose of HepB vaccine (without HBIG) within 12 hours of birth. Only single-antigen HepB vaccine should be used for the birth dose (Table 3).
- If the mother is determined to be HBsAg-positive, the infant should receive HBIG as soon as possible but no later than age seven days, and the vaccine series should be completed according to the recommended schedule for infants born to HBsAg-positive mothers. The final dose in the series should not be administered before age 24 weeks (164 days). If the mother is determined to be HBsAg-negative, the vaccine series should be completed according to the recommended schedule for infants born to HBsAg-negative mothers. The final dose in the series should not be administered before age 24 weeks (164 days).
- Because of the potentially decreased immunogenicity of vaccine in infants weighing <2,000 grams, these infants should receive both single-antigen HepB vaccine and HBIG, administered at different injection sites (e.g., separate limbs), if the mother’s HBsAg status cannot be determined within 12 hours of birth. The birth dose of vaccine should not be counted as part of the 3 doses required to complete the vaccine series; 3 additional doses of vaccine (for a total of 4 doses) should be administered according to a recommended schedule on the basis of the mother’s HBsAg test result. The final dose in the series should not be administered before age 24 weeks (164 days).
- If it is not possible to determine the mother’s HBsAg status (e.g., when a parent or person with lawful custody safely surrenders an infant confidentially shortly after birth), the vaccine series should be completed according to a recommended schedule for infants born to HBsAg-positive mothers (new recommendation). The final dose in the series should not be administered before age 24 weeks (164 days). These infants should receive postvaccination serologic testing at age 9–12 months, and revaccination if necessary (new recommendation).
- Anti-HBs testing should be performed using a method that allows detection of the protective concentration of anti-HBs (≥10 mIU/mL). Testing should not be performed before age nine months to avoid detection of passive anti-HBs from HBIG administered at birth and to maximize the likelihood of detecting late HBV infection. Anti-HBc testing of infants is not recommended because passively acquired maternal anti-HBc might be detected in infants born to HBsAg-positive mothers up to age 24 months.
- HBsAg-negative infants with anti-HBs levels ≥10 mIU/mL are protected and need no further medical management.
- HBsAg-negative infants with anti-HBs <10 mIU/mL should be revaccinated with a single dose of HepB vaccine and receive postvaccination serologic testing 1–2 months later (new recommendation). Infants whose anti-HBs remains <10 mIU/mL following single dose revaccination should receive two additional doses of HepB vaccine to complete the second series, followed by postvaccination serologic testing 1–2 months after the final dose.
- Based on clinical circumstances or family preference, HBsAg-negative infants with anti-HBs <10 mIU/mL may instead be revaccinated with a second, complete 3-dose series, followed by postvaccination serologic testing performed 1–2 months after the final dose of vaccine.
- Available data do not suggest a benefit from administering additional HepB vaccine doses to infants who have not attained anti-HBs ≥10 mIU/mL following receipt of two complete HepB vaccine series.
- HBsAg-positive infants should be referred for appropriate follow-up.
- Infants born to mothers with unknown HBsAg status may be breastfed beginning immediately after birth.
- For infants transferred to a different facility after birth (e.g., a hospital with a higher level of neonatal care), staff at the transferring and receiving facilities should communicate regarding the infant’s HepB vaccination and HBIG receipt status to ensure prophylaxis is administered in a timely manner (new recommendation).
Persons Recommended for HepB Vaccination
Universal Vaccination of Infants
- All infants should receive the HepB vaccine series as part of the recommended childhood immunization schedule, beginning at birth as a safety net (Box 4; Table 3).
- For all medically stable infants weighing ≥2,000 grams at birth and born to HBsAg-negative mothers, the first dose of vaccine should be administered within 24 hours of birth (new recommendation). Only single-antigen HepB vaccine should be used for the birth dose.
- Infants weighing <2,000 grams and born to HBsAg-negative mothers should have their first vaccine dose delayed to the time of hospital discharge or age 1 month (even if weight is still <2,000 grams). For these infants, a copy of the original laboratory report indicating that the mother was HBsAg negative during this pregnancy should be placed in the infant’s medical record. Infants weighing <2,000 grams at birth have a decreased response to HepB vaccine administered before age 1 month (118).
- For infants transferred to a different facility after birth (e.g., a hospital with a higher level of neonatal care), staff at the transferring and receiving facilities should communicate regarding the infant’s HepB vaccination and HBIG receipt status to ensure prophylaxis is administered in a timely manner (new recommendation).
- The final dose in the vaccine series should not be administered before age 24 weeks (164 days).
- In populations with currently or previously high rates of childhood HBV infection (e.g., Alaska Natives; Pacific Islanders; and immigrant families from Asia, Africa, and countries with intermediate or high endemic rates of infection), the first dose of HepB vaccine should be administered at birth and the final dose at age 6–12 months.
Vaccination of Children and Adolescents
- HepB vaccination is recommended for all unvaccinated children and adolescents aged <19 years (Box 4).
- Children and adolescents who have not previously received HepB vaccine should be vaccinated routinely at any age (i.e., children and adolescents are recommended for catch-up vaccination) (Table 4).
Vaccination of Adults
- HepB vaccination is recommended for all unvaccinated adults at risk for HBV infection and for all adults requesting protection from HBV infection. Acknowledgement of a specific risk factor should not be a requirement for vaccination (Box 4).
- Adults recommended to receive HepB vaccine:
- Persons at risk for infection by sexual exposure (e.g., sex partners of HBsAg-positive persons, sexually active persons who are not in a mutually monogamous relationship [e.g., persons with more than one sex partner during the previous 6 months], persons seeking evaluation or treatment for a sexually transmitted infection, and MSM).
- Persons with a history of current or recent injection drug use are at increased risk for HBV infection. An increased incidence of HBV incidence among young adults in rural U.S. communities has been associated with an increase in injection drug use.
- Other persons at risk for infection by percutaneous or mucosal exposure to blood (household contacts of HBsAg-positive persons; residents and staff of facilities for developmentally disabled persons; health care and public safety personnel with reasonably anticipated risk for exposure to blood or blood-contaminated body fluids, hemodialysis patients and predialysis, peritoneal dialysis, and home dialysis patients; persons with diabetes mellitus aged <60 years and persons with diabetes mellitus aged ≥60 years at the discretion of the treating clinician).
- Others (international travelers to countries with high or intermediate levels [HBsAg prevalence of ≥2%] [Box 3] of endemic HBV infection, persons with HCV infection, persons with chronic liver disease [including, but not limited to, those with cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an ALT or AST level greater than twice the upper limit of normal] [new recommendation], persons with HIV infection, incarcerated persons, all other persons seeking protection from HBV infection without acknowledgement of a specific risk factor).
Vaccination of Pregnant Women
- Pregnant women who are identified as being at risk for HBV infection during pregnancy (e.g., having more than one sex partner during the previous 6 months, been evaluated or treated for an STI, recent or current injection-drug use, or having had an HBsAg-positive sex partner) should be vaccinated.
- Pregnant women at risk for HBV infection during pregnancy should be counseled concerning other methods to prevent HBV infection.
Implementation Strategies
Delivery Hospital Policies and Procedures
- All delivery hospitals and birthing facilities should implement policies and procedures to ensure identification of infants born to HBsAg-positive mothers and infants born to mothers with unknown HBsAg status, initiation of prophylaxis for these infants, and routine birth dose for medically stable infants weighing ≥2,000 grams within 24 hours of birth. Such policies and procedures should include standing orders and electronic medical record reminders or prompts.
Case-Management Programs to Prevent Perinatal HBV Infection
- States and localities should establish case-management programs, including appropriate policies, procedures, laws, and regulations to ensure that all pregnant women are tested for HBsAg during each pregnancy and that those who are HBsAg-positive are tested for HBV DNA to guide maternal antiviral therapy. Infants born to HBsAg-positive women and women with unknown HBsAg status also should receive case management.
Settings Providing Services to Adults
- In settings in which a high proportion of persons have risk factors for HBV infection (e.g., health care settings targeting services to injection-drug users, correctional facilities, institutions and nonresidential day care facilities for developmentally disabled persons), all adults should be assumed to be at risk for HBV infection and should be offered HepB vaccination if they have not previously completed vaccination.
- HCP should implement standing orders to administer HepB vaccine as part of routine services to adults who have not completed the vaccine series and make HepB vaccination a standard component of evaluation and treatment for STIs and HIV/AIDS.
- When feasible, HepB vaccination should be offered in outreach and other settings in which services are provided to persons at risk for HBV infection (e.g., needle-exchange programs, HIV testing sites, HIV prevention programs, and homeless shelters).
- In medical settings, HCP should implement standing orders to identify adults recommended for HepB vaccination and administer vaccination as part of routine services.
Postexposure Prophylaxis
This section provides recommendations for management of persons who are exposed to HBV through a distinct, identifiable exposure to blood or body fluids that contain blood, in occupational and nonoccupational settings.
- Wounds and skin sites that have been in contact with blood or body fluids should be washed with soap and water; mucous membranes should be flushed with water. Using antiseptics (e.g., 2%–4% chlorhexidine) for wound care or expressing fluid by squeezing the wound further have not been shown to reduce the risk for HBV transmission; however, the use of antiseptics is not contraindicated. The application of caustic agents (e.g., bleach) or the injection of antiseptics or disinfectants into the wound is not recommended.
Occupational Settings
Vaccinated HCP
- For vaccinated HCP (who have written documentation of a complete HepB vaccine series) with subsequent documented anti-HBs ≥10 mIU/mL, testing the source patient for HBsAg is unnecessary. No postexposure prophylaxis for HBV is necessary, regardless of the source patient’s HBsAg status (Table 5).TABLE 5Postexposure management of health care personnel after occupational percutaneous or mucosal exposure to blood or body fluids, by health care personnel HepB vaccination and response status
- For vaccinated HCP (who have written documentation of a complete HepB vaccine series) without previous anti-HBs testing, the HCP should be tested for anti-HBs and the source patient (if known) should be tested for HBsAg as soon as possible after the exposure. Anti-HBs testing should be performed using a method that allows detection of the protective concentration of anti-HBs (≥10 mIU/mL). Testing the source patient and the HCP should occur simultaneously; testing the source patient should not be delayed while waiting for the HCP anti-HBs test results, and likewise, testing the HCP should not be delayed while waiting for the source patient’s HBsAg results (Table 5).
- If the HCP has anti-HBs <10 mIU/mL and the source patient is HBsAg-positive or has an unknown HBsAg status, the HCP should receive 1 dose of HBIG and be revaccinated as soon as possible after the exposure. HepB vaccine may be administered simultaneously with HBIG at a separate anatomical injection site (e.g., separate limb). The HCP should then receive the second 2 doses of HepB vaccine to complete the second series (likely 6 doses total when accounting for the original series) according to the vaccination schedule. So the HCP’s vaccine response status can be documented for future exposures, anti-HBs testing should be performed 1–2 months after the final vaccine dose.
- If the HCP has anti-HBs <10 mIU/mL and the source patient is HBsAg-negative, the HCP should receive an additional single HepB vaccine dose, followed by repeat anti-HBs testing 1–2 months later. HCP whose anti-HBs remains <10 mIU/mL should undergo revaccination with two more doses (likely 6 doses total when accounting for the original series). So the HCP’s vaccine response status can be documented for future exposures, anti-HBs testing should be performed 1–2 months after the final dose of vaccine.
- If the HCP has anti-HBs ≥10 mIU/mL at the time of the exposure, no postexposure HBV management is necessary, regardless of the source patient’s HBsAg status.
- For vaccinated HCP with anti-HBs <10 mIU/mL after two complete HepB vaccine series, the source patient should be tested for HBsAg as soon as possible after the exposure. If the source patient is HBsAg-positive or has unknown HBsAg status, the HCP should receive 2 doses of HBIG (1,10). The first dose should be administered as soon as possible after the exposure, and the second dose should be administered 1 month later. HepB vaccine is not recommended for the exposed HCP who has previously completed two HepB vaccine series. If the source patient is HBsAg-negative, neither HBIG nor HepB vaccine is necessary (Table 5).
Unvaccinated HCP
- For unvaccinated or incompletely vaccinated HCP, the source patient should be tested for HBsAg as soon as possible after the exposure. Testing unvaccinated or incompletely vaccinated HCP for anti-HBs is not necessary and is potentially misleading, because anti-HBs ≥10 mIU/mL as a correlate of vaccine-induced protection has only been determined for persons who have completed an approved vaccination series (107) (Table 5).
- If the source patient is HBsAg-positive or has an unknown HBsAg status, the HCP should receive 1 dose of HBIG and 1 dose of HepB vaccine administered as soon as possible after the exposure. HepB vaccine may be administered simultaneously with HBIG at a separate anatomical injection site (e.g., separate limb). The HCP should complete the HepB vaccine series according to the vaccination schedule. To document the HCP’s vaccine response status for future exposures, anti-HBs testing should be performed approximately 1–2 months after the final vaccine dose. Anti-HBs testing should be performed using a method that allows detection of the protective concentration of anti-HBs (≥10 mIU/mL). Because anti-HBs testing of HCP who received HBIG should be performed after anti-HBs from HBIG is no longer detectable (6 months after administration), it might be necessary to defer anti-HBs testing for a period longer than 1–2 months after the last vaccine dose in these situations (Table 5).
- HCP with anti-HBs ≥10 mIU/mL after receipt of the primary vaccine series are considered immune. Immunocompetent persons have long-term protection and do not need further periodic testing to assess anti-HBs levels.
- HCP with anti-HBs <10 mIU/mL after receipt of the primary series should be revaccinated. For these HCP, administration of a second complete series on an appropriate schedule, followed by anti-HBs testing 1–2 months after the final dose, is usually more practical than conducting serologic testing after each additional dose of vaccine. So the HCP’s vaccine response status can be documented for future exposures, anti-HBs testing should be performed 1–2 months after the final vaccine dose.
- If the source patient is HBsAg-negative, the HCP should complete the HepB vaccine series according to the vaccination schedule. So the HCP’s vaccine response status can be documented for future exposures, anti-HBs testing should be performed approximately 1–2 months after the final vaccine dose (Table 5).
- HCP with anti-HBs ≥10 mIU/mL after receipt of the primary vaccine series are considered immune. Immunocompetent persons have long-term protection and do not need further periodic testing to assess anti-HBs levels.
- HCP with anti-HBs <10 mIU/mL after receipt of the primary series should be revaccinated. For these HCP, administration of a second complete series on an appropriate schedule, followed by anti-HBs testing 1–2 months after the final dose, is usually more practical than conducting serologic testing after each additional dose of vaccine. So the HCP’s vaccine response status can be documented for future exposures, anti-HBs testing should be performed 1–2 months after the final vaccine dose.
Clinical Management of Exposed HCP
- HCP who have anti-HBs <10 mIU/mL (or who are unvaccinated or incompletely vaccinated) and sustain an exposure to a source patient who is HBsAg-positive or has an unknown HBsAg status should undergo baseline testing for HBV infection as soon as possible after the exposure, and follow-up testing approximately 6 months later. Testing immediately after the exposure should consist of total anti-HBc, and follow-up testing approximately 6 months later should consist of HBsAg and total anti-HBc (Table 5).
- HCP exposed to a source patient who is HBsAg-positive or has an unknown HBsAg status do not need to take special precautions to prevent secondary transmission during the follow-up period; however, they should refrain from donating blood, plasma, organs, tissue, or semen (10). The exposed HCP does not need to modify sexual practices or refrain from becoming pregnant (10). If an exposed HCP is breastfeeding, she does not need to discontinue (7,10). No modifications to an exposed HCP’s patient-care responsibilities are necessary to prevent transmission to patients based solely on exposure to a source patient who is HBsAg-positive or has an unknown HBsAg status.
Previously Vaccinated HCP
- Providers should only accept written, dated records as evidence of HepB vaccination (151).
- An increasing number of HCP have received routine HepB vaccination during childhood. No postvaccination serologic testing is recommended after routine infant or adolescent HepB vaccination. Because vaccine-induced anti-HBs wanes over time, testing HCP for anti-HBs years after vaccination might not distinguish vaccine nonresponders from responders. Pre-exposure assessment of current or past anti-HBs results upon hire or matriculation, followed by one or more additional doses of HepB vaccine for HCP with anti-HBs <10 mIU/mL and retesting anti-HBs, if necessary, helps to ensure that HCP will be protected if they have an exposure to HBV-containing blood or body fluids (Box 5; Figure 3).
- HCP who cannot provide documentation of 3 doses of HepB vaccine should be considered unvaccinated and should complete the vaccine series. Postvaccination serologic testing for anti-HBs is recommended 1–2 months after the third vaccine dose. HCP who are inadvertently tested before receiving 3 documented doses of HepB vaccine and have anti-HBs ≥10 mIU/mL should not be considered immune because anti-HBs ≥10 mIU/mL is a known correlate of protection only when testing follows a documented 3-dose series. Health care facilities are encouraged to try to locate vaccine records for HCP and to enter all vaccine doses in their state immunization information system.
Nonoccupational Settings
HBsAg-Positive Source
This section provides recommendations for management of persons who are exposed to HBV through a distinct, identifiable exposure to blood or body fluids that contain blood, in nonoccupational settings (Table 6). The exposed person does not need to undergo postvaccination serologic testing following vaccination based solely on being exposed.
- Exposed persons who have written documentation of a complete HepB vaccine series and who did not receive postvaccination testing should receive a single dose of HepB vaccine.
- Exposed persons who are in the process of being vaccinated but who have not completed the vaccine series should receive a dose of HBIG and complete the HepB vaccine series (it is not necessary to restart the HepB vaccine series). HepB vaccine may be administered simultaneously with HBIG at a separate anatomical injection site (e.g., separate limb).
- Exposed unvaccinated persons should receive both HBIG and HepB vaccine as soon as possible after exposure (preferably within 24 hours). HepB vaccine may be administered simultaneously with HBIG at a separate anatomical injection site (e.g., separate limbs). Studies are limited on the maximum interval after exposure during which postexposure prophylaxis is effective, but the interval is unlikely to exceed 7 days for percutaneous exposure and 14 days for sexual exposures. The HepB vaccine series should be completed according to the vaccination schedule.
HBsAg-Unknown Source
- Exposed persons with written documentation of a complete HepB vaccine series require no further treatment.
- Exposed persons who are in the process of being vaccinated but who are not fully vaccinated should complete the HepB vaccine series (it is not necessary to restart the vaccination series).
- Exposed unvaccinated persons should receive the HepB vaccine series with the first dose administered as soon as possible after exposure, preferably within 24 hours. Studies are limited on the maximum interval after exposure during which postexposure prophylaxis is effective, but the interval is unlikely to exceed 7 days for percutaneous exposure and 14 days for sexual exposures. The vaccine series should be completed according to the vaccination schedule.
Immunization Management Issues
Prevaccination Testing
- Vaccination of persons immune to HBV because of current or previous infection or HepB vaccination does not increase the risk for adverse events (8). However, in populations that have high rates of previous HBV infection, prevaccination testing might reduce costs by avoiding vaccination of persons who are already immune. Prevaccination testing consists of testing for HBsAg, anti-HBs, and anti-HBc. Serologic testing should not be a barrier to vaccination of susceptible persons, especially in populations that are difficult to access. Testing is not a requirement for vaccination, and in settings where testing is not feasible, vaccination of recommended persons should continue.
- The first dose of HepB vaccine should typically be administered immediately after collection of the blood for serologic testing. Prevaccination testing is recommended for the following persons (Box 6):
- household, sexual, or needle-sharing contacts of HBsAg-positive persons;
- HIV-positive persons;
- persons with elevated alanine aminotransferase (ALT)/aspartate aminotransferase (AST) of unknown etiology;
- hemodialysis patients (refer to 2001 CDC recommendations [88] for additional information);
- MSM; and
- past or current injection-drug users.
Testing for HBV Infection
- Testing for HBV infection (consisting of testing for HBsAg, anti-HBs, and anti-HBc) is also recommended for the following persons:
- persons born in countries of high and intermediate HBV endemicity (HBsAg prevalence ≥2%);
- U.S.-born persons not vaccinated as infants whose parents were born in countries with high HBV endemicity (≥8%);
- persons needing immunosuppressive therapy, including chemotherapy, immunosuppression related to organ transplantation, and immunosuppression for rheumatologic or gastroenterologic disorders; and
- donors of blood, plasma, organs, tissues, or semen.
- All pregnant women should be tested for HBsAg during each pregnancy. Pregnant women with positive HBsAg tests should be tested for HBV DNA.
Postvaccination Serologic Testing
- Serologic testing for immunity is not necessary after routine vaccination of infants, children, or adults.
- Testing for anti-HBs after vaccination is recommended for the following persons whose subsequent clinical management depends on knowledge of their immune status (Box 7):
- infants born to HBsAg-positive women and infants born to women whose HBsAg status remains unknown (e.g., infants safely surrendered shortly after birth). Postvaccination serologic testing should consist of testing for anti-HBs and HBsAg;
- HCP and public safety workers at risk for blood or body fluid exposure;
- hemodialysis patients (and other persons who might require outpatient hemodialysis), HIV-infected persons, and other immunocompromised persons (e.g., hematopoietic stem-cell transplant recipients or persons receiving chemotherapy), to determine the need for revaccination and the type of follow-up testing; and
- sex partners of HBsAg-positive persons, to determine the need for revaccination and for other methods of protection against HBV infection.
- Testing should be performed 1–2 months after administration of the final dose of the vaccine series using a method that allows determination of a protective concentration of anti-HBs (≥10 mIU/mL).
- Persons found to have anti-HBs concentrations of ≥10 mIU/mL after the primary vaccine series are considered to be immune.
- Immunocompetent persons have long-term protection and do not need further periodic testing to assess anti-HBs levels.
- Immunocompromised persons might need annual testing to assess anti-HBs concentrations (See Revaccination).
- Persons found to have anti-HBs concentrations of <10 mIU/mL after the primary vaccine series should be revaccinated. Administration of all doses in the second series, on an appropriate schedule, followed by anti-HBs testing 1–2 months after the final dose, is usually more practical than serologic testing after one or more doses of vaccine (except for when revaccinating infants born to HBsAg-positive mothers).
- Persons who do not have a protective concentration of anti-HBs after revaccination should be tested for HBsAg.
- If the HBsAg test result is positive, the person should receive appropriate management, and any household, sexual, or needle-sharing contacts should be identified and vaccinated. Prevaccination testing (consisting of anti-HBc, HBsAg, and anti-HBs) to identify infected persons is recommended for household, sexual, or needle-sharing contacts of HBsAg-positive persons; serologic testing should not be a barrier to vaccination, and the first HepB vaccine dose should be administered immediately after collection of the blood for serologic testing.
- Persons who test negative for HBsAg should be considered susceptible to HBV infection and should be counseled about precautions to prevent HBV infection and the need to obtain HBIG postexposure prophylaxis for any known or likely exposure to an HBsAg-positive source (10).
- Testing HCP with documentation of complete HepB vaccination for anti-HBs upon hire or matriculation (i.e., pre-exposure assessment of prior response to HepB vaccination), followed by one or more additional doses of HepB vaccine for HCP with anti-HBs <10 mIU/mL, helps to ensure that HCP will be protected if they have an exposure to HBV-containing blood or body fluids.
- Anti-HBs levels of ≥10 mIU/mL are generally considered seroprotective; however, different assays have different assay cutoff values based on which reported levels of anti-HBs might vary depending on the assay used. Refer to the package insert of the test for the determination of actual/correct levels of anti-HBs antibodies.
Revaccination
- Revaccination (i.e., booster dose, challenge dose, or revaccination with a complete series) is not generally recommended for persons with a normal immune status who were vaccinated as infants, children, adolescents, or adults. Available data do not suggest a maximum number of booster doses. Revaccination when anti-HBs is <10 mIU/mL is recommended for the following persons:
- Infants born to HBsAg-positive mothers. HBsAg-negative infants with anti-HBs <10 mIU/mL should be revaccinated with a single dose of HepB vaccine, and retested 1–2 months later. Infants whose anti-HBs remains <10 mIU/mL following single dose revaccination should receive two additional doses of HepB vaccine on a vaccine schedule to complete the second series, followed by anti-HBs testing 1–2 months later. Alternatively, these infants may be revaccinated with a second 3-dose series and retested (HBsAg and anti-HBs) 1–2 months after the final dose of vaccine.
- HCP. Completely vaccinated HCP with anti-HBs <10 mIU/mL should receive an additional dose of HepB vaccine, followed by anti-HBs testing 1–2 months later. HCP whose anti-HBs remains <10 mIU/mL should complete the second series (usually 6 doses total), followed by repeat anti-HBs testing 1–2 months after the final dose. Alternatively, it might be more practical for very recently vaccinated HCP with anti-HBs <10 mIU/mL to receive the second complete series (usually 6 doses total), followed by anti-HBs testing 1–2 months after the final dose.
- Hemodialysis patients. For hemodialysis patients treated in outpatient centers, the need for booster doses should be assessed by annual anti-HBs testing. A booster dose should be administered when anti-HBs levels decline to <10 mIU/mL. Anti-HBs testing 1–2 months following the booster dose to assess response is not recommended.
- Other immunocompromised persons. For other immunocompromised persons (e.g., HIV-infected persons, hematopoietic stem-cell transplant recipients, and persons receiving chemotherapy), the need for booster doses has not been determined. Annual anti-HBs testing and booster doses should be considered for persons with an ongoing risk for exposure.
Interrupted Schedules and Minimum Dosing Intervals
- For all ages, when the HepB vaccine schedule is interrupted, the vaccine series does not need to be restarted. If the series is interrupted after the first dose, the second dose should be administered as soon as possible, and the second and third doses should be separated by an interval of at least 8 weeks. If only the third dose has been delayed, it should be administered as soon as possible. The final dose of vaccine must be administered at least 8 weeks after the second dose and should follow the first dose by at least 16 weeks; the minimum interval between the first and second doses is 4 weeks. Inadequate doses of Hep B vaccine or doses received after a shorter-than-recommended dosing interval should be readministered, using the correct dosage or schedule.
- Vaccine doses administered ≤4 days before the minimum interval or age are considered valid. Because of the unique accelerated schedule for Twinrix, the 4-day guideline does not apply to the first 3 doses of this vaccine when administered on a 0-day, 7-day, 21–30-day, and 12-month schedule (new recommendation).
- In infants, administration of the final dose is not recommended before age 24 weeks.
Other Immunization Management Issues
- No differences in immunogenicity have been observed when one or 2 doses of HepB vaccine produced by one manufacturer are followed by doses from a different manufacturer (8). Whenever feasible, the same vaccine should be used for the subsequent doses; however, if a different brand is administered, the dose should be considered valid and does not need to be repeated.
- Providers should only accept dated records as evidence of HepB vaccination.
- Although vaccinations should not be postponed if records cannot be found, an attempt to locate missing records should be made by contacting previous health care providers, reviewing state or local immunization information systems, and searching for a personally held record. If records cannot be located within a reasonable time, these persons should be considered susceptible and started on the age-appropriate vaccination schedule. An anti-HBs ≥10 mIU/mL is a serologic correlate of protection only when following a documented, complete series.
- In all settings, vaccination should be initiated even though completion of the series might not be ensured.
- Adverse events occurring after administration of any vaccine should be reported to VAERS. Reports should be submitted to VAERS online, by facsimile, or by mail. More information about VAERS is available by calling 1-800-822-7967 (toll-free) or online at https://vaers.hhs.gov.
Future Directions
ACIP and CDC will review these recommendations as new epidemiology or other information related to HepB vaccines (including licensure of additional HepB-containing vaccines), HepB vaccine adverse events, and the experience gained in the implementation of these recommendations becomes available. Revised recommendations will be developed as needed.
Acknowledgments
Mona Doshani, Alaya Koneru, Henry Roberts, PhD, Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC.
Advisory Committee on Immunization Practices
Membership as of September 16, 2016
Chair: Nancy Bennett, MD, University of Rochester School of Medicine and Dentistry, Rochester, New York.
Executive Secretary: Amanda Cohn, MD, National Center for Immunization and Respiratory Diseases, CDC, Atlanta, Georgia.
Members: Robert Atmar, MD, Baylor College of Medicine, Houston, Texas; Edward Belongia, MD, Marshfield Clinic Research Foundation, Marshfield, Wisconsin; Echezona Ezeanolue, MD, University of Nevada, Las Vegas, Nevada; Paul Hunter, MD, City of Milwaukee Health Department, Milwaukee, Wisconsin; Allison Kempe, MD, University of Colorado School of Medicine, Denver, Colorado; Grace Lee, MD, Boston Children’s Hospital, Boston, Massachusetts; Kelly Moore, MD, Tennessee Department of Health, Nashville, Tennessee; Cynthia Pellegrini, March of Dimes, District of Columbia; Arthur Reingold, MD, University of California, Berkeley School of Public Health, Berkeley, California; Laura Riley, MD, Harvard Medical School, Boston, Massachusetts; José Romero, MD, University of Arkansas for Medical Sciences, Little Rock, Arkansas; David Stephens, MD, Emory University, Atlanta, Georgia; Peter Szilagyi, MD, University of California, Los Angeles, California; Emmanuel (Chip) Walter, Jr., MD, Duke University School of Medicine, Durham, North Carolina.
Ex Officio Members: Bruce Gellin, MD, National Vaccine Program Office, District of Columba; Richard Gorman, MD, National Institutes of Health, Bethesda, Maryland; Amy Groom, MPH, Indian Health Service, Albuquerque, New Mexico; Mary Beth Hance, Centers for Medicare and Medicaid Services, Baltimore, Maryland; Jane Kim, MD, Department of Veterans Affairs, Durham, North Carolina; Narayan Nair, MD, Health Resources and Services Administration, Rockville, Maryland; Eric Sergienko, MD, Department of Defense, Atlanta, Georgia; Wellington Sun, MD, Food and Drug Administration, Bethesda, Maryland.
Liaison Representatives: American Academy of Family Physicians, Margot Savoy, MD, Wilmington, Delaware; American Academy of Pediatrics, Carrie Byington, MD, Salt Lake City, Utah; American Academy of Pediatrics, Red Book Editor, David Kimberlin, MD, Birmingham, Alabama; American Academy of Physician Assistants, Marie-Michèle Léger, MPH, Alexandria, Virginia; American College Health Association, Susan Even, MD, Columbia, Missouri; American College of Nurse Midwives, Carol Hayes, MN, MPH, Atlanta, Georgia; American College of Nurse Midwives Alternate, Pamela Meharry, PhD, Chicago, Illinois; American College of Obstetricians and Gynecologists, Kevin Ault, MD, Kansas City, Kansas; American College of Physicians, Sandra Fryhoffer, MD, Atlanta, Georgia; American College of Physicians Alternate, Gregory Poland, MD, Rochester, Minnesota; American Geriatrics Society, Kenneth Schmader, MD, Durham, North Carolina; America’s Health Insurance Plans, Mark Netoskie, MD, Houston, Texas; American Medical Association, Sandra Fryhoffer, Atlanta, Georgia; American Nurses Association, Charles Rittle, DNP, Pittsburgh, Pennsylvania; American Osteopathic Association, Stanley Grogg, DO, Tulsa, Oklahoma; American Pharmacists Association, Stephan L. Foster, PharmD, Memphis, Tennessee; Association of Immunization Managers, Christine Finley, MPH, Burlington, Vermont; Association for Prevention and Teaching Research, Paul McKinney, MD, Louisville, Kentucky; Association of State and Territorial Health Officials, Terry Dwelle, MD, Bismarck, North Dakota; Biotechnology Industry Organization, Phyllis Arthur, District of Columbia; Council of State and Territorial Epidemiologists, Christine Hahn, MD, Boise, Idaho; Canadian National Advisory Committee on Immunization, Ian Gemmill, MD, Kingston, Ontario, Canada; Infectious Diseases Society of America, Kathleen Neuzil, MD, Seattle, Washington; Infectious Disease Society of America Alternate, Carol Baker, MD, Houston, Texas; National Association of County and City Health Officials, Matthew Zahn, MD, Santa Ana, California; National Association of County and City Health Officials, Jeffrey Duchin, MD, Seattle, Washington; National Association of Pediatric Nurse Practitioners, Patricia Stinchfield, MPH, St. Paul, Minnesota; National Foundation for Infectious Diseases, William Schaffner, MD, Nashville, Tennessee; National Immunization Council and Child Health Program, Mexico; Ignacio Villasenor Ruiz, MD, Mexico; National Medical Association, Patricia Whitley-Williams, MD, New Brunswick, New Jersey; National Vaccine Advisory Committee, Kimberly Thompson, ScD, Orlando, Florida; Pediatric Infectious Diseases Society, Sean O’Leary, MD, Denver, Colorado; Pediatric Infectious Diseases Society Alternate, Mark Sawyer, MD, San Diego, California; Pharmaceutical Research and Manufacturers of America, David Johnson, MD, Swiftwater, Pennsylvania; Society for Adolescent Health and Medicine, Amy Middleman, MD, Houston, Texas; Society for Healthcare Epidemiology of America, David J. Weber, MD, University of North Carolina, Chapel Hill, North Carolina.
The ACIP Hepatitis Work Group
Membership as of September 16, 2016
Chair: Arthur Reingold, MD, University of California, Berkeley School of Public Health, Berkeley, California
Members: Natali Aziz, MD, MD, Stanford University School of Medicine, Stanford, California; Sharon Balter, MD, New York City Department of Health and Mental Hygiene, New York, New York; Elizabeth Barnett, MD, Boston University School of Medicine, Boston, Massachusetts; Kathleen Harriman, PhD, California Department of Public Health, Richmond, California; Susan Even, MD, University of Missouri, Columbia, Missouri; Christine Finley, Vermont Department of Health, Burlington, Vermont; Robert Frenck, MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Kathleen Harriman, PhD, California Department of Public Health, Richmond, California; Susan M. Lett, MD, Massachusetts Department of Public Health, Jamaica Plain, Massachusetts; Marian Major, PhD, Food and Drug Administration, Silver Spring, Maryland; Brian McMahon, MD, Alaska Native Tribal Health Consortium, Anchorage, Alaska; Amy Middleman, MD, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma; David A. Nace, MD, University of Pittsburgh, Pittsburgh, Pennsylvania; Gregory Poland, MD, Mayo Clinic, Rochester, Minnesota; Pamela Rockwell, DO, University of Michigan, Ann Arbor, Michigan; José Romero, MD, University of Arkansas for Medical Sciences, Little Rock, Arkansas; Brenna Simons-Petrusa, PhD, Alaska Native Tribal Health Consortium, Anchorage, Alaska; Ann Thomas, MD, Oregon Health Authority, Portland, Oregon; David J. Weber, MD, University of North Carolina, Chapel Hill, North Carolina, Matthew Zahn, MD, Children’s Hospital of Orange County, Orange, California; Jennifer Zipprich, PhD, California Department of Public Health, Richmond, California.
Contributors (CDC): Carolyn Bridges, MD, Maria Cano, MD, Melissa Collier, MD, Mona Doshani, MD, Mark Gershman, MD, Penina Haber, MPH, Aaron Harris, MD, Beth Hibbs, MPH, Scott Holmberg, MD, Ruth Jiles, PhD, David Kim, MD, Alaya Koneru, MPH, Noele Nelson, MD, PhD, Sarah Schillie, MD, Philip Spradling, MD, Claudia Vellozzi, MD, John Ward, MD, Tureka Watson, MS, Donna L. Weaver, MN, Matthew Wise, PhD.
Corresponding author: Sarah Schillie, Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. Telephone: 404-718-8608; E-mail: Ggi1@cdc.gov.
1Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; 2University of California, Berkeley School of Public Health, Berkeley, California; 3Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, CDC
Disclosure of Relationships: The 2016–2017 ACIP Hepatitis Vaccines Work Group members wish to disclose that they have no financial or competing interests with the manufacturers of commercial products or suppliers of commercial services related to hepatitis B (HepB) vaccines. Content will not include any discussion of the unlabeled use of a product or a product under investigational use, with the following exceptions:• use of Pediarix vaccine for infants born to hepatitis B surface antigen (HBsAg)–positive mothers or mothers with an unknown HBsAg status to complete the vaccine series after receipt of a birth dose of single-antigen HepB vaccine and hepatitis B immune globulin (HBIG);• alternate 3-dose vaccine administration schedules heeding to minimum intervals of 4 weeks between the first and second dose, 8 weeks between the second and third dose, and 16 weeks between the first and third dose;• modified dosing regimens (e.g., doubling of standard dose and administration of additional doses) in certain circumstances (e.g., for persons with immunocompromising conditions); and• antiviral therapy during pregnancy for the prevention of perinatal hepatitis B virus (HBV) transmission.
* A list of the members appears on page 30.
† Code of Federal Regulations. 21 CFR §600.80. Revised April 1, 2010. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm? fr=600.80.
References
- CDC. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001;50(No. RR-11):1–52. PubMed
- Preboth M. PHS guidelines for management of occupational exposure to HBV, HCV and HIV: management of occupational blood exposures. Am Fam Physician 2001;64:2012–4. PubMed
- Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet 1981;317:550–1. CrossRef PubMed
- CDC. Viral hepatitis—statistics and surveillance. Atlanta, GA: US Department of Health and Human Services, CDC; 2017. https://www.cdc.gov/hepatitis/statistics/
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. CrossRef PubMed
- Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:e44–100. CrossRef PubMed
- Mast EE, Margolis HS, Fiore AE, et al. ; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(No. RR-16):1–31. PubMed
- Mast EE, Weinbaum CM, Fiore AE, et al. ; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006;55(No. RR-16):1–33, quiz CE1–4. PubMed
- CDC. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011;60:1709–11. PubMed
- Schillie S, Murphy TV, Sawyer M, et al. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013;62(No. RR-10):1–19. PubMed
- Schillie S, Murphy TV, Fenlon N, Ko S, Ward JW. Update: shortened interval for postvaccination serologic testing of infants born to hepatitis b-infected mothers. MMWR Morb Mortal Wkly Rep 2015;64:1118–20. CrossRef PubMed
- Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015;33:4398–405. CrossRef PubMed
- Zhou W, Pool V, Iskander JK, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. MMWR Surveill Summ 2003;52(No. SS-1):1–24. PubMed
- McNeil MM, Gee J, Weintraub ES, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine 2014;32:5390–8. CrossRef PubMed
- CDC. Surveillance data for acute viral hepatitis—United States, 2008. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. https://www.cdc.gov/hepatitis/statistics/2008surveillance/index.htm
- Harris AM, Iqbal K, Schillie S, et al. Increases in acute hepatitis B virus infections—Kentucky, Tennessee, and West Virginia, 2006–2013. MMWR Morb Mortal Wkly Rep 2016;65:47–50. CrossRef PubMed
- Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology 2016;63:388–97. CrossRef PubMed
- Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis 2010;202:192–201. CrossRef PubMed
- Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology 2012;56:422–33. CrossRef PubMed
- Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B—United States, 1974–2008. PLoS One 2011;6:e27717. CrossRef PubMed
- CDC. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1991;40(No. RR-13):1–25. PubMed
- CDC. Recommendation of the Immunization Practices Advisory Committee (ACIP). Inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep 1982;31:317–22, 327–8. PubMed
- CDC. Changing patterns of groups at high risk for hepatitis B in the United States. MMWR Morb Mortal Wkly Rep 1988;37:429–32, 437. PubMed
- CDC. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health-care settings. MMWR Morb Mortal Wkly Rep 1988;37:377–82, 387–8. PubMed
- CDC. Prevention of perinatal transmission of hepatitis B virus: prenatal screening of all pregnant women for hepatitis B surface antigen. MMWR Morb Mortal Wkly Rep 1988;37:341–6, 351. PubMed
- CDC. Update: recommendations to prevent hepatitis B virus transmission—United States. MMWR Morb Mortal Wkly Rep 1999;48:33–4. PubMed
- Kolasa MS, Tsai Y, Xu J, Fenlon N, Schillie S. Hepatitis B surface antigen testing among pregnant women, United States 2014. Pediatr Infect Dis J 2017;36:e175–80. CrossRef PubMed
- Kubo A, Shlager L, Marks AR, et al. Prevention of vertical transmission of hepatitis B: an observational study. Ann Intern Med 2014;160:828–35. CrossRef PubMed
- Schillie S, Walker T, Veselsky S, et al. Outcomes of infants born to women infected with hepatitis B. Pediatrics 2015;135:e1141–7. CrossRef PubMed
- Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination coverage among children aged 19–35 months—United States, 2016. MMWR Morb Mortal Wkly Rep 2017;66:1171–7. CrossRef PubMed
- CDC. Newborn hepatitis B vaccination coverage among children born January 2003-June 2005—United States. MMWR Morb Mortal Wkly Rep 2008;57:825–8. PubMed
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy people 2020. Washington, DC: US Department of Health and Human Services; 2014. https://www.healthypeople.gov/
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep 2017;66:874–82. CrossRef PubMed
- Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations—United States, 2015. MMWR Surveill Summ 2017;66(No. SS-11):1–28. CrossRef PubMed
- Agerton TB, Mahoney FJ, Polish LB, Shapiro CN. Impact of the bloodborne pathogens standard on vaccination of healthcare workers with hepatitis B vaccine. Infect Control Hosp Epidemiol 1995;16:287–91. CrossRef PubMed
- Fan L, Owusu-Edusei K Jr, Schillie SF, Murphy TV. Cost-effectiveness of testing hepatitis B-positive pregnant women for hepatitis B e antigen or viral load. Obstet Gynecol 2014;123:929–37. CrossRef PubMed
- CDC; The American College of Obstetricians and Gynecologists. Screening pregnant women for hepatitis B virus (HBV) infection. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. https://www.cdc.gov/hepatitis/hbv/pdfs/prenatalhbsagtesting.pdf
- Pan CQ, Duan ZP, Bhamidimarri KR, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol 2012;10:452–9. CrossRef PubMed
- Pan CQ, Han GR, Jiang HX, et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol 2012;10:520–6. CrossRef PubMed
- Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol 2007;85:16–23. CrossRef PubMed
- Gish RG, Given BD, Lai CL, et al. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res 2015;121:47–58. CrossRef PubMed
- Hoofnagle JH, Di Bisceglie AM. Serologic diagnosis of acute and chronic viral hepatitis. Semin Liver Dis 1991;11:73–83. CrossRef PubMed
- Krugman S, Overby LR, Mushahwar IK, Ling CM, Frösner GG, Deinhardt F. Viral hepatitis, type B. Studies on natural history and prevention re-examined. N Engl J Med 1979;300:101–6. CrossRef PubMed
- Long SS, Prober CG, Fischer M. Principles and practice of pediatric infectious diseases. 5th ed. Amsterdam, the Netherlands: Elsevier; 2017.
- Magnius LO, Norder H. Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology 1995;38:24–34. CrossRef PubMed
- Kramvis A. Genotypes and genetic variability of hepatitis B virus. Intervirology 2014;57:141–50. CrossRef PubMed
- Kramvis A. The clinical implications of hepatitis B virus genotypes and HBeAg in pediatrics. Rev Med Virol 2016;26:285–303. CrossRef PubMed
- Allain JP. Occult hepatitis B virus infection. Transfus Clin Biol 2004;11:18–25. CrossRef PubMed
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014;384:2053–63. CrossRef PubMed
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985;151:599–603. CrossRef PubMed
- Dienstag JL. Immunopathogenesis of the extrahepatic manifestations of hepatitis B virus infection. Springer Semin Immunopathol 1981;3:461–72. CrossRef PubMed
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983;322:1099–102. CrossRef PubMed
- Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci 1993;253:197–201. CrossRef PubMed
- Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis 1995;20:992–1000. CrossRef PubMed
- Hadler SC, Judson FN, O’Malley PM, et al. Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis 1991;163:454–7. CrossRef PubMed
- Beasley RP, Lin CC, Hwang LY, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981;318:1129–33. CrossRef PubMed
- Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34:1329–39. CrossRef PubMed
- McMahon BJ, Alberts SR, Wainwright RB, Bulkow L, Lanier AP. Hepatitis B-related sequelae. Prospective study in 1400 hepatitis B surface antigen-positive Alaska native carriers. Arch Intern Med 1990;150:1051–4. CrossRef PubMed
- Jonas MM, Lok AS, McMahon BJ, et al. Antiviral therapy in management of chronic hepatitis B viral infection in children: a systematic review and meta-analysis. Hepatology 2016;63:307–18. CrossRef PubMed
- Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 2016;63:284–306. CrossRef PubMed
- McMahon BJ. Chronic hepatitis B virus infection. Med Clin North Am 2014;98:39–54. CrossRef PubMed
- Wang C, Ji D, Chen J, et al. Hepatitis due to reactivation of hepatitis B virus in endemic areas among patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol 2017;15:132–6. CrossRef PubMed
- Nelson NP, Jamieson DJ, Murphy TV. Prevention of perinatal hepatitis B virus transmission. J Pediatric Infect Dis Soc 2014;3(Suppl 1):S7–12. CrossRef PubMed
- Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine 2013;31:2506–16. CrossRef PubMed
- Pondé RA. Atypical serological profiles in hepatitis B virus infection. Eur J Clin Microbiol Infect Dis 2013;32:461–76. CrossRef PubMed
- Pondé RA, Cardoso DD, Ferro MO. The underlying mechanisms for the ‘anti-HBc alone’ serological profile. Arch Virol 2010;155:149–58. CrossRef PubMed
- Franks AL, Berg CJ, Kane MA, et al. Hepatitis B virus infection among children born in the United States to Southeast Asian refugees. N Engl J Med 1989;321:1301–5. CrossRef PubMed
- Mortensen E, Kamali A, Schirmer PL, et al. Are current screening protocols for chronic hepatitis B virus infection adequate? Diagn Microbiol Infect Dis 2016;85:159–67. CrossRef PubMed
- Calisti G, Herman O, Powley M, Haque T. Persistence of hepatitis B surface antigen in blood in a chronic haemodialysis patient following vaccination booster. BMJ Case Rep 2014;2014(jun10 1):bcr2013202191. CrossRef PubMed
- Grob P, Jilg W, Bornhak H, et al. Serological pattern “anti-HBc alone”: report on a workshop. J Med Virol 2000;62:450–5. CrossRef PubMed
- Silva AE, McMahon BJ, Parkinson AJ, Sjogren MH, Hoofnagle JH, Di Bisceglie AM. Hepatitis B virus DNA in persons with isolated antibody to hepatitis B core antigen who subsequently received hepatitis B vaccine. Clin Infect Dis 1998;26:895–7. CrossRef PubMed
- Candotti D, Lin CK, Belkhiri D, et al. Occult hepatitis B infection in blood donors from South East Asia: molecular characterisation and potential mechanisms of occurrence. Gut 2012;61:1744–53. CrossRef PubMed
- De Feo TM, Poli F, Mozzi F, Moretti MP, Scalamogna M; Collaborative Kidney, Liver and Heart North Italy Transplant Program Study Groups. Risk of transmission of hepatitis B virus from anti-HBC positive cadaveric organ donors: a collaborative study. Transplant Proc 2005;37:1238–9. CrossRef PubMed
- Council of State and Territorial Epidemiologists. Hepatitis B, perinatal virus infection 2017 case definition. Atlanta, GA: Council of State and Territorial Epidemiologists; 2017. https://wwwn.cdc.gov/nndss/conditions/hepatitis-b-perinatal-virus-infection/case-definition/2017/
- Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378:571–83. CrossRef PubMed
- CDC. Viral hepatitis—CDC recommendations for specific populations and settings. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. https://www.cdc.gov/hepatitis/populations/stds.htm
- Hurie MB, Mast EE, Davis JP. Horizontal transmission of hepatitis B virus infection to United States-born children of Hmong refugees. Pediatrics 1992;89:269–73. PubMed
- Chaudhary RK, Perry E, Cleary TE. Prevalence of hepatitis B infection among residents of an institution for the mentally retarded. Am J Epidemiol 1977;105:123–6. CrossRef PubMed
- Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998-2008. Ann Intern Med 2009;150:33–9. CrossRef PubMed
- Williams RE, Sena AC, Moorman AC, et al. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine 2012;30:3147–50. CrossRef PubMed
- Woodruff BA, Vazquez E. Prevalence of hepatitis virus infections in an institution for persons with developmental disabilities. Am J Ment Retard 2002;107:278–92. CrossRef PubMed
- Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health 2001;91:31–7. CrossRef PubMed
- Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 2008;57(No. RR-8):1–20. PubMed
- Weinbaum C, Lyerla R, Margolis HS. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR Recomm Rep 2003;52(No. RR-1):1–36, quiz CE1–4. PubMed
- Dienstag JL, Ryan DM. Occupational exposure to hepatitis B virus in hospital personnel: infection or immunization? Am J Epidemiol 1982;115:26–39. CrossRef PubMed
- Hadler SC, Doto IL, Maynard JE, et al. Occupational risk of hepatitis B infection in hospital workers. Infect Control 1985;6:24–31. CrossRef PubMed
- US Department of Labor. Occupational Safety and Health Administration. OSHA Law and Regulations. https://www.osha.gov/law-regs.html
- CDC. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001;50(No. RR-5):1–43. PubMed
- Alter MJ, Favero MS, Maynard JE. Impact of infection control strategies on the incidence of dialysis-associated hepatitis in the United States. J Infect Dis 1986;153:1149–51. CrossRef PubMed
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial 2005;18:52–61. CrossRef PubMed
- Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep 2015;64:453–8. PubMed
- Bell BP. Hepatitis A and hepatitis B vaccination of patients with chronic liver disease. Acta Gastroenterol Belg 2000;63:359–63. PubMed
- Johnson DF, Leder K, Torresi J. Hepatitis B and C infection in international travelers. J Travel Med 2013;20:194–202. CrossRef PubMed
- Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis 2003;188:571–7. CrossRef PubMed
- Chun HM, Fieberg AM, Hullsiek KH, et al. ; Infectious Disease Clinical Research Program HIV Working Group. Epidemiology of Hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years. Clin Infect Dis 2010;50:426–36. CrossRef PubMed
- Homann C, Krogsgaard K, Pedersen C, Andersson P, Nielsen JO. High incidence of hepatitis B infection and evolution of chronic hepatitis B infection in patients with advanced HIV infection. J Acquir Immune Defic Syndr 1991;4:416–20. PubMed
- Thio CL. Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis 2003;23:125–36. CrossRef PubMed
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006;44(Suppl):S6–9. CrossRef PubMed
- Thio CL, Seaberg EC, Skolasky R Jr, et al. ; Multicenter AIDS Cohort Study. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360:1921–6. CrossRef PubMed
- Reilly ML, Schillie SF, Smith E, et al. Increased risk of acute hepatitis B among adults with diagnosed diabetes mellitus. J Diabetes Sci Technol 2012;6:858–66. CrossRef PubMed
- Schillie SF, Xing J, Murphy TV, Hu DJ. Prevalence of hepatitis B virus infection among persons with diagnosed diabetes mellitus in the United States, 1999–2010. J Viral Hepat 2012;19:674–6. CrossRef PubMed
- Polish LB, Shapiro CN, Bauer F, et al. Nosocomial transmission of hepatitis B virus associated with the use of a spring-loaded finger-stick device. N Engl J Med 1992;326:721–5. CrossRef PubMed
- Emini EA, Ellis RW, Miller WJ, McAleer WJ, Scolnick EM, Gerety RJ. Production and immunological analysis of recombinant hepatitis B vaccine. J Infect 1986;13(Suppl A):3–9. CrossRef PubMed
- Stephenne J. Development and production aspects of a recombinant yeast-derived hepatitis B vaccine. Vaccine 1990;8(Suppl):S69–73, discussion S79–80.
- CDC. Update: expanded availability of thimerosal preservative-free hepatitis B vaccine. MMWR Morb Mortal Wkly Rep 2000;49:642–51
- US Food and Drug Administration. Licensed biological products with supporting documents. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2017. https://www.fda.gov/biologicsbloodvaccines/ucm133705.htm
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999;179:489–92. CrossRef PubMed
- Bruce MG, Bruden D, Hurlburt D, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis 2016;214:16–22. CrossRef PubMed
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011;53:68–75 . CrossRef PubMed
- Simons BC, Spradling PR, Bruden DJ, et al. A longitudinal hepatitis B vaccine cohort demonstrates long-lasting hepatitis B virus (HBV) cellular immunity despite loss of antibody against HBV surface antigen. J Infect Dis 2016;214:273–80. CrossRef PubMed
- André FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. Am J Med 1989;87(3a):S14–20. CrossRef PubMed
- Schillie SF, Spradling PR, Murphy TV. Immune response of hepatitis B vaccine among persons with diabetes: a systematic review of the literature. Diabetes Care 2012;35:2690–7. CrossRef PubMed
- Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B Vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med 1998;15:1–8. CrossRef PubMed
- Shaw FE Jr, Guess HA, Roets JM, et al. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 1989;7:425–30. CrossRef PubMed
- Bowman S, Grau LE, Singer M, Scott G, Heimer R. Factors associated with hepatitis B vaccine series completion in a randomized trial for injection drug users reached through syringe exchange programs in three US cities. BMC Public Health 2014;14:820. CrossRef PubMed
- Drachman R, Isacsohn M, Rudensky B, Drukker A. Vaccination against hepatitis B in children and adolescent patients on dialysis. Nephrol Dial Transplant 1989;4:372–4. CrossRef PubMed
- Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ 2006;332:328–36. CrossRef PubMed
- Patel DM, Butler J, Feldman S, Graves GR, Rhodes PG. Immunogenicity of hepatitis B vaccine in healthy very low birth weight infants. J Pediatr 1997;131:641–3. CrossRef PubMed
- Marsano LS, West DJ, Chan I, et al. A two-dose hepatitis B vaccine regimen: proof of priming and memory responses in young adults. Vaccine 1998;16:624–9. CrossRef PubMed
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001;107:626–31. CrossRef PubMed
- Fabrizi F, Dixit V, Messa P, Martin P. Hepatitis B virus vaccine in chronic kidney disease: improved immunogenicity by adjuvants? A meta-analysis of randomized trials. Vaccine 2012;30:2295–300. CrossRef PubMed
- Hovi L, Valle M, Siimes MA, Jalanko H, Saarinen UM. Impaired response to hepatitis B vaccine in children receiving anticancer chemotherapy. Pediatr Infect Dis J 1995;14:931–4. CrossRef PubMed
- Zuin G, Principi N, Tornaghi R, et al. Impaired response to hepatitis B vaccine in HIV infected children. Vaccine 1992;10:857–60. CrossRef PubMed
- World Health Organization. Information sheet observed rate of vaccine reactions: hepatitis B vaccine. Geneva, Switzerland: World Health Organization; 2012. http://www.who.int/vaccine_safety/initiative/tools/Hep_B_Vaccine_rates_information_sheet.pdf
- Bohlke K, Davis RL, Marcy SM, et al. ; Vaccine Safety Datalink Team. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics 2003;112:815–20. CrossRef PubMed
- Stratton K, Ford A, Rusch E, Clayton EW, eds. Adverse effects of vaccines: evidence and causality. Washington, DC: National Academy Press; 2012.
- Stratton K, Almario D, McCormick MC, editors. Immunization safety review: hepatitis B vaccine and demyelinating neurological disorders. Washington, DC: National Academies Press; 2002.
- Halsey NA, Duclos P, Van Damme P, Margolis H; Viral Hepatitis Prevention Board. Hepatitis B vaccine and central nervous system demyelinating diseases. Pediatr Infect Dis J 1999;18:23–4. CrossRef PubMed
- DeStefano F, Weintraub ES, Chen RT. Hepatitis B vaccine and risk of multiple sclerosis. Pharmacoepidemiol Drug Saf 2007;16:705–7, author reply 707–8. CrossRef PubMed
- Ray P, Black S, Shinefield H, et al. ; Vaccine Safety Datalink Team. Risk of rheumatoid arthritis following vaccination with tetanus, influenza and hepatitis B vaccines among persons 15–59 years of age. Vaccine 2011;29:6592–7. CrossRef PubMed
- Rowhani-Rahbar A, Klein NP, Lewis N, et al. Immunization and Bell’s palsy in children: a case-centered analysis. Am J Epidemiol 2012;175:878–85. CrossRef PubMed
- Yu O, Bohlke K, Hanson CA, et al. Hepatitis B vaccine and risk of autoimmune thyroid disease: a Vaccine Safety Datalink study. Pharmacoepidemiol Drug Saf 2007;16:736–45. CrossRef PubMed
- Naleway AL, Belongia EA, Donahue JG, Kieke BA, Glanz JM; Vaccine Safety Datalink. Risk of immune hemolytic anemia in children following immunization. Vaccine 2009;27:7394–7. CrossRef PubMed
- McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol 2016;137:868–78. CrossRef PubMed
- Baxter R, Lewis E, Fireman B, DeStefano F, Gee J, Klein NP. Case-centered analysis of optic neuritis after vaccines. Clin Infect Dis 2016;63:79–81. CrossRef PubMed
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013;57:197–204. CrossRef PubMed
- Baxter R, Lewis N, Bohrer P, Harrington T, Aukes L, Klein NP. Sudden-onset sensorineural hearing loss after immunization: a case-centered analysis. Otolaryngol Head Neck Surg 2016;155:81–6. CrossRef PubMed
- 138.Haber P, Moro PL, Ng C, et al. Safety of currently licensed hepatitis B surface antigen vaccines in the United States, Vaccine adverse event reporting system (VAERS), 2005–2015. Vaccine 2017. http://www.sciencedirect.com/science/article/pii/S0264410X1731722X
- Hadler SC, de Monzon MA, Lugo DR, Perez M. Effect of timing of hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine 1989;7:106–10. CrossRef PubMed
- Halsey NA, Moulton LH, O’Donovan JC, et al. Hepatitis B vaccine administered to children and adolescents at yearly intervals. Pediatrics 1999;103:1243–7. CrossRef PubMed
- Wiström J, Ahlm C, Lundberg S, Settergren B, Tärnvik A. Booster vaccination with recombinant hepatitis B vaccine four years after priming with one single dose. Vaccine 1999;17:2162–5. CrossRef PubMed
- Jilg W, Schmidt M, Deinhardt F. Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis 1989;160:766–9. CrossRef PubMed
- Tan KL, Goh KT, Oon CJ, Chan SH. Immunogenicity of recombinant yeast-derived hepatitis B vaccine in nonresponders to perinatal immunization. JAMA 1994;271:859–61. CrossRef PubMed
- Brown RS Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology 2016;63:319–33. CrossRef PubMed
- Jourdain G, Ngo-Giang-Huong N, Cressey TR, et al. Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen. BMC Infect Dis 2016;16:393. CrossRef PubMed
- Han GR, Jiang HX, Yue X, et al. Efficacy and safety of telbivudine treatment: an open-label, prospective study in pregnant women for the prevention of perinatal transmission of hepatitis B virus infection. J Viral Hepat 2015;22:754–62. CrossRef PubMed
- Fan L, Owusu-Edusei K Jr, Schillie SF, Murphy TV. Cost-effectiveness of active-passive prophylaxis and antiviral prophylaxis during pregnancy to prevent perinatal hepatitis B virus infection. Hepatology 2016;63:1471–80. CrossRef PubMed
- Barbosa C, Smith EA, Hoerger TJ, et al. Cost-effectiveness analysis of the national Perinatal Hepatitis B Prevention Program. Pediatrics 2014;133:243–53. CrossRef PubMed
- Hoerger TJ, Schillie S, Wittenborn JS, et al. Cost-effectiveness of hepatitis B vaccination in adults with diagnosed diabetes. Diabetes Care 2013;36:63–9 . CrossRef PubMed
- Hoerger TJ, Bradley C, Schillie SF, Reilly M, Murphy TV. Cost-effectiveness of ensuring hepatitis B protection for previously vaccinated healthcare personnel. Infect Control Hosp Epidemiol 2014;35:845–54. CrossRef PubMed
- CDC. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60(No. RR-2):1–64. PubMed
 BOX 1. Abbreviations used in this report
BOX 1. Abbreviations used in this report
AASLD American Association for the Study of Liver Diseases
ACIPAdvisory Committee on Immunization Practices
anti-HBcantibody to hepatitis B core antigen
anti-HBeantibody to hepatitis B e antigen
anti-HBsantibody to hepatitis B surface antigen
HBeAghepatitis B e antigen
HBIGhepatitis B immune globulin
HBsAghepatitis B surface antigen
HBVhepatitis B virus
HBV DNAhepatitis B virus deoxyribonucleic acid
HCPhealth care personnel
HCVhepatitis C virus
HepBhepatitis B
HIVhuman immunodeficiency virus
IDSAInfectious Diseases Society of America
IDUInjection-drug use
IgMImmunoglobulin class M
IgGImmunoglobulin class G
MSMmen who have sex with men
NNDSSNational Notifiable Diseases Surveillance System
PHBPPPerinatal Hepatitis B Prevention Program
PWIDpersons who inject drugs
QALYquality-adjusted life-year
STIsexually transmitted infection
VAERSVaccine Adverse Events Reporting System
VSDVaccine Safety Datalink
 FIGURE 1. Incidence of hepatitis B virus infection — National Notifiable Diseases Surveillance System, United States, 1980–2015
FIGURE 1. Incidence of hepatitis B virus infection — National Notifiable Diseases Surveillance System, United States, 1980–2015
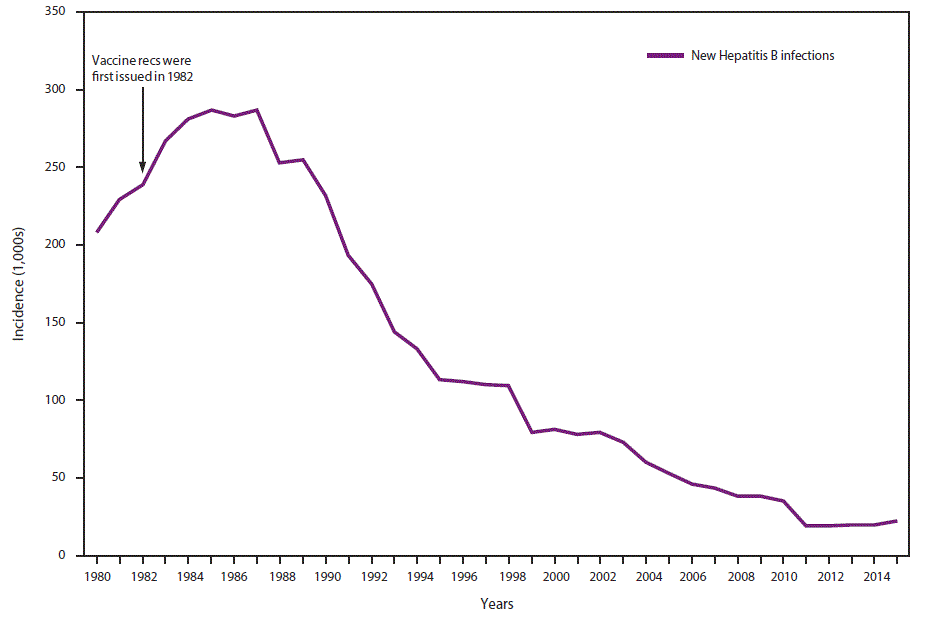
 BOX 2. Strategy to eliminate HBV transmission in the United States*
BOX 2. Strategy to eliminate HBV transmission in the United States*
- Screening of all pregnant women for HBsAg
- HBV DNA testing for HBsAg-positive pregnant women, with suggestion of maternal antiviral therapy to reduce perinatal transmission when HBV DNA is >200,000 IU/mL
- Prophylaxis (HepB vaccine and HBIG) for infants born to HBsAg-positive† women
- Universal vaccination of all infants beginning at birth§,¶ as a safeguard for infants born to HBV-infected mothers not identified prenatally
- Routine vaccination of previously unvaccinated children aged <19 years
- Vaccination of adults at risk for HBV infection, including those requesting protection from HBV without acknowledgment of a specific risk factor
*Sources: Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(No. RR-16):1–31; Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part II: immunization of adults. MMWR Recomm Rep 2006;55(No. RR-16):1–33.
†Refer to Table 3 for prophylaxis recommendations for infants born to women with unknown HBsAg status.
§Within 24 hours of birth for medically stable infants weighing ≥2,000 grams.
¶Refer to Table 3 for birth dose recommendations for infants weighing <2,000 grams.
Abbreviations: – = negative; + = positive; anti-HBc = antibody to hepatitis B core antigen; anti-HBs = antibody to hepatitis B surface antigen; HBsAg = hepatitis B surface antigen; HBV DNA = hepatitis B virus deoxyribonucleic acid; IgM = immunoglobulin class M.
 FIGURE 2. Acute hepatitis B virus infection with recovery
FIGURE 2. Acute hepatitis B virus infection with recovery
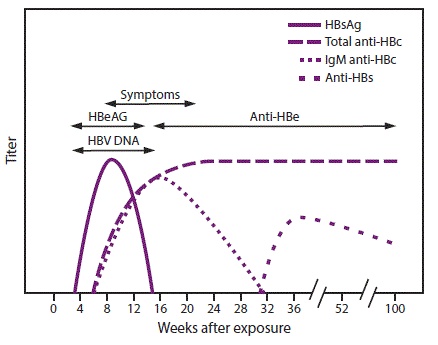
Abbreviations: anti-HBc = antibody to hepatitis B core antigen; anti-HBe = antibody to hepatitis B e antigen; anti-HBs = antibody to hepatitis B surface antigen; HBeAg = hepatitis B e antigen; HBsAg = hepatitis B surface antigen; HBV DNA = hepatitis B virus deoxyribonucleic acid; IgM = immunoglobulin class M.
 BOX 3. Prevalence of chronic hepatitis B virus infection, by country*
BOX 3. Prevalence of chronic hepatitis B virus infection, by country*
High (≥8% prevalence): Angola, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Congo, Côte d’Ivoire, Djibouti, Equatorial Guinea, Gabon, Gambia, Ghana, Guinea, Haiti, Kiribati, Kyrgyzstan, Laos, Liberia, Malawi, Mali, Mauritania, Mongolia, Mozambique, Namibia, Nauru, Niger, Nigeria, Niue, Papua New Guinea, Senegal, Sierra Leone, Solomon Islands, Somalia, South Sudan, Sudan, Swaziland, Togo, Tonga, Uganda, Vanuatu, Vietnam, Yemen, and Zimbabwe.
Intermediate (5%–7.9% prevalence): Albania, Bhutan, Cape Verde, China, Democratic Republic of the Congo, Ethiopia, Kazakhstan, Kenya, Marshall Islands, Moldova, Oman, Romania, Rwanda, Samoa, South Africa, Tajikistan, Tanzania, Thailand, Tunisia, Tuvalu, Uzbekistan, and Zambia.
Low Intermediate (2%–4.9% prevalence): Algeria, Azerbaijan, Bangladesh, Belarus, Belize, Brunei Darussalam, Bulgaria, Cambodia, Colombia, Cyprus, Dominican Republic, Ecuador, Eritrea, Federated States of Micronesia, Fiji, Georgia, Italy, Jamaica, Kosovo, Libya, Madagascar, Myanmar, New Zealand, Pakistan, Palau, Philippines, Peru, Russia, Saudi Arabia, Singapore, South Korea, Sri Lanka, Suriname, Syria, Tahiti, and Turkey.
Low (≤1.9% prevalence): Afghanistan, Argentina, Australia, Austria, Bahrain, Barbados, Belgium, Bolivia, Bosnia and Herzegovina, Brazil, Canada, Chile, Costa Rica, Croatia, Cuba, Czech Republic, Denmark, Egypt, France, Germany, Greece, Guatemala, Hungary, Iceland, India, Indonesia, Iran, Iraq, Ireland, Israel, Japan, Jordan, Kuwait, Lebanon, Lithuania, Malaysia, Mexico, Morocco, Nepal, Netherlands, Nicaragua, Norway, Palestine, Panama, Poland, Portugal, Qatar, Serbia, Seychelles, Slovakia, Slovenia, Spain, Sweden, Switzerland, Ukraine, UK, United Arab Emirates, United States of America, and Venezuela.
No data: Andorra, Antigua and Barbuda, Armenia, The Bahamas, Botswana, Chad, Comoros, Cook Islands, Dominica, El Salvador, Finland, Grenada, Guinea- Bissau, Guyana, Honduras, Latvia, Lesotho, Lithuania, Luxembourg, Macedonia, Maldives, Malta, Mauritius, Monaco, Montenegro, North Korea, Paraguay, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, San Marino, Sao Tome and Principe, Timor-Leste, Trinidad and Tobago, Turkmenistan, and Uruguay.
* Source: CDC. Travelers health: infectious diseases related to travel. Atlanta, GA: US Department of Health and Human Services, CDC; 2017.
Abbreviation: N/A = not applicable.
* Pediarix is approved for use in persons aged 6 weeks through 6 years (prior to the 7th birthday).
† Twinrix is approved for use in persons aged ≥18 years.
§ Adult formulation administered on a 2-dose schedule.
Abbreviations: HBIG = hepatitis B immune globulin; HBsAg = hepatitis B surface antigen.
* Mothers should have blood drawn and tested for HBsAg as soon as possible after admission for delivery; if the mother is found to be HBsAg positive, the infant should receive HBIG as soon as possible but no later than age 7 days.
† Pediarix should not be administered before age 6 weeks.
§ HBIG should be administered at a separate anatomical site from vaccine.
¶ The final dose in the vaccine series should not be administered before age 24 weeks (164 days).
* Refer to package inserts for further information. For all ages, when the HepB vaccine schedule is interrupted, the vaccine series does not need to be restarted. If the series is interrupted after the first dose, the second dose should be administered as soon as possible, and the second and third doses should be separated by an interval of at least 8 weeks. If only the third dose has been delayed, it should be administered as soon as possible. The final dose of vaccine must be administered at least 8 weeks after the second dose and should follow the first dose by at least 16 weeks; the minimum interval between the first and second doses is 4 weeks. Inadequate doses of hepatitis B vaccine or doses received after a shorter-than-recommended dosing interval should be readministered, using the correct dosage or schedule. Vaccine doses administered ≤4 days before the minimum interval or age are considered valid. Because of the unique accelerated schedule for Twinrix, the 4-day guideline does not apply to the first three doses of this vaccine when administered on a 0-day, 7-day, 21–30-day, and 12-month schedule (new recommendation).
†A 2-dose schedule of Recombivax adult formulation (10 µg) is licensed for adolescents aged 11–15 years. When scheduled to receive the second dose, adolescents aged >15 years should be switched to a 3-dose series, with doses 2 and 3 consisting of the pediatric formulation administered on an appropriate schedule.
§ Twinrix is approved for use in persons aged ≥18 years and is available on an accelerated schedule with doses administered at 0, 7, 21–30 days, and 12 months.
¶ A 4-dose schedule of Engerix administered in two 1 mL doses (40 µg) on a 0-, 1-, 2-, and 6-month schedule is recommended for adult hemodialysis patients.
 BOX 4. Persons recommended to receive hepatitis B vaccination
BOX 4. Persons recommended to receive hepatitis B vaccination
• All infants
• Unvaccinated children aged <19 years
• Persons at risk for infection by sexual exposure
- Sex partners of hepatitis B surface antigen (HBsAg)–positive persons
- Sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., persons with more than one sex partner during the previous 6 months)
- Persons seeking evaluation or treatment for a sexually transmitted infection
- Men who have sex with men
- Persons at risk for infection by percutaneous or mucosal exposure to blood
- Current or recent injection-drug users
- Household contacts of HBsAg-positive persons
- Residents and staff of facilities for developmentally disabled persons
- Health care and public safety personnel with reasonably anticipated risk for exposure to blood or blood-contaminated body fluids
- Hemodialysis patients and predialysis, peritoneal dialysis, and home dialysis patients
- Persons with diabetes aged 19–59 years; persons with diabetes aged ≥60 years at the discretion of the treating clinician
- Others
- International travelers to countries with high or intermediate levels of endemic hepatitis B virus (HBV) infection (HBsAg prevalence of ≥2%)
- Persons with hepatitis C virus infection
- Persons with chronic liver disease (including, but not limited to, persons with cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase [ALT] or aspartate aminotransferase [AST] level greater than twice the upper limit of normal)
- Persons with HIV infection
- Incarcerated persons
- All other persons seeking protection from HBV infection
Abbreviations: anti HBs = antibody to hepatitis B surface antigen; HBIG = hepatitis B immune globulin; HBsAg = hepatitis B surface antigen; HCP = health care personnel; N/A = not applicable.
* Not indicated.
 FIGURE 3. Pre-exposure evaluation for health care personnel previously vaccinated with complete, ≥3-dose HepB vaccine series who have not had postvaccination serologic testing*
FIGURE 3. Pre-exposure evaluation for health care personnel previously vaccinated with complete, ≥3-dose HepB vaccine series who have not had postvaccination serologic testing*
![rr6701a1-F3 The figure above shows an algorithm depicting the procedure for conducting pre-exposure evaluation for health care personnel previously vaccinated with complete, greater than or equal to 3-dose hepatitis B vaccine series who have not had postvaccination serologic testing. Such testing should be performed 1–2 months after the last dose of vaccine using a quantitative method that allows detection of the protective concentration of anti-HBs (≥10 mIU/mL) (e.g., enzyme-linked immunosorbent assay [ELISA]).](/mmwr/volumes/67/rr/figures/rr6701a1-F3.gif?_=09140)
* Should be performed 1–2 months after the last dose of vaccine using a quantitative method that allows detection of the protective concentration of anti-HBs (≥10 mIU/mL) (e.g., enzyme-linked immunosorbent assay [ELISA]).
The figure above shows an algorithm depicting the procedure for conducting pre-exposure evaluation for health care personnel previously vaccinated with complete, greater than or equal to 3-dose hepatitis B vaccine series who have not had postvaccination serologic testing. Such testing should be performed 1–2 months after the last dose of vaccine using a quantitative method that allows detection of the protective concentration of anti-HBs (≥10 mIU/mL) (e.g., enzyme-linked immunosorbent assay [ELISA]).
 BOX 5. Testing anti-HBs for health care personnel (HCP) vaccinated in the past
BOX 5. Testing anti-HBs for health care personnel (HCP) vaccinated in the past
The issue: An increasing number of HCP have received routine hepatitis B (HepB) vaccination during childhood. No postvaccination serologic testing is recommended after routine infant or adolescent HepB vaccination. Because vaccine-induced antibody to hepatitis B surface antigen (anti-HBs) wanes over time, testing HCP for anti-HBs years after vaccination might not distinguish vaccine nonresponders from responders.
Guidance for health care institutions: Health care institutions may measure anti-HBs upon hire or matriculation for HCP who have documentation of a complete HepB vaccine series in the past (e.g., as part of routine infant or adolescent vaccination). HCP with anti-HBs <10 mIU/mL should receive one or more additional doses of HepB vaccine and retesting (Figure 3). Institutions that decide to not measure anti-HBs upon hire or matriculation for HCP who have documentation of a complete HepB vaccine series in the past should ensure timely assessment and postexposure prophylaxis following an exposure (Table 5).
Considerations: The risk for occupational HBV infection for vaccinated HCP might be low enough in certain settings so that assessment of anti-HBs status and appropriate follow-up should be done at the time of exposure to potentially infectious blood or body fluids. This approach relies on HCP recognizing and reporting blood and body fluid exposures and therefore may be applied on the basis of documented low risk, implementation, and cost considerations. Certain HCP occupations have lower risk for occupational blood and body fluid exposures (e.g., occupations involving counseling versus performing procedures), and nontrainees have lower risks for blood and body fluid exposures than trainees. Some settings also will have a lower prevalence of HBV infection in the patient population served than in other settings, which will influence the risk for HCP exposure to HBsAg-positive blood and body fluids.
Abbreviations: HepB = hepatitis B; HBsAg = hepatitis B surface antigen; HBIG = hepatitis B immune globulin.
* Exposures include percutaneous (e.g., bite or needlestick) or mucosal exposure to blood or body fluids, sex or needle-sharing contact, or victim of sexual assault/abuse.
 BOX 6. Persons recommended to receive serologic testing prior to vaccination*
BOX 6. Persons recommended to receive serologic testing prior to vaccination*
- Household, sexual, or needle contacts of hepatitis B surface antigen (HBsAg)–positive persons†
- HIV-positive persons†
- Persons with elevated alanine aminotransferase/aspartate aminotransferase of unknown etiology†
- Hemodialysis patients†
- Men who have sex with men†
- Past or current persons who inject drugs†
- Persons born in countries of high and intermediate hepatitis B virus (HBV) endemicity (HBsAg prevalence ≥2%)
- U.S.-born persons not vaccinated as infants whose parents were born in countries with high HBV endemicity (≥8%)
- Persons needing immunosuppressive therapy, including chemotherapy, immunosuppression related to organ transplantation, and immunosuppression for rheumatologic or gastroenterologic disorders
- Donors of blood, plasma, organs, tissues, or semen
* Serologic testing comprises testing for hepatitis B surface antigen (HBsAg), antibody to HBsAg, and antibody to hepatitis B core antigen.
† Denotes persons also recommended for hepatitis B vaccination. Serologic testing should occur prior to vaccination. Serologic testing should not be a barrier to vaccination of susceptible persons. The first dose of vaccine should typically be administered immediately after collection of the blood for serologic testing.
 BOX 7. Persons recommended to receive postvaccination serologic testing* following a complete series of HepB vaccination
BOX 7. Persons recommended to receive postvaccination serologic testing* following a complete series of HepB vaccination
• Infants born to hepatitis B surface antigen (HBsAg)–positive mothers or mothers whose HBsAg status remains unknown (e.g., when a parent or person with lawful custody safely surrenders an infant confidentially shortly after birth infants safely surrendered at or shortly after birth)†
• Health care personnel and public safety workers
• Hemodialysis patients and others who might require outpatient hemodialysis (e.g., predialysis, peritoneal dialysis, and home dialysis)
• HIV-infected persons
• Other immunocompromised persons (e.g., hematopoietic stem-cell transplant recipients or persons receiving chemotherapy)
• Sex partners of HBsAg-positive persons
* Postvaccination serologic testing for persons other than infants born to HBsAg-positive (or HBsAg-unknown) mothers consists of anti-HBs.
† Postvaccination serologic testing for infants born to HBsAg-positive (or HBsAg-unknown) mothers consists of anti-HBs and HBsAg. Persons with anti-HBs <10 mIU/mL after the primary vaccine series should be revaccinated. Infants born to HBsAg-positive mothers or mothers with an unknown HBsAg status should be revaccinated with a single dose of HepB vaccine and receive postvaccination serologic testing 1–2 months later. Infants whose anti-HBs remains <10 mIU/mL following single dose revaccination should receive two additional doses of HepB vaccine, followed by postvaccination serologic testing 1–2 months after the final dose. Based on clinical circumstances or family preference, HBsAg-negative infants with anti-HBs <10 mIU/mL may instead be revaccinated with a second, complete 3-dose series, followed by postvaccination serologic testing performed 1–2 months after the final dose of vaccine. For others with anti-HBs <10 mIU/mL after the primary series, administration of 3 additional HepB vaccine doses on an appropriate schedule, followed by anti-HBs testing 1–2 months after the final dose, is usually more practical than serologic testing after ≥1 dose of vaccine.
Suggested citation for this article: Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67(No. RR-1):1–31. DOI: http://dx.doi.org/10.15585/mmwr.rr6701a1.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.