 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
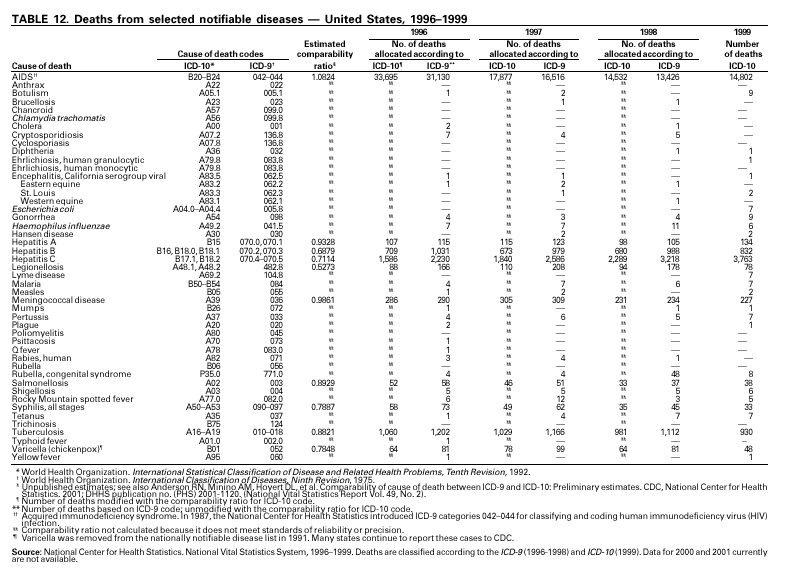
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Summary of Notifiable Diseases --- United States, 2001The following CDC staff members contributed to this report: Samuel L. Groseclose, D.V.M., M.P.H. in collaboration with John D. Hatmaker PrefaceThe MMWR Summary of Notifiable Diseases, United States, 2001 contains, in tabular and graphic form, the official statistics for the reported occurrence of nationally notifiable diseases in the United States for 2001. These statistics are collected and compiled from reports sent by state health departments to the National Notifiable Diseases Surveillance System (NNDSS), which is operated by CDC in collaboration with the Council of State and Territorial Epidemiologists (CSTE). The Summary is located on the Internet at http://www2.cdc.gov/mmwr/summary.html. This site also includes publications from past years. Because the dates of onset or diagnosis for notifiable diseases are not always reported, these surveillance data are presented by the year and week they were reported to CDC by public health officials in state and territorial health departments. The data are finalized and published each year in the Summary for use by state and local health departments; schools of medicine and public health; communications media; local, state, and federal agencies; and other agencies or persons interested in following the trends of reportable diseases in the United States. This publication also documents which diseases are considered national priorities for notification and the annual number of reported cases of such diseases. The Highlights section presents information on selected nationally notifiable diseases to provide a context in which to interpret surveillance and disease-trend data and to provide further information on the epidemiology and prevention of selected diseases. Part 1 contains tables showing incidence data for each of the diseases considered nationally notifiable during 2001.* The tables provide the number of cases of notifiable diseases reported to CDC for 2001, as well as the distribution of cases by month and geographic location and by patient’s age, sex, race, and Hispanic ethnicity. The data are final totals reported as of June 21, 2002, unless otherwise noted. Nationally notifiable diseases that are reportable in <40 states also do not appear in these tables. Ehrlichiosis, human, other or unspecified agent, is not reported in any tables because data are incomplete. In all tables, leprosy is listed as Hansen disease, and tickborne typhus fever is listed as Rocky Mountain spotted fever (RMSF). In addition, syphilis (all stages) includes the following categories: latent; early latent; late latent; latent of unknown duration; neurosyphilis; late, with clinical manifestations other than neurosyphilis; syphilitic stillbirth, and congenital syphilis. Part 2 contains graphs and maps that depict summary data for many of the notifiable diseases described in tabular form in Part 1. Part 3 contains tables that list the number of cases of notifiable diseases reported to CDC since 1970. This section also includes a table enumerating deaths associated with specified notifiable diseases reported to the National Center for Health Statistics (NCHS), CDC, during 1996–1999.† The Selected Reading section presents general and disease-specific references for
notifiable infectious diseases. These references provide additional information on
surveillance and epidemiologic issues, diagnostic issues, or disease control activities.
BackgroundThe infectious diseases designated as notifiable at the national level during 2001 are listed in the following table. A notifiable disease is one for which regular, frequent, and timely information regarding individual cases is considered necessary for the prevention and control of the disease. This section briefly summarizes the history of the reporting of nationally notifiable diseases in the United States. In 1878, Congress authorized the U.S. Marine Hospital Service (the forerunner of the Public Health Service [PHS]) to collect morbidity reports regarding cholera, small-pox, plague, and yellow fever from U.S. consuls overseas. The intention was to use this information to institute quarantine measures to prevent the introduction and spread of these diseases into the United States. In 1879, a specific Congressional appropriation was made for the collection and publication of reports of these notifiable diseases. Congress expanded the authority for weekly reporting and publication of these reports in 1893 to include data from states and municipal authorities. To increase the uniformity of the data, Congress enacted a law in 1902 directing the Surgeon General to provide forms for the collection and compilation of data and for the publication of reports at the national level. In 1912, state and territorial health authorities — in conjunction with PHS — recommended immediate telegraphic reporting of five infectious diseases and the monthly reporting, by letter, of 10 additional diseases. The first annual summary of The Notifiable Diseases in 1912 included reports of 10 diseases from 19 states, the District of Columbia, and Hawaii. By 1928, all states, the District of Columbia, Hawaii, and Puerto Rico were participating in national reporting of 29 specified diseases. At their annual meeting in 1950, state and territorial health officers authorized the Council of State and Territorial Epidemiologists (CSTE) to determine which diseases should be reported to PHS. In 1961, CDC assumed responsibility for the collection and publication of data concerning nationally notifiable diseases. The list of nationally notifiable diseases is revised periodically. For example, a diseases might be added to the list as a new pathogen emerges, or a disease might be deleted as its incidence declines. Public health officials at state health departments and CDC continue to collaborate in determining which diseases should be nationally notifiable. CSTE, with input from CDC, makes recommendations annually for additions and deletions. Although disease reporting is mandated by legislation or regulation at the state and local levels, state reporting to CDC is voluntary. Thus, the list of diseases considered notifiable varies slightly by state. All states generally report the internationally quarantinable diseases (i.e., cholera, plague, and yellow fever) in compliance with the World Health Organization’s International Health Regulations. 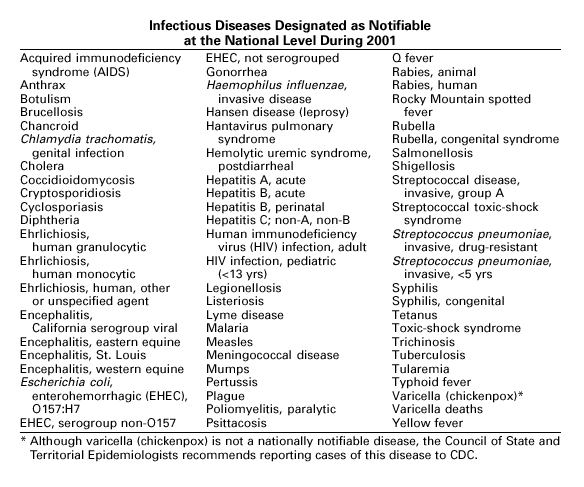
Data SourcesProvisional data concerning the reported occurrence of notifiable diseases are published weekly in the MMWR. After each reporting year, staff in state health departments finalize reports of cases for that year with local or county health departments and reconcile the data with reports previously sent to CDC throughout the year. These data are compiled in final form in the Summary. Notifiable disease reports are the authoritative and archival counts of cases. They must be approved by the appropriate epidemiologist from each submitting state or territory before being published in the Summary. Although useful for detailed epidemiologic analyses, data published in CDC Surveillance Summaries or other surveillance reports produced by CDC programs might not agree exactly with data reported in the annual summary because of differences in the timing of reports, the source of the data, or the case definitions. Data in the Summary were derived primarily from reports transmitted to the Division of Public Health Surveillance and Informatics, Epidemiology Program Office, CDC, from health departments in the 50 states, five territories, New York City, and the District of Columbia through the National Electronic Telecommunications System for Surveillance (NETSS). More information regarding NETSS and notifiable diseases, including case definitions for these conditions, is available on the Internet at http://www.cdc.gov/epo/dphsi/phs.htm. Policies for reporting notifiable disease cases can vary by disease or reporting jurisdiction, depending on case status classification (i.e., confirmed, probable, or suspected). Final data for selected diseases (presented in Parts 1, 2, and 3) are from the surveillance records of the CDC programs listed below. Requests for further information regarding these data should be directed to the appropriate program.
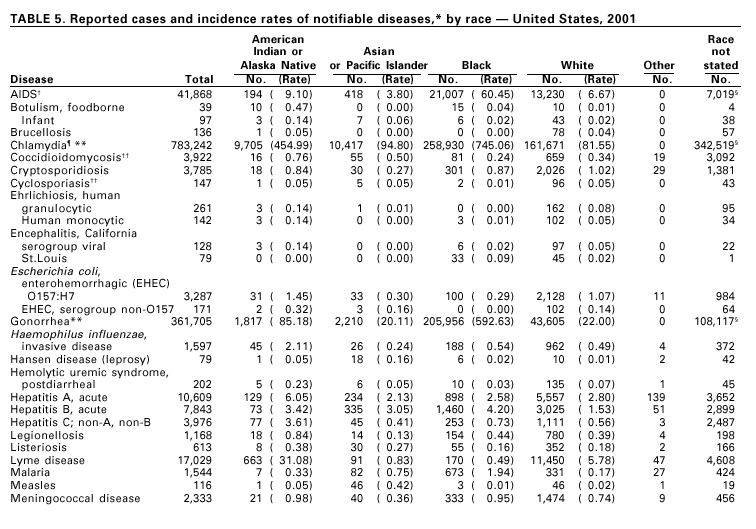
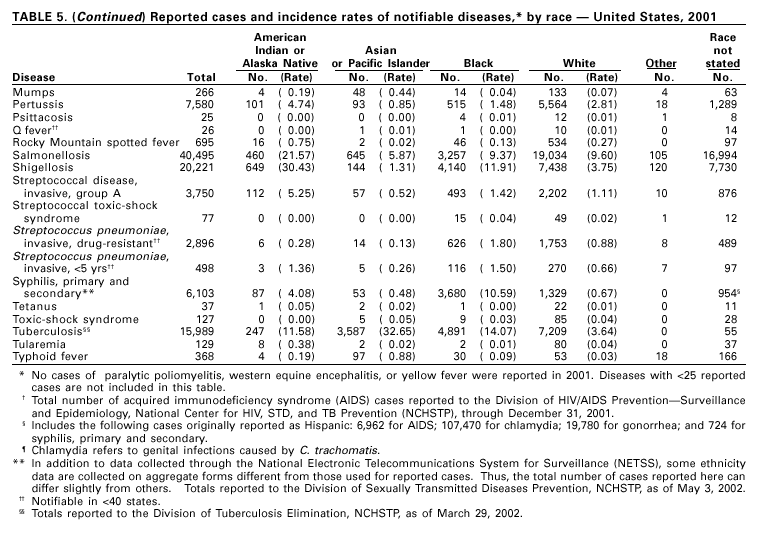
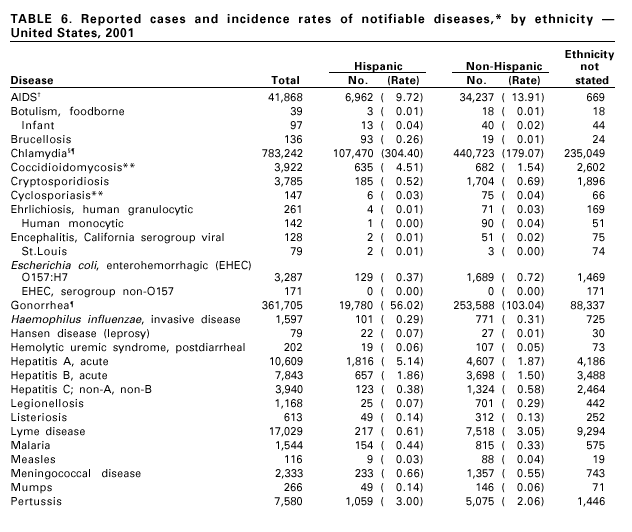
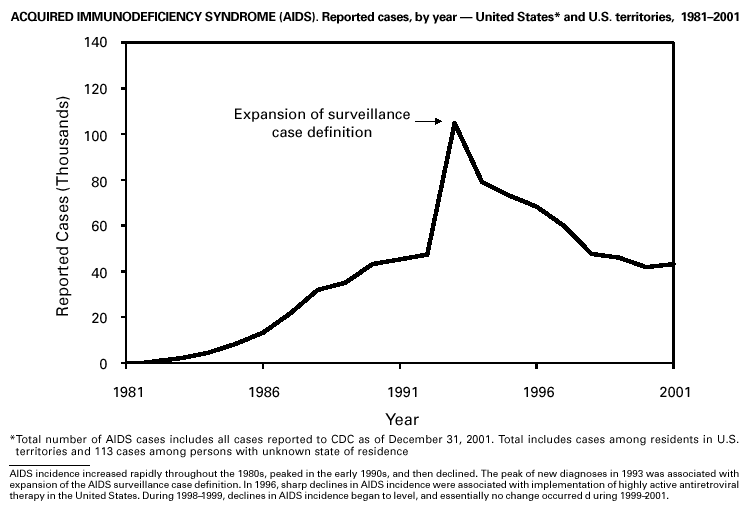
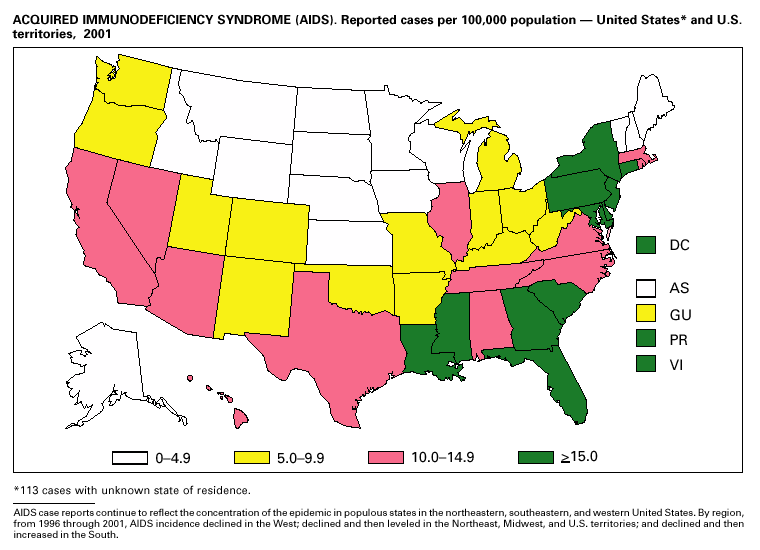
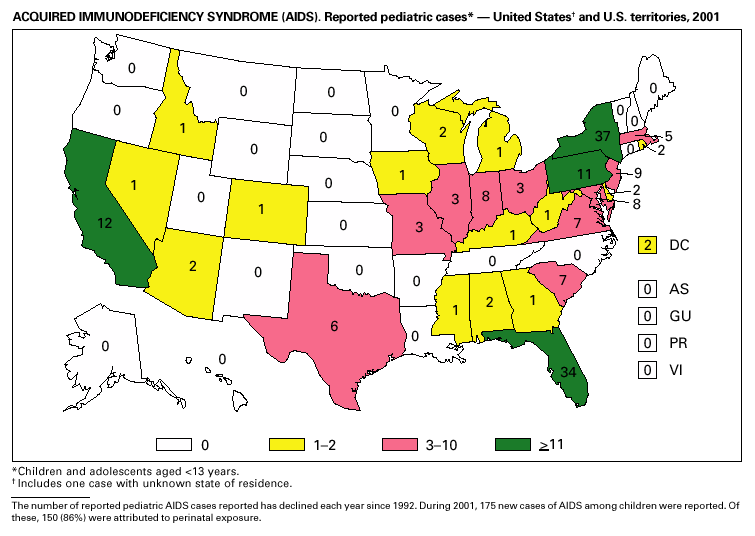
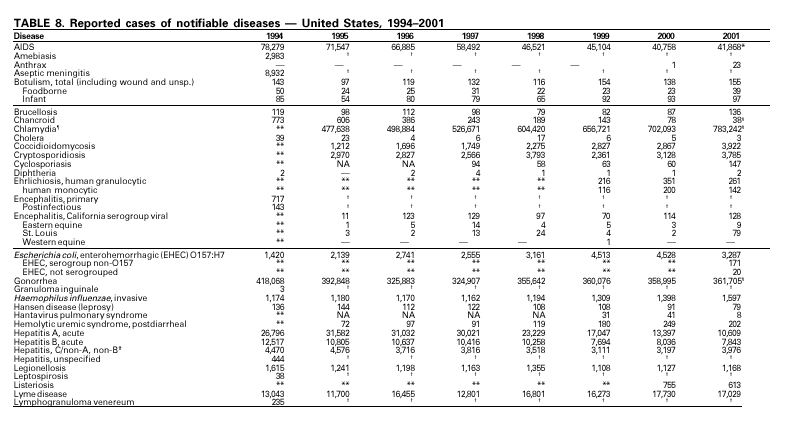
Disease totals for the United States, unless otherwise stated, do not include data for American Samoa, Guam, Puerto Rico, the U.S. Virgin Islands, or the Commonwealth of the Northern Mariana Islands. Population estimates for the states are from the April 1, 2000, population estimates from the Population Division, U.S. Bureau of the Census. Population numbers for territories are 2000 estimates from the U.S. Bureau of the Census, International Data Base Data Access Display Mode. More information regarding census estimates is available at http://eire.census.gov/popest/data/states/tables/ST-EST2002-01.php and http:// www.census.gov/ipc/www/idbprint.html. The choice of population denominators for incidence rates reported in the MMWR is based on 1) the consistency in the incidence rates reported by various CDC programs, and 2) the availability of census population data at the time of preparation for MMWR publications. Rates in the Summary are presented as incidence rates per 100,000 population, based on data for the U.S. total-resident population. However, population data from states in which diseases were not notifiable or disease data were not available were excluded from rate calculations. Interpreting Data Incidence data in the Summary are presented by the date of report to CDC as deter-mined by the MMWR week and year assigned by the state or territorial health department. As a result, annual incidence data in the Summary represent cases with onset during the MMWR year assigned to the case, or during previous years. In addition, data in the Summary are reported by the state in which the patient resides at the time of diagnosis. For many of the nationally notifiable infectious diseases, surveillance data are independently reported to EPO and other CDC programs. Thus, surveillance data reported by other CDC programs may vary from data reported in the Summary because of differences in 1) the date used to aggregate data (e.g., date of report, date of disease occurrence), 2) the timing of reports, 3) the source of the data, 4) surveillance case definitions, and 5) policies regarding case jurisdiction (i.e., which state should report the case to CDC). The data reported in the Summary are useful for analyzing disease trends and determining relative disease burdens. However, these data must be interpreted in light of reporting practices. Some diseases that cause severe clinical illness (e.g., plague and rabies) are most likely reported accurately if they were diagnosed by a clinician. However, persons who have diseases that are clinically mild and infrequently associated with serious consequences (e.g., salmonellosis) might not seek medical care from a health-care provider. Even if these less severe diseases are diagnosed, they are less likely to be reported. The degree of completeness of data reporting also is influenced by the diagnostic facilities available; the control measures in effect; public awareness of a specific disease; and interests, resources, and priorities of state and local officials responsible for disease control and public health surveillance. Finally, factors such as changes in the case definitions for public health surveillance, introduction of new diagnostic tests, or discovery of new disease entities can cause changes in disease reporting that are independent of the true incidence of disease. Public health surveillance data are published for selected racial and ethnic population groups because these variables can be risk markers for certain notifiable diseases. Race and ethnicity data can also be used to highlight populations for focused prevention efforts. However, caution must be used when drawing conclusions from reported race and ethnicity data. Certain racial/ethnic population groups have differential patterns of access to health care, potentially resulting in data that are not representative of disease incidence in these populations. Surveillance data reported to NNDSS are either in individual case-specific form or summary form (aggregated data for a group of cases). Summary data often lack demographic information (e.g., race); therefore, the demographic-specific incidence rates presented in the Summary may be underestimated. In addition, not all race and ethnicity data are collected uniformly for all diseases. For example, in NCHSTP, the Division of HIV/AIDS Prevention — Surveillance and Epidemiology and the Division of Sexually Transmitted Diseases Prevention collect race/ethnicity data using a single variable. A person’s race/ethnicity is reported as American Indian/Alaska Native, Asian/Pacific Islander, black non-Hispanic, white non-Hispanic, or Hispanic. Additionally, although the recommended standard for classifying a person’s race or ethnicity is based on self-reporting, this procedure might not always be followed. Highlights for 2001This section presents information on the public health importance of selected nationally notifiable diseases reported from the states to CDC, including a) domestic and some international disease outbreaks, b) active surveillance findings, c) changes in data reporting practices, d) the impact of prevention programs, e) the emergence of antimicrobial resistance, and f) changes in immunization policies. This information is intended to provide a context in which to interpret surveillance and disease-trend data and to provide further information on the epidemiology and prevention of selected diseases. AIDS Since the use of highly active antiretroviral therapy (HAART) in the United States became widespread in 1996, the number of persons diagnosed with acquired immunodeficiency syndrome (AIDS) has declined. The number of deaths among persons with AIDS has also declined substantially; as a result, the number of persons living with AIDS has increased (1). By December 2001, a total of 807,075 adults and 9,074 children had been reported with AIDS. In 1996, sharp declines in AIDS incidence occurred for the first time; during 1998– 1999, declines in AIDS incidence began to level, and essentially no change occurred from 1999 through 2000. Through December 2001, 462,653 adult and 5,257 pediatric AIDS cases resulted in death. Since 1996, the number of deaths among persons with AIDS declined sharply and continued to decline each year through 2000. The number of persons living with AIDS, approximately 362,827, was the highest ever reported; of these persons, 78% were men and 61% were black or Hispanic. Of the 282,250 adult and adolescent men with AIDS, 57% were men who have sex with men, 24% were injecting drug users, 9% were exposed through heterosexual contact, and 8% were both men who have sex with men and injecting drug users. Of the 76,696 adult and adolescent women with AIDS, 59% were exposed through heterosexual contact and 38% through injecting drug use (2). To provide better data for prevention of human immunodeficiency virus (HIV) infection (the virus that causes AIDS), CDC and CSTE recommend that national surveillance include the monitoring of both HIV infection and AIDS (3,4). CDC supports several supplemental surveillance projects that collect data on barriers to preventing AIDS cases and deaths of persons with AIDS, including access to HIV testing and treatment in accordance with current public health service guidelines.
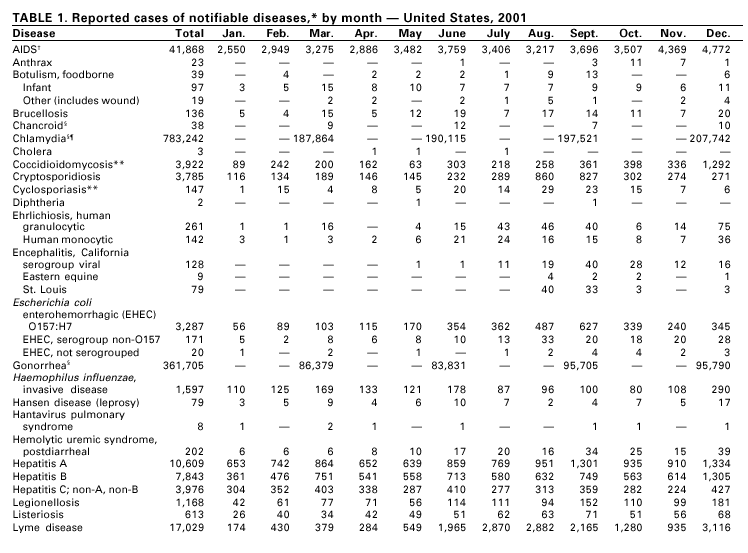
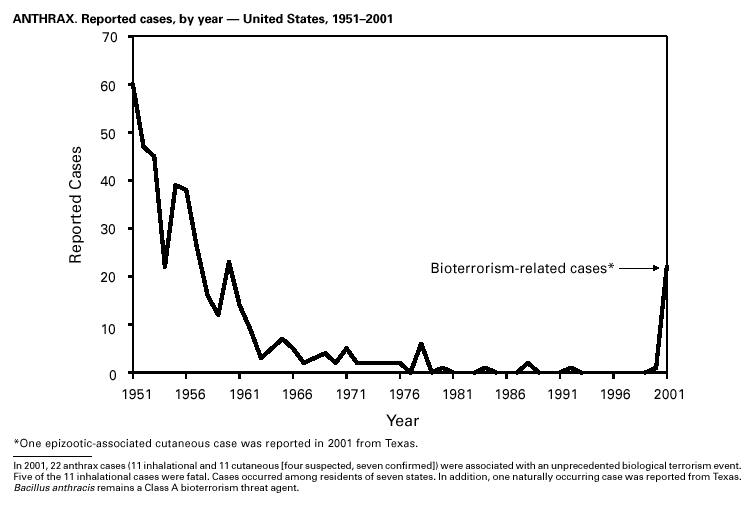
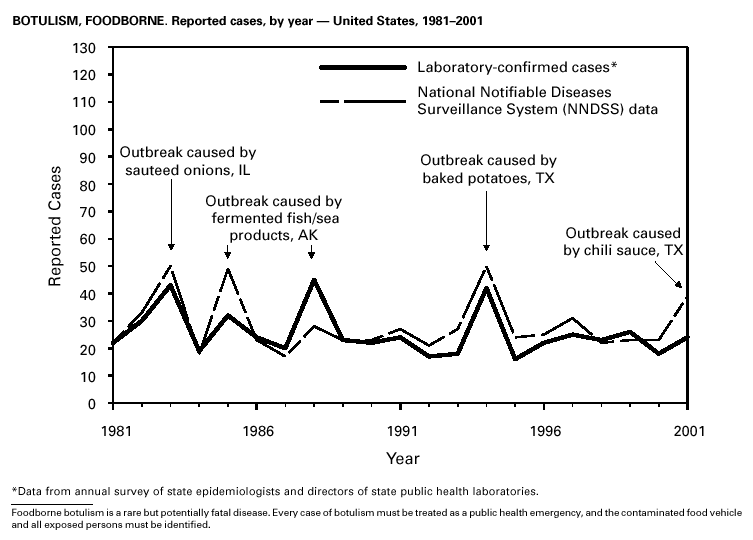
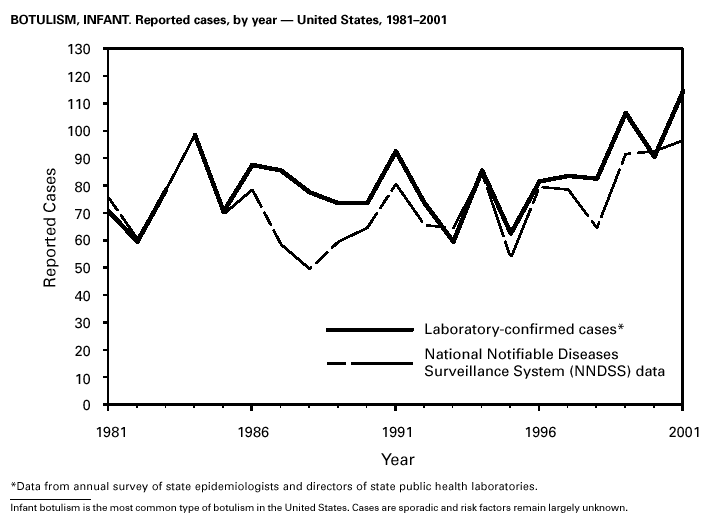
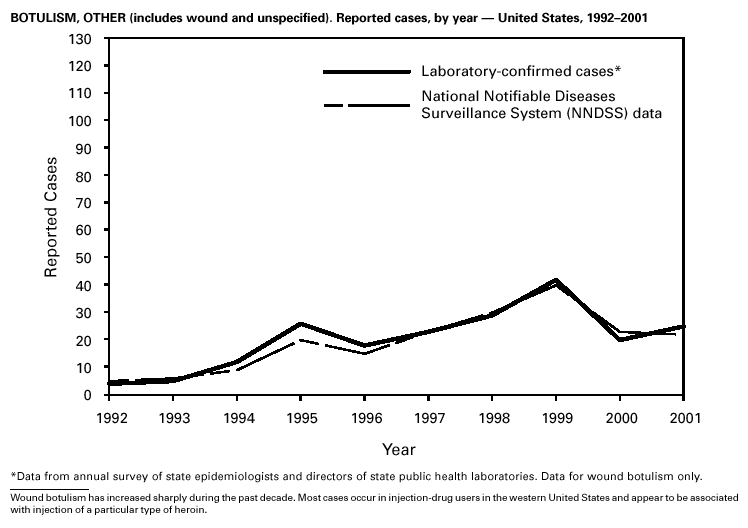
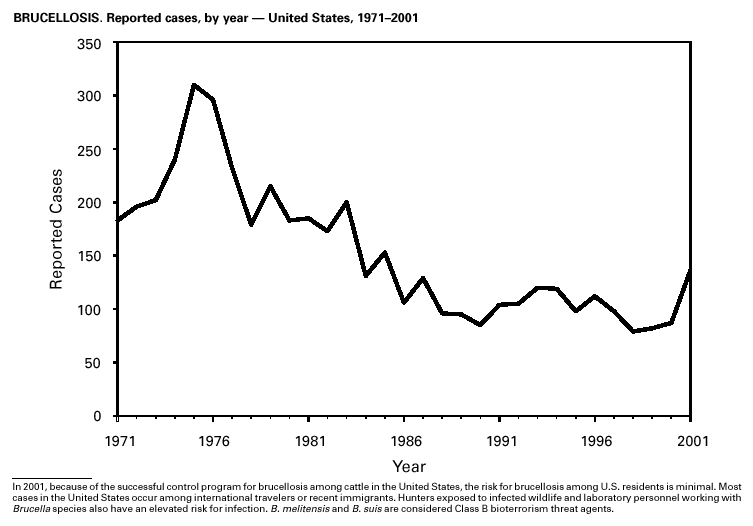
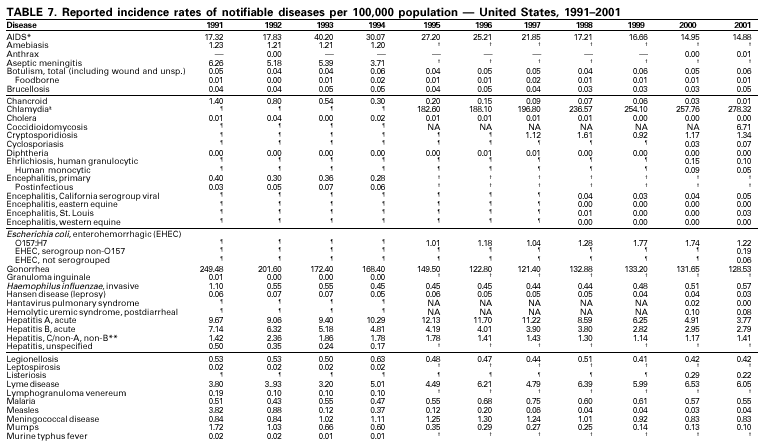
In September and October 2001, in an unprecedented biological terrorism event, letters containing Bacillus anthracis spores were sent through the U.S. Postal Service to various addresses in several states. Eleven inhalational and 11 cutaneous (four suspected and seven confirmed) anthrax cases resulted. Five of the 11 inhalational infections were fatal. These bioterrorism-associated cases occurred among residents of seven states along the East Coast of the United States: Connecticut, one case; Florida, two cases; Maryland, three cases; New Jersey, five cases; New York City, eight cases (includes a case in a New Jersey resident exposed in New York City); Pennsylvania, one case; and Virginia, two cases. In addition to the 22 bioterrorism-associated cases, one naturally occurring case of cutaneous anthrax (associated with direct exposure to livestock that had died of anthrax) was reported from Texas in the summer of 2001. B. anthracis remains a Category A bioterrorism threat agent. BotulismThirty-nine cases of foodborne botulism were reported in 2001 through NNDSS. An outbreak of foodborne botulism in Texas involving nine culture-confirmed and seven clinically diagnosed cases was caused by commercially produced chili sauce and likely occurred because of time and temperature abuse of the food at a retail salvage store. The highest annual frequency of infant botulism, 97 cases, was reported in 2001. The number of wound botulism cases reported in 2001 was 19. Botulism surveillance conducted by the Foodborne and Diarrheal Diseases Branch, NCID, indicated 33 foodborne cases, 112 cases of infant botulism, and 23 cases of wound botulism. Clostridium botulinum toxin is a Category A bioterrorism threat agent. BrucellosisIn 2001, the control program for brucellosis among cattle in the United States has nearly eliminated Brucella abortus infection from U.S. herds. Therefore, at present, the risk of contracting brucellosis either from occupational exposure to livestock in the United States or from domestically produced food products is minimal. However, a risk remains for infection with both B. abortus and B. melitensis from consumption of unpasteurized goat and cow milk products, in particular those produced outside the United States. Most cases in the United States are now seen in international travelers or recent immigrants. Hunters exposed to infected wildlife and laboratory personnel working with Brucella species also have an elevated risk for infection. B. melitensis and B. suis are considered Category B bioterrorism threat agents. ChancroidDuring 2001, a total of 38 cases of chancroid were reported (rate: 0.01 cases/100,000 population), representing a 51% decline from 2000 and a continuing decline since 1987 (1). However, chancroid is difficult to culture and could be substantially underdiagnosed. Several studies that used DNA amplification tests (which are not commercially available) have identified this infection in cities where it was previously undetected (2).
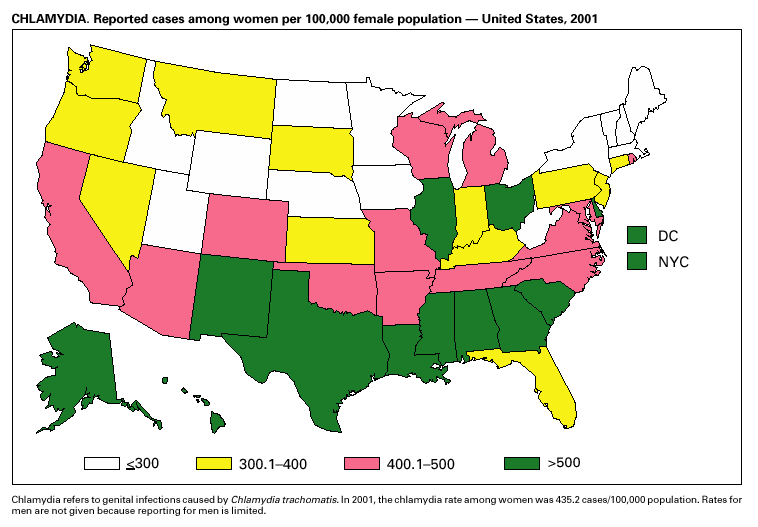
During 2001, a total of 783,242 cases of genital chlamydial infection were reported (rate: 278.32/100,000). This rate was the highest since voluntary case reporting began in the mid-1980s and the highest since genital chlamydial infection became a nationally notifiable disease in 1995 (1). This increase could be caused in part by the continued expansion of chlamydia screening programs and increased use of more sensitive diagnostic tests for this condition.
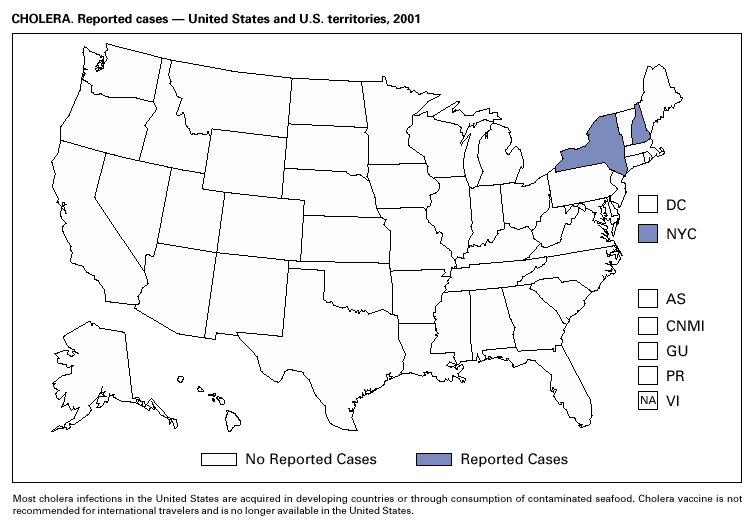
During 1995–2000, 61 laboratory-confirmed cases of cholera, all caused by Vibrio cholerae O1, were reported to CDC. Thirty-five (57%) patients were hospitalized, and one died. Thirty-seven (61%) infections were acquired outside the United States, whereas six (10%) were acquired through consumption of contaminated seafood harvested in Gulf Coast waters (1). Only three laboratory-confirmed cases of cholera were reported to CDC in 2001. All were caused by V. cholerae O1 and were acquired outside the United States. All three isolates were resistant to trimethoprim-sulfamethoxazole, sulfisoxazole, streptomycin, and furazolidone. Thus, foreign travel continues to account for most cholera cases in the United States, and antimicrobial resistance is common among V. cholerae O1 strains isolated from ill travelers. Production and sale of the only licensed cholera vaccine in the United States ceased in 2001.
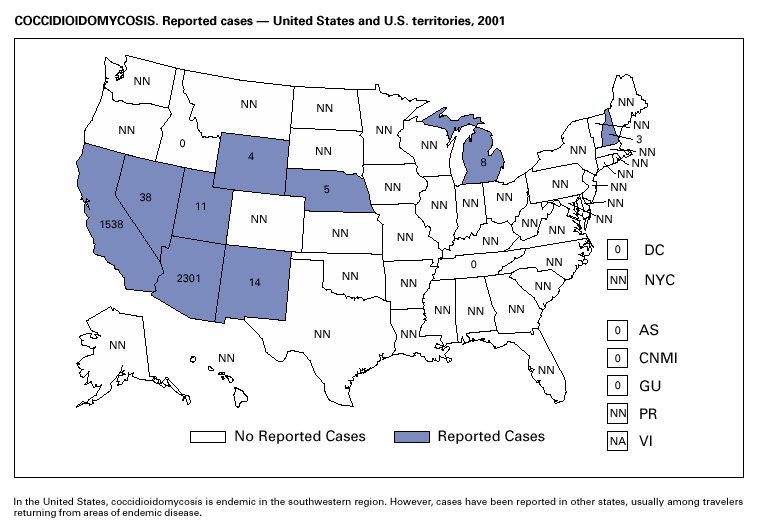
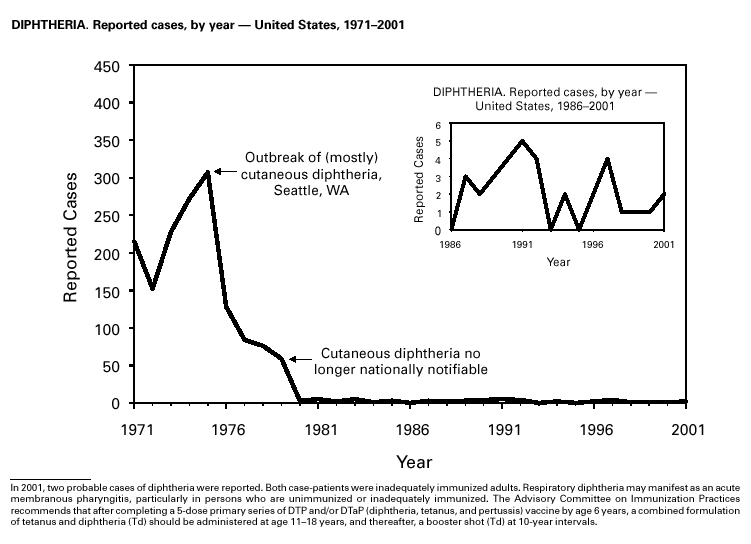
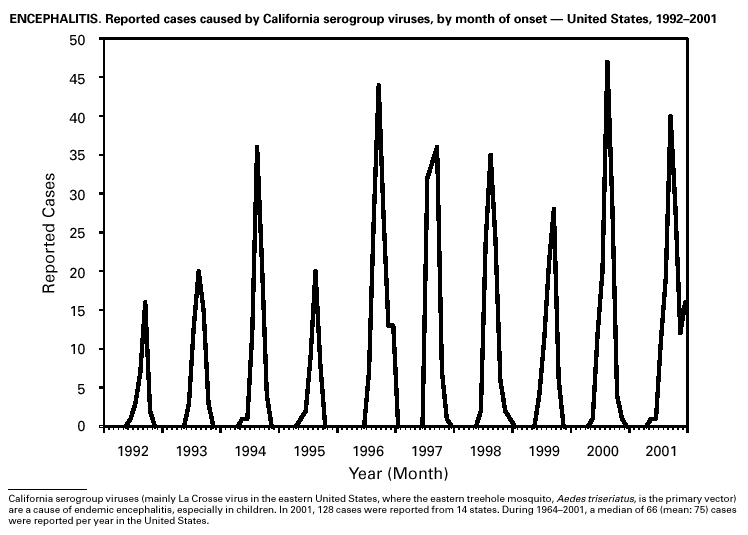
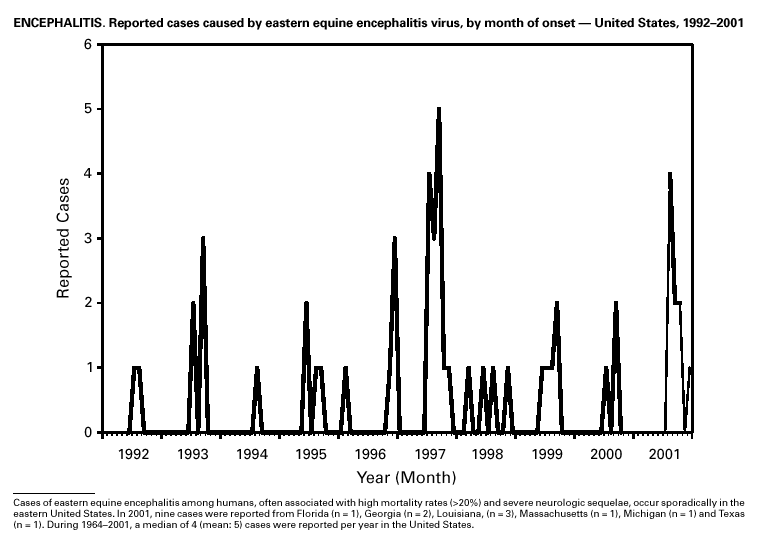
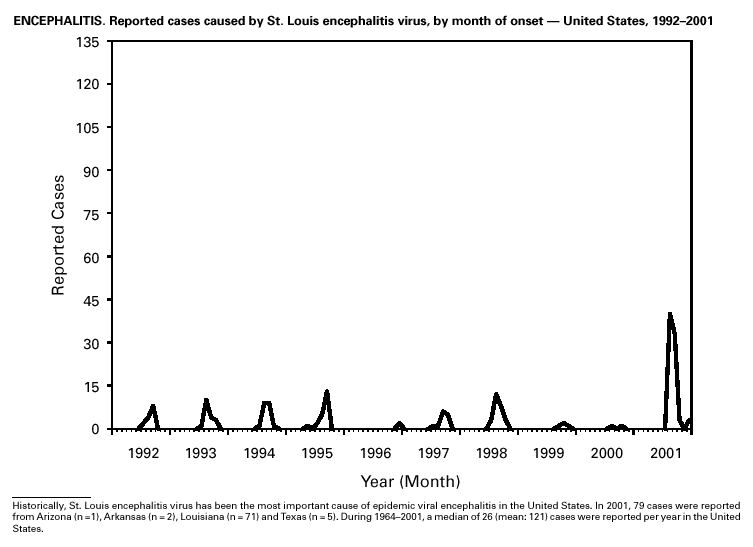
In recent years, Arizona has experienced a significant increase in the incidence rates of coccidioidomycosis, from 18/100,000 in 1997 to 42/100,000 in 2001. This increase is likely related to demographic and climactic changes. Physicians should maintain a high suspicion for acute coccidioidomycosis, especially for persons with a flu-like illness who live in or have visited endemic-disease areas. DiphtheriaDuring 2001, two probable diphtheria cases were reported to CDC. Both patients had membranous pharyngitis. The first was a man aged 59 years from Montana. A specimen for culture was not obtained from this patient. The second patient was a woman aged 19 years from Michigan. Although a throat swab culture from this patient did not yield Corynebacterium diphtheriae, a weakly positive Taqman polymerase chain reaction test result was obtained from the membranous tissue. Neither patient had a history of recent travel or had contact with international or local visitors. Both patients survived. Encephalitis, ArboviralIn 2001, epizootic and epidemic West Nile virus (WNV) activity continued in the United States, and geographic limits of reported viral activity extended to western Arkansas and southern Florida (1). WNV-infected birds, mosquitoes, or horses were detected in 27 states and the District of Columbia; 16 of these states had not previously reported WNV activity. In Florida, dead infected birds were collected as late as December 26, suggesting the potential for winter transmission in southern regions. An unprecedented equine WNV epizootic occurred in Florida and Georgia and resulted in 511 reported equine cases. Culex ( Cx. pipiens, Cx. restuans, and Cx. salinarius) mosquitoes were again the most commonly identified mosquito vectors of WNV. WNV was also detected in several human-feeding mosquito species ( Cx. nigripalpus, Ochlerotatus sollicitans, Oc. tainiorhynchus, and Coquillitidia perturbans), raising concerns about increased human risk in areas where these species are common (2,3). A total of 66 human cases of WNV disease were reported from 39 counties in 10 states (64 patients with WNV meningoencephalitis and two persons with uncomplicated WNV fever). In 2001, 79 human cases of St. Louis encephalitis (SLE) were reported from Arizona (n = 1), Arkansas (n = 2), Louisiana, (n = 71), and Texas (n = 5). Epidemic SLE activity in Louisiana was centered in the city of Monroe (4).
In 2001, the National Notifiable Diseases Surveillance System expanded surveillance of Escherichia coli O157:H7 to include other serogroups of Shiga toxin-producing E. coli under the inclusive name enterohemorrhagic E. coli (EHEC). Surveillance categories for EHEC include 1) EHEC O157:H7; 2) EHEC, serogroup non-O157; and 3) EHEC, not serogrouped. During 2001, 3,485 cases of EHEC infection were reported from 50 states, Guam and Puerto Rico. These cases included 3,294 due to EHEC O157:H7, 171 due to EHEC, serogroup non-O157, and 20 due to EHEC that were not serogrouped. Approximately 50% of stools are tested for E. coli O157, and few stool specimens are tested in a way that would identify other Shiga toxin-producing E. coli (1). The number of cases reported for EHEC should be interpreted as an underestimate in a maturing surveillance system.
Healthy cattle are the main animal reservoir for E. coli O157:H7 and other Shiga toxin-producing E. coli, and they harbor the organism as part of the bowel flora. Most reported outbreaks are caused by contaminated food or water. However, direct transmission from animals and their environment to humans in settings such as petting zoos, open farms, and animal exhibits represents a growing public health concern (2).
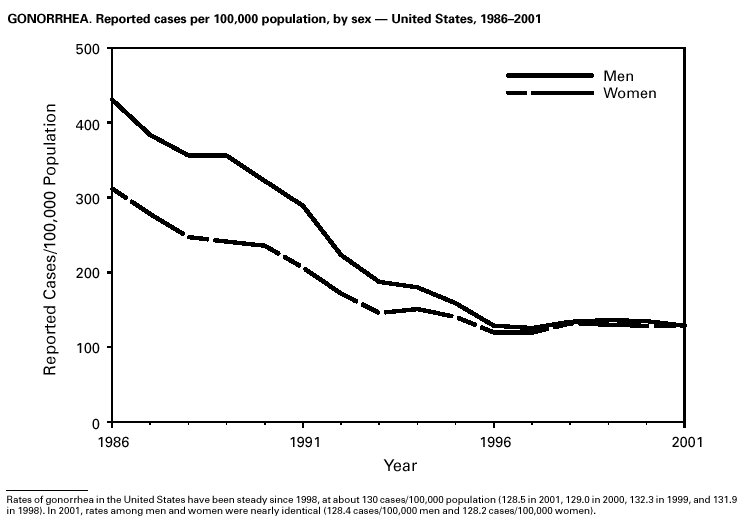
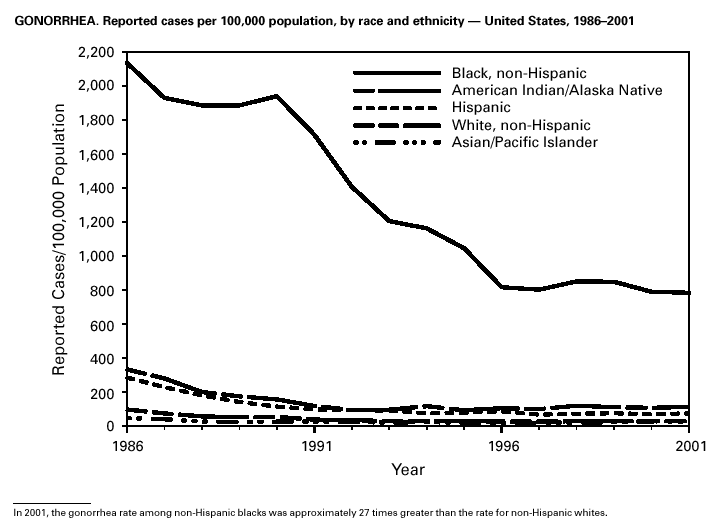
During 2001, a total of 361,705 cases of gonorrhea were reported (rate: 128.53/ 100,000). The 2001 rate was similar to rates for 2000 (129.04/100,000), 1999 (132.32/ 100,000), and 1998 (131.89/100,000) (1) and has remained stable among men and women. Nevertheless, increases have been observed in some areas among men who have sex with men (2). Decreased susceptibility to the fluoroquinolone antibiotics and azithromycin has been reported from some regions (3). In 2001, the prevalence of fluoroquinolone-resistant Neisseria gonorrhoeae infections increased in California. As a result, fluoroquinolones are no longer advised for treatment of gonorrhea in Hawaii or California or for infections that may have been acquired in those states (4).
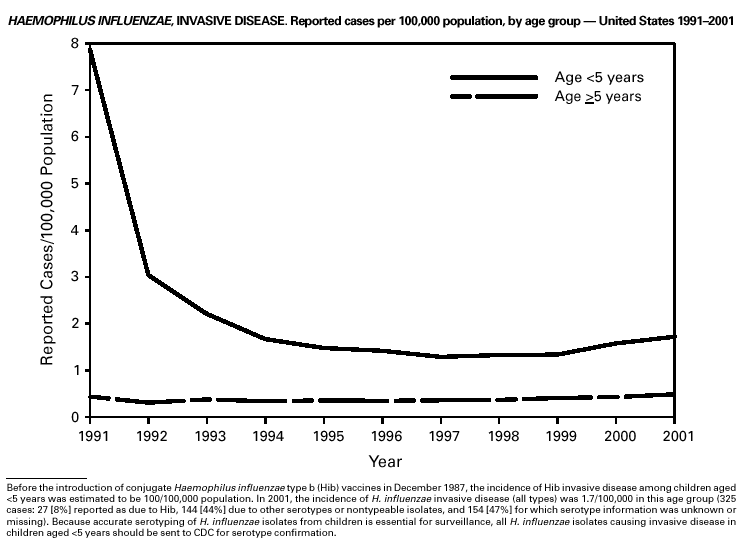
Since 1990, when Haemophilus influenzae type b (Hib) conjugate vaccines were licensed for use in infants beginning at age 2 months, Hib has become a rare cause of invasive disease (e.g., meningitis) among children aged <5 years in the United States (1). Surveillance information is used to monitor the effectiveness of immunization programs and vaccines and to assess progress toward disease elimination. To continue to assess progress toward the elimination of Hib invasive disease, accurate laboratory information is essential to correctly identify the serotype of the causative H. influenzae (Hi) isolate (2). Serotyping Hi by slide agglutination can sometimes be inaccurate, especially since it is not performed frequently in most laboratories. Recently, CDC reported discrepancies in Hi slide agglutination serotyping results obtained by state health department laboratories participating in active surveillance and those obtained by CDC. In this study, 28 (70%) of 40 Hi isolates that had been reported as Hib to CDC were actually identified at CDC as nontypeable Hi (2). Because of these discrepancies, CDC requests state health department laboratories to send all Hi invasive disease isolates from children aged <5 years to CDC for testing to reconfirm serotype.
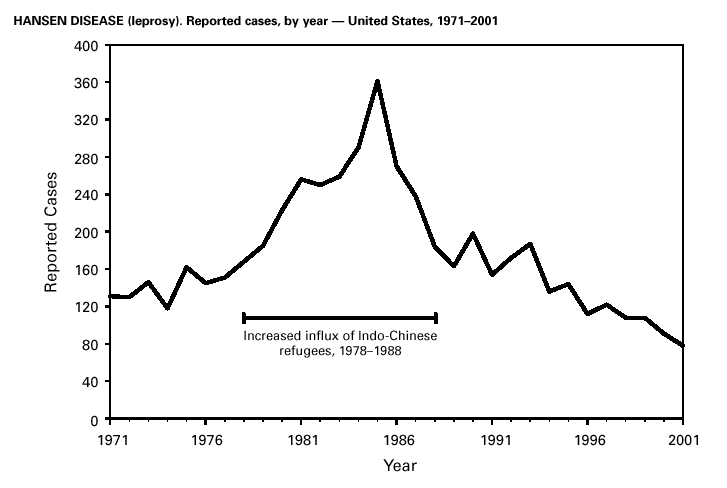
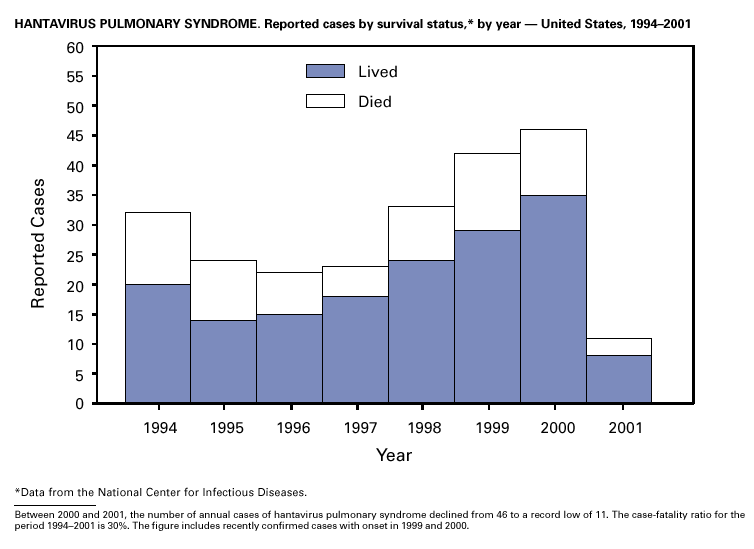
A total of 81 Hansen disease cases were reported to CDC through the NNDSS database from 20 states, Puerto Rico and American Samoa in 2001; three states (California, Hawaii and New York) accounted for 74% of the total number of cases reported. In contrast, 110 Hansen disease cases were reported to the National Hansen Disease Program from 27 states and Puerto Rico in 2001; six states (Texas, New York, Louisiana, Washington, Florida and California) accounted for 71% of the total number of cases reported. These data suggest that the annual number of cases in the United States may not be declining and underscore the need for coordination between the multiple surveillance systems as well as the need to continue to identify and treat patients with Hansen disease. Hantavirus Pulmonary SyndromeDuring 2001, a total of 11 cases of hantavirus pulmonary syndrome (HPS) were confirmed in eight states through the Hantavirus Pulmonary Syndrome National Surveillance System and Registry. Three (27%) cases were fatal. This is the lowest number of annual cases reported since the disease was recognized in 1993. Previously, the average number of cases per year was 34 (range: 22–48). As of December 31, 2001, a total of 313 cases have been confirmed in 31 states, including 32 cases that were retrospectively identified back to 1959. Hantaviruses are rodent borne, and human infection most commonly occurs through inhalation of virus particles from infectious rodent droppings, urine, or saliva. Preventing exposure to rodent hosts remains the most effective way of preventing morbidity and mortality from HPS because treatment for the disease is largely supportive (1).
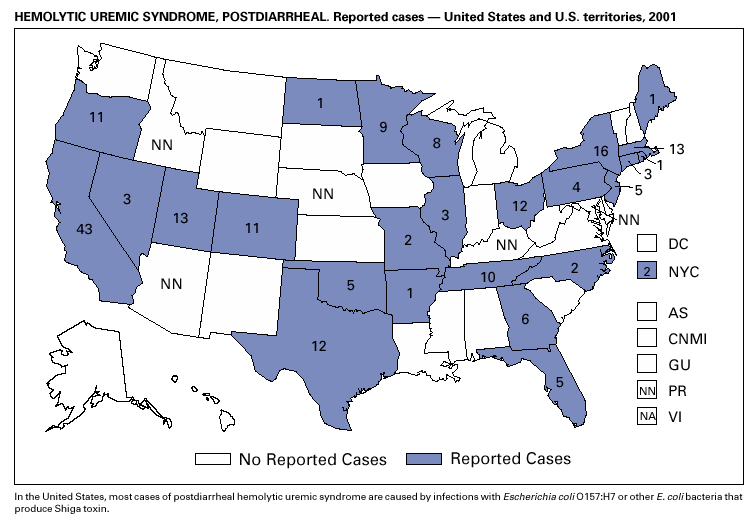
During 2001, the sixth year of national reporting, 28 states reported 202 cases of postdiarrheal hemolytic uremic syndrome (HUS). The median age of patients was 5 years (range: <1–79), and 66% were female. Illness was seasonal, with 43% of cases occurring from June through September. Although the number of reported cases in 2001 decreased compared with 2000 (249 cases), it was greater than in 1999 (181 cases); thus, a trend is not possible to determine. At least five states, the District of Columbia, and two territories did not list HUS as a notifiable disease in 2000, contributing to substantial underreporting. Postdiarrheal HUS is a life-threatening illness characterized by hemolytic anemia, throm-bocytopenia, and renal injury. In the United States, most cases are caused by infection with Escherichia coli O157:H7; some are caused by other Shiga toxin-producing E. coli (1,2).
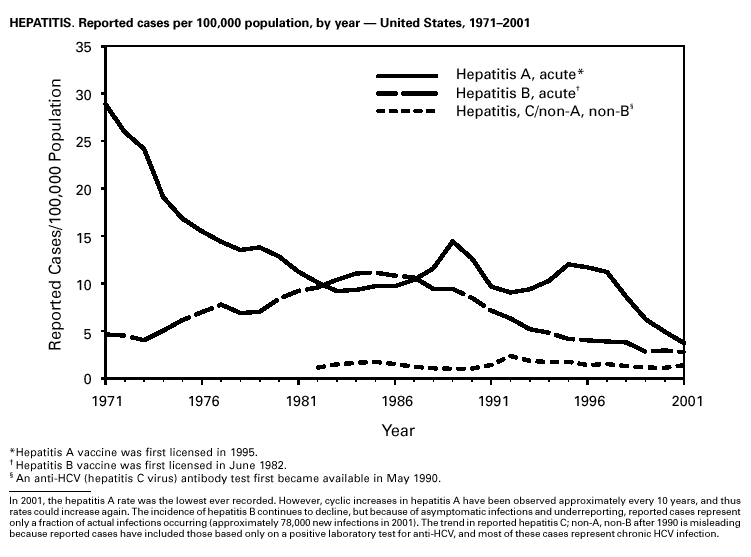
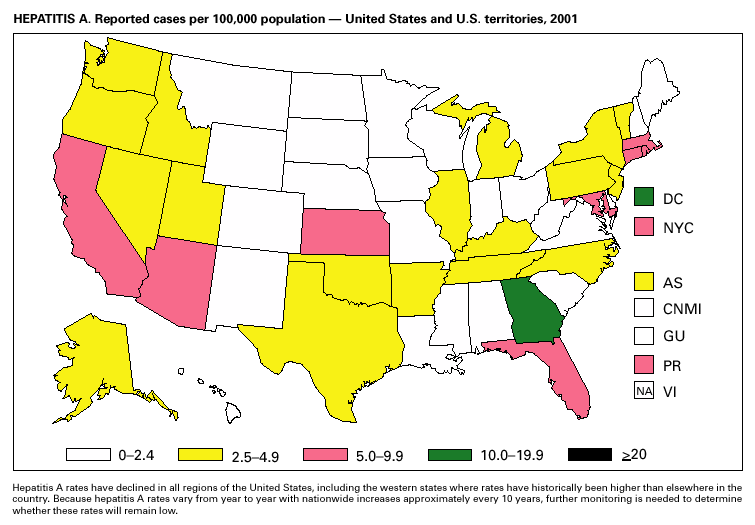
Hepatitis A vaccine is recommended for persons at increased risk of acquiring hepatitis A (e.g., illegal drug users, men who have sex with men [MSM]) and also for children in states and counties that have historically had consistently elevated rates of hepatitis A (1). After routine childhood vaccination was recommended, the overall hepatitis A rate has declined steadily, and in 2001 it was the lowest yet recorded (4.0/100,000). Because hepatitis A rates tend to vary from year to year and from region to region, continued monitoring of hepatitis A incidence is needed to determine whether this low rate is due to routine immunization or natural variability in infection rates. However, declines in rates have been greater among children and in the states where routine childhood vaccination is recommended, suggesting an impact of childhood vaccination. Despite declining overall rates, some states reported increasing rates in 2000– 2001. In several states, these increases were related to outbreaks occurring among high-risk adults, including MSM, and cases among adults in high-risk groups represent an increasing proportion of reported cases nationwide. For example, cases among MSM increased from 4% (1990) to 8% (1995) to12% (2000). Hepatitis BDuring 2001, a total of 7,843 acute hepatitis B cases were reported, representing a >60% decrease since 1990 (21,102 cases). Surveillance data are being used to monitor the impact of the national strategy for eliminating hepatitis B virus (HBV) infection. Healthy People 2010 objectives call for a 75%–90% reduction in the national incidence of hepatitis B among adults (baseline: 15–24 cases/100,000), a 99% reduction among children aged 2–18 years (baseline: 945 cases/year), and a 75% reduction in the number of perinatal HBV infections (baseline: 1,682 infections/year) (1). The effect of routine infant and adolescent vaccination can already be seen in the declining rate of disease among persons aged <19 years. In contrast, the continued high incidence among persons in other risk groups for which vaccination is recommended, e.g., injection drug users and persons engaging in high-risk sexual behaviors, indicates that programs for reaching these populations need to be developed or strengthened.
Cases of hepatitis C reported to CDC are considered unreliable because 1) no serologic marker for acute infection exists, and 2) most health departments do not have the resources to determine if a positive laboratory report for hepatitis C virus (HCV) infection represents acute infection, chronic infection, repeated testing of a person previously reported, or a false-positive result (1). Historically, the most reliable national estimates of acute disease incidence have come from sentinel surveillance. After adjusting for underreporting and asymptomatic infections, the annual number of new infections has decreased >80% since 1989 to 25,000 cases in 2001 (CDC, unpublished data, 2002). Because surveillance for acute hepatitis C can be used to evaluate the effectiveness of prevention efforts and identify missed opportunities for prevention, efforts are under way to help states establish and improve surveillance. HIV Infection, Adult*Persons with HIV infection are living longer without progressing to AIDS. As a result, AIDS incidence is decreasing and no longer provides the most accurate information on the HIV epidemic. Recommendations for implementing national HIV case surveillance were published in December 1999, and the revised surveillance case definition became effective January 1, 2000 (1). By December 31, 2001, 37 areas had laws or regulations requiring confidential reporting by name of adults/adolescents with confirmed HIV infection. Nine areas (Washington, DC, Hawaii, Illinois, Kentucky, Maryland, Massachusetts, Puerto Rico, Rhode Island, and Vermont) had implemented a code-based system to conduct case surveillance for HIV infection. Other areas (Delaware, Maine, Montana, Oregon, and Washington) had implemented a name-to-code system to conduct HIV infection surveillance: names are collected initially and later are converted to codes. Data on cases of HIV infection from those areas conducting code-based or name-to code systems are not included in this report pending evaluations demonstrating acceptable performance under CDC guidelines and the development of methods to report such data to CDC (2). Trend analysis is possible by examining data from the 25 states* that have continually conducted HIV surveillance since 1994. These 25 states represent 24% of all AIDS cases diagnosed in the United States. During 1994–2000, HIV infection was diagnosed in 128,813 persons from the 25 states. The number of persons newly diagnosed each year with HIV infection declined steadily during 1994–1997. From 1997 through 2000, case counts have been stable in all age, race/ethnicity and HIV exposure categories. The largest declines were observed in the following groups: persons aged 25–44 years, men who have sex with men, and injection-drug users. The majority (55%) of persons with newly diagnosed HIV in these 25 states were black non-Hispanic, and 36% were white non-Hispanic. Because persons with newly diagnosed HIV infections include those who may have had previously unrecognized infections for a long time, these data do not represent incident infections. However, the stability in the number of infections diagnosed each year during the latter part of the 1990s and the small declines in the proportion of persons presenting with AIDS indicate that improvements in the targeting of HIV counseling and testing are needed to facilitate earlier diagnoses. Early diagnosis is a critical factor in ensuring that infected persons are linked to effective treatment and prevention services to reduce further transmission and improve quality of life (3).
____________________ As of December 2001, 39 areas conducted name-based surveillance for HIV infection among children aged <13 years. In 2001, 543 children whose infection had not progressed to AIDS and 175 children who had AIDS were reported (1). These states also received reports of perinatally exposed children who required follow-up with health-care providers to determine their HIV infection status. In 2000, an estimated 6,075–6,422 infants were born to HIV-positive mothers in the United States. Of these infants, an estimated 280–370 were infected with HIV, representing a decline of >80% from the 1991 peak of 1,760 estimated HIV-positive U.S. births (2). Declines in perinatal HIV infections have been attributed to the use of zidovudine to reduce perinatal HIV transmission (3) and to nationwide efforts to implement routine, voluntary prenatal HIV testing for all pregnant women (4). Continued declines in perinatal HIV infections may be difficult to sustain unless new HIV infections in women of childbearing age are reduced.
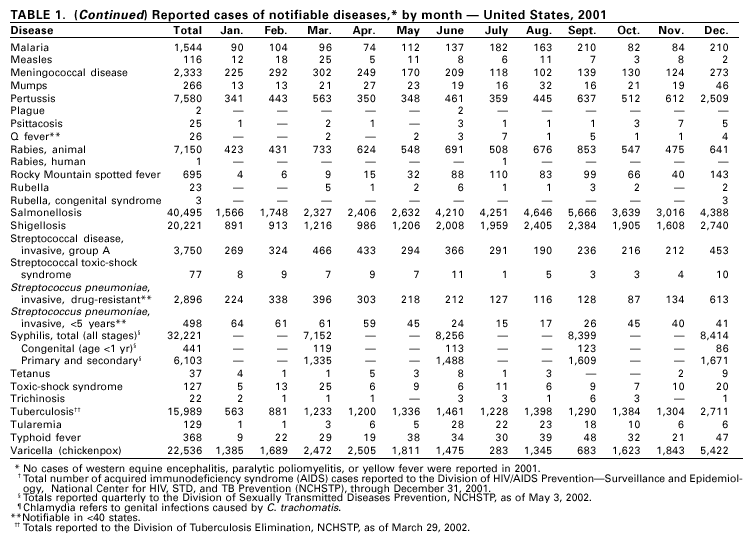
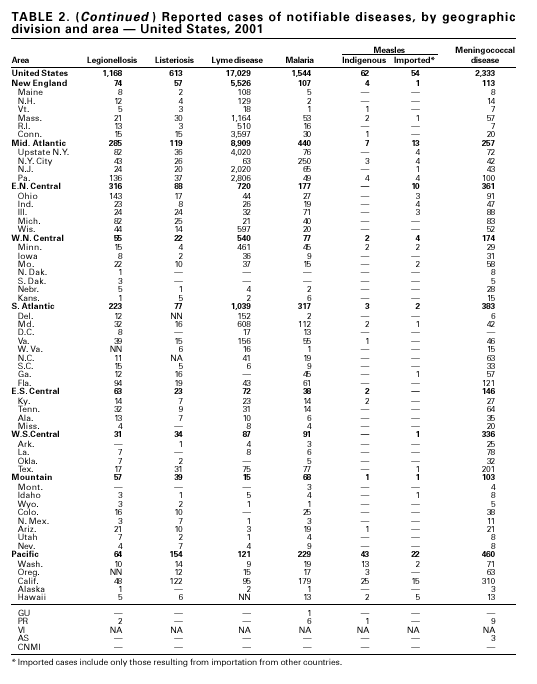
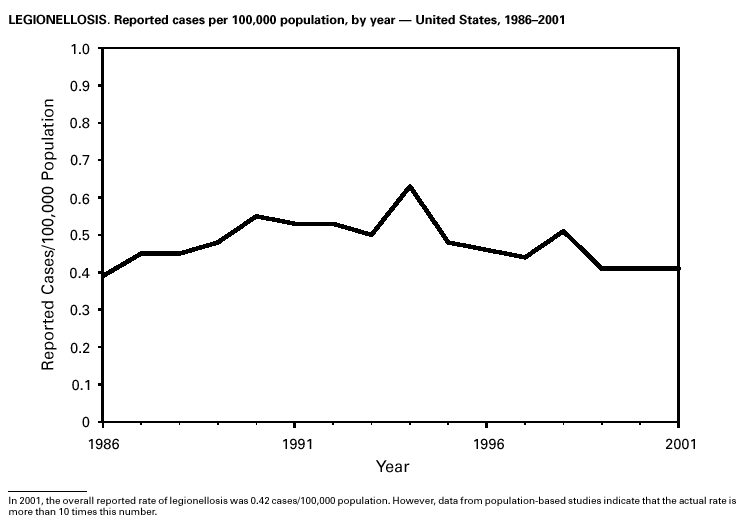
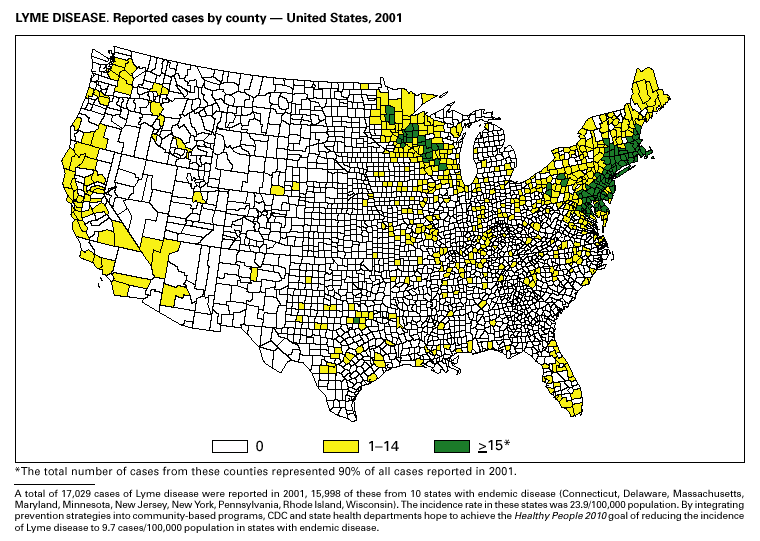
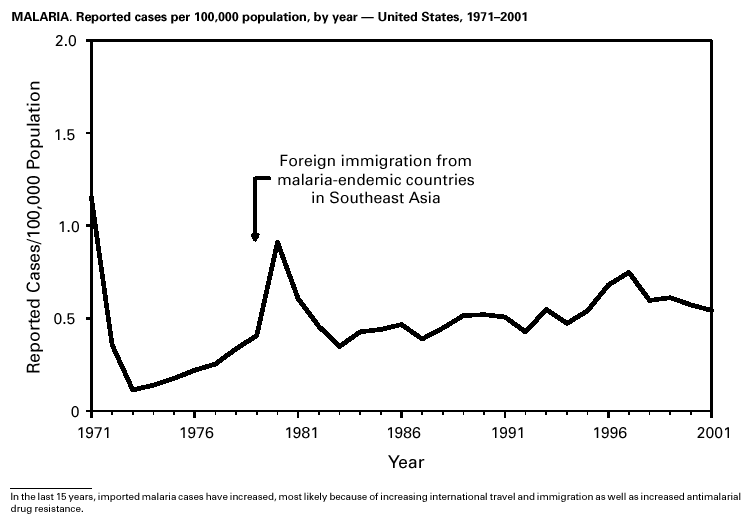
During 2001, 17,029 cases of Lyme disease were reported, most from the northeastern and north-central United States. During 1991–2000, the reported incidence of Lyme disease nearly doubled (1). LYMErix ® , the Lyme disease vaccine produced by GlaxoSmithKline Pharmaceuticals, was removed from the market in February 2002 and is no longer available. CDC promotes community-based Lyme disease prevention using strategies aimed at reducing vector tick densities and preventing human infection and is currently funding such projects in Connecticut, Massachusetts, New Jersey, and New York. MalariaDuring 2001, 1,544 malaria cases were reported in the United States. Most cases were imported, with twice as many cases occurring among U.S. residents traveling to malarious areas as occurred among foreign residents immigrating to or visiting the United States (1). Although the number of reported cases was similar to 2000 (1,591) (2), the annual number of cases has increased during the past 15 years. This increase was likely caused by increases in both international travel (3) and immigration (4), as well as the spread and intensification of antimalarial drug resistance globally (5).
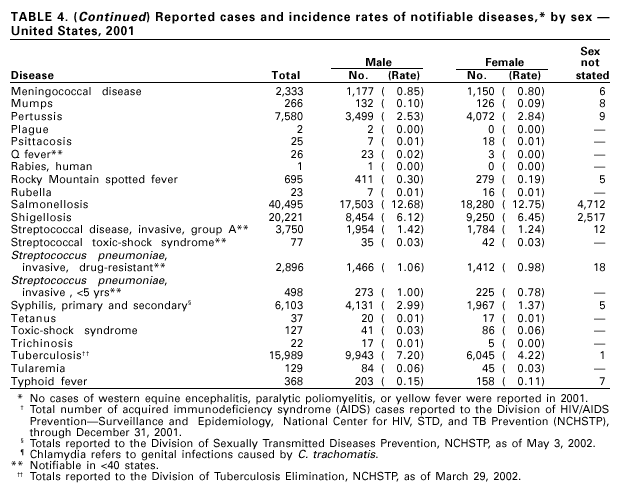
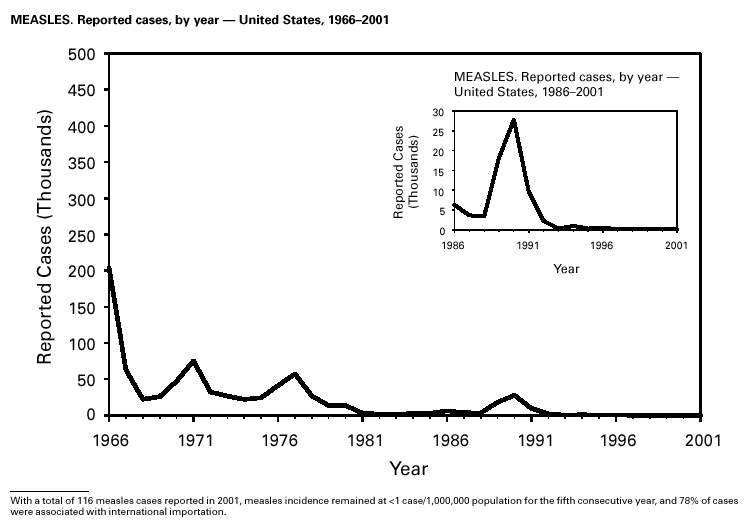
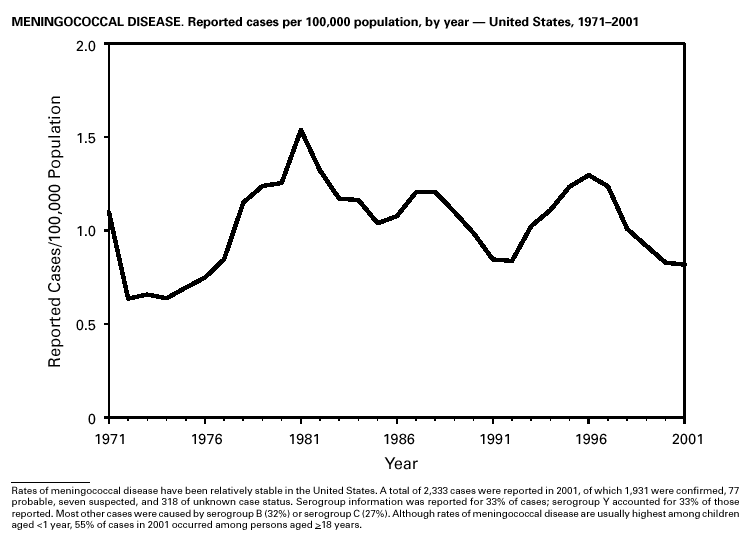
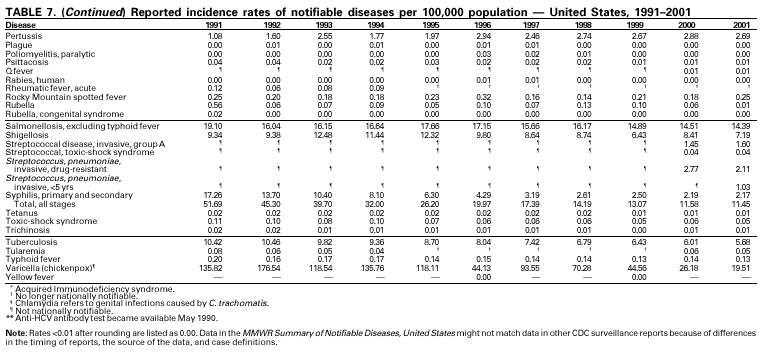
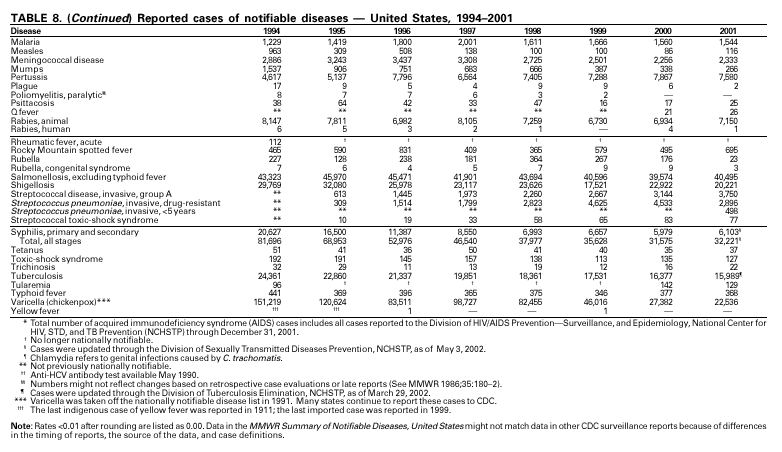
A total of 116 confirmed measles cases were reported in 2001; cases occurred in 22 states. Fifty-four of the cases were internationally imported, and exposure to these cases resulted in 25 additional cases. Twelve other cases had virologic evidence of importation (i.e., genotypic analysis of measles viruses indicated an imported source). The remaining 25 cases were classified as unknown source cases because no link to importation was detected. The majority of confirmed measles cases (61 cases) occurred in persons aged >20 years; 29 cases occurred in persons 5–19 years, and 26 occurred in children aged <5 years. Ten outbreaks, ranging in size from 3 to 14 cases, accounted for 49% of cases (n = 57). All 10 outbreaks were linked to international importation; nine had an epidemiologic link to imported cases and one had virologic evidence of importation. Meningococcal diseaseRates of meningococcal disease have been relatively stable in the United States. A total of 2,333 cases were reported in 2001, of which 1,931 were confirmed, 77 probable, seven suspected, and 318 of unknown case status. Serogroup information was reported for 33% of cases, and serogroup Y accounted for 33% of those reported. Most other cases were caused by serogroup B (32%) or serogroup C (27%). Although rates of meningococcal disease are usually highest among children aged <1 year, 55% of cases in 2001 occurred among persons aged >18 years. Using the technology applied to the development of Haemophilus influenzae type b (Hib) conjugate vaccines, several companies are in the final stages of developing and testing meningococcal conjugate vaccines with various serogroup-specific formulas and in combination with other antigens for licensure in the United States (1). Three serogroup C meningococcal conjugate vaccines were licensed and integrated into routine childhood immunization in the United Kingdom in 2000; early results confirm 85%–95% efficacy in infants, toddlers and teenagers (2) and suggest herd immunity.
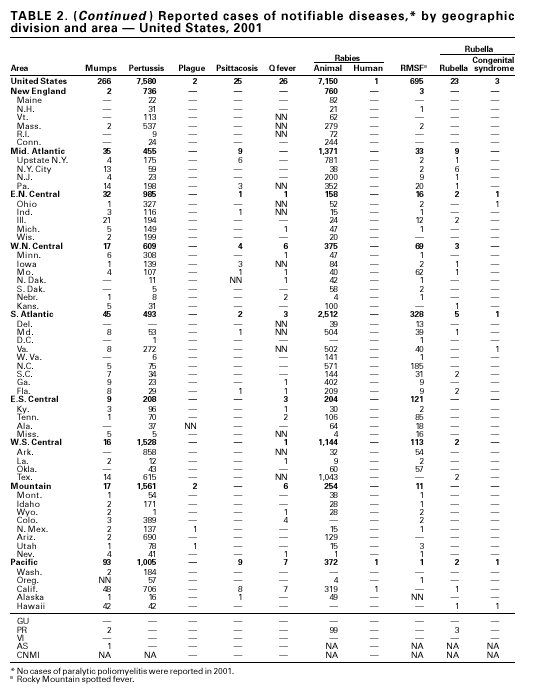
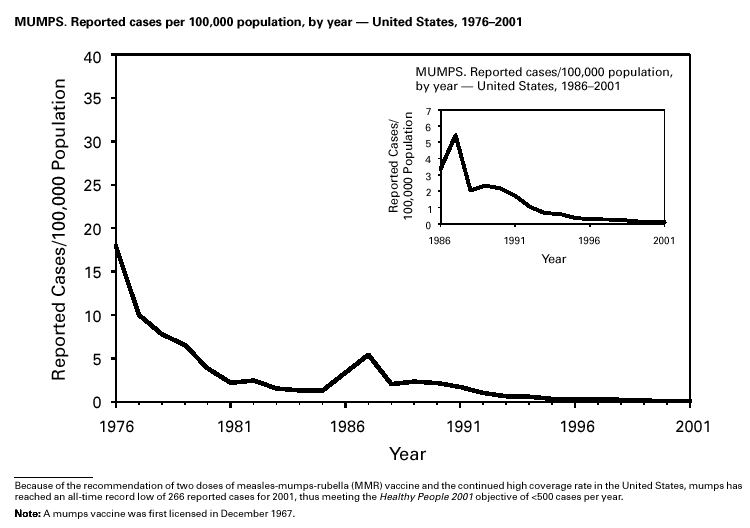
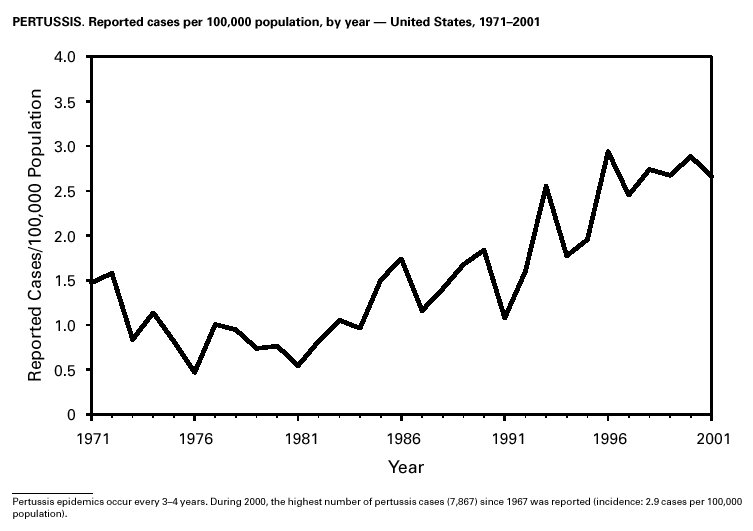
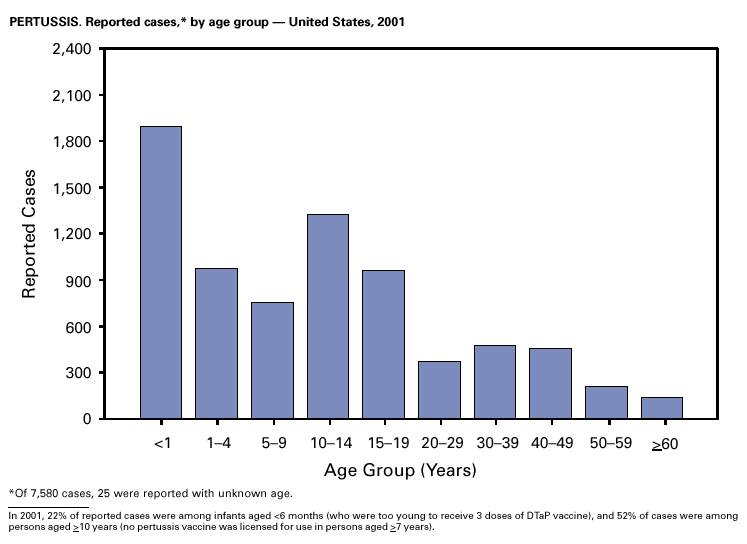
Because of the recommendation of two doses of Measles/Mumps/Rubella vaccine and its high coverage rate in the United States, mumps is at record low levels. During the 1990s, mumps cases declined substantially, from 5,292 reported cases in 1990 to 266 reported cases in 2001, meeting the Healthy People 2000 objective of <500 cases per year (1). PertussisDuring 2001, a total of 7,580 cases of pertussis were reported. Of these, 22% occurred among infants aged <6 months, who were too young to have received the recommended three doses of diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine; 3% occurred among children aged 6–11 months; 13% among preschool-aged children (i.e., those aged 1–4 years); 10% among children aged 5–9 years; 30% among persons aged 10–19 years; and 22% among persons aged >20 years. Since 1995, the coverage rate with >3 doses of a pertussis-containing vaccine has been >94% among U.S. children aged 19–35 months (1). Since 1980, the number of reported cases of pertussis in infants aged <7 months and in adolescents and adults has increased markedly in some states (2). The reasons for this rise are unknown but could include increased awareness of pertussis among health-care providers, increased use of more sensitive diagnostic tests, better reporting of cases to health departments, and possibly an increase in circulating pertussis. In contrast, the incidence of reported pertussis among children aged 7 months to 9 years has not increased markedly and suggests protection against pertussis. Adolescents and adults can become susceptible to disease because vaccine-induced immunity is believed to wane approximately 5–10 years after pertussis vaccination.
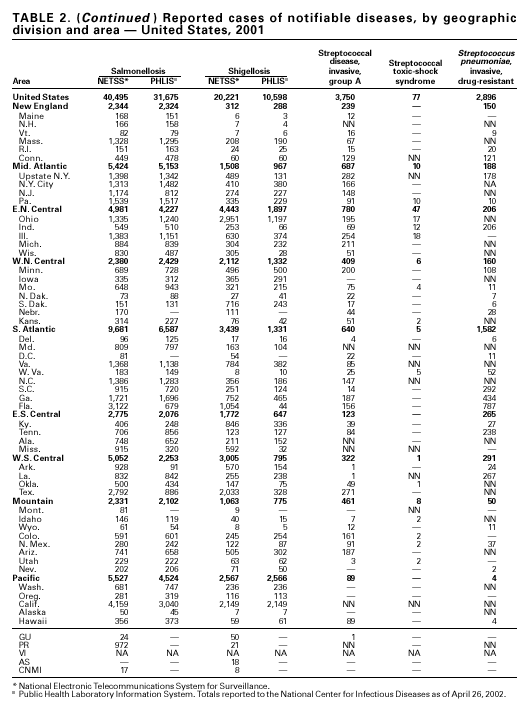
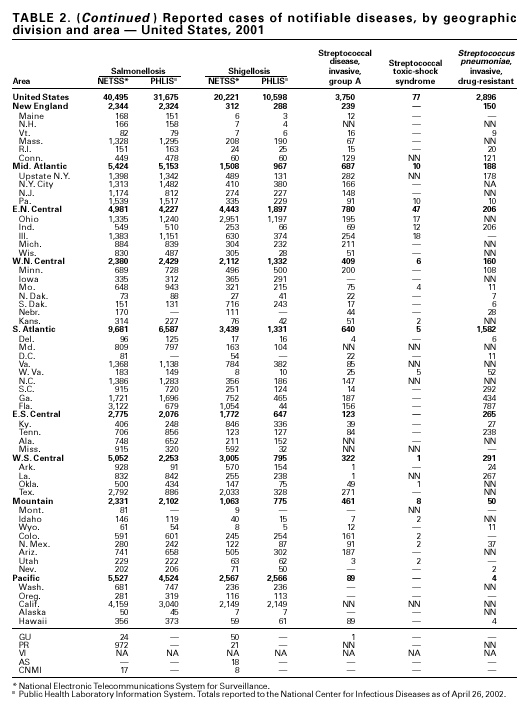
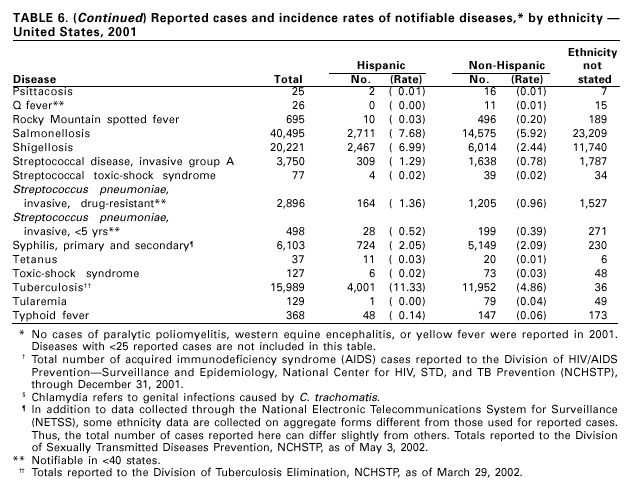
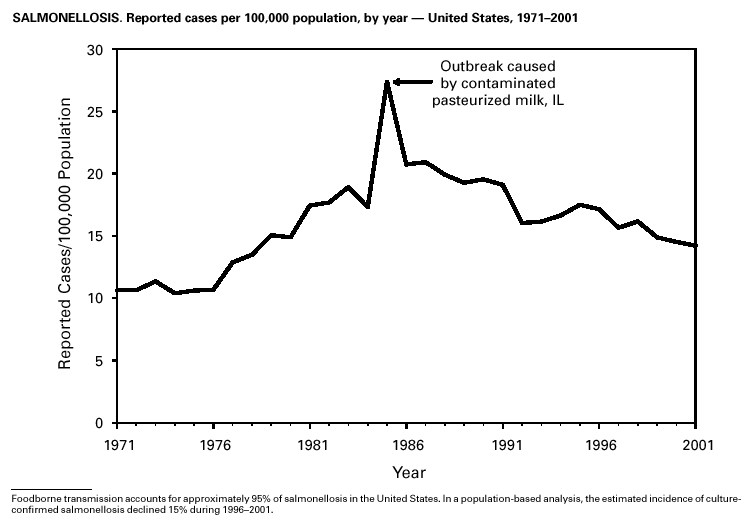
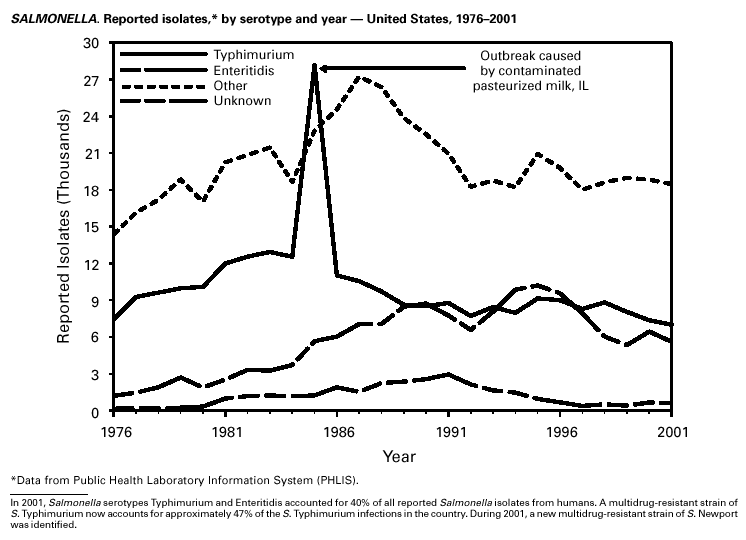
Because of the success of the U.S. rubella vaccination program, rubella is at a record low level, with 23 reported cases in 2001. Rubella now mostly occurs among adults born in countries that do not have routine rubella vaccination programs or that have only recently implemented such programs. In 2000 and 2001, 10 mothers of the 11 children with reported congenital rubella syndrome were foreign-born Hispanics. SalmonellosisA total of 40,495 salmonellosis cases were reported in 2001, an 11% decrease from 46,831 cases in 1995. Salmonella isolates are reported through the Public Health Laboratory Information System by serotype (1). Of >2,000 known Salmonella serotypes, the three most commonly reported in 2001 were S. Typhimurium, S. Enteritidis, and S. Newport; these accounted for 50% of isolates reported. During the 5-year period 1997–2001, the number of S. Newport isolates increased from 5% to 10% of all reported Salmonella isolates. The increasing number of S. Newport infections in the United States is concurrent with the emergence and rapid dissemination of multidrug-resistant strains of S. Newport with resistance to at least nine antimicrobial drugs. Some strains are also resistant to third-generation cephalosporins such as ceftriaxone, which may be used to treat serious infections. Several outbreaks caused by multidrug-resistant S. Newport have been investigated, including one in which raw or undercooked ground beef was implicated (2).
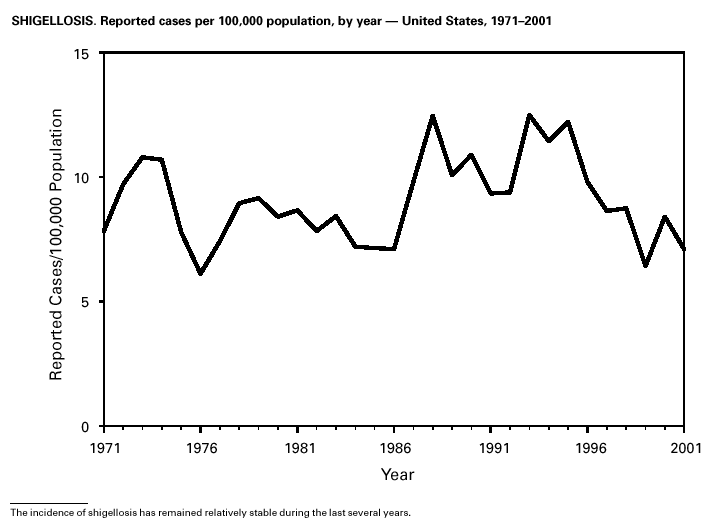
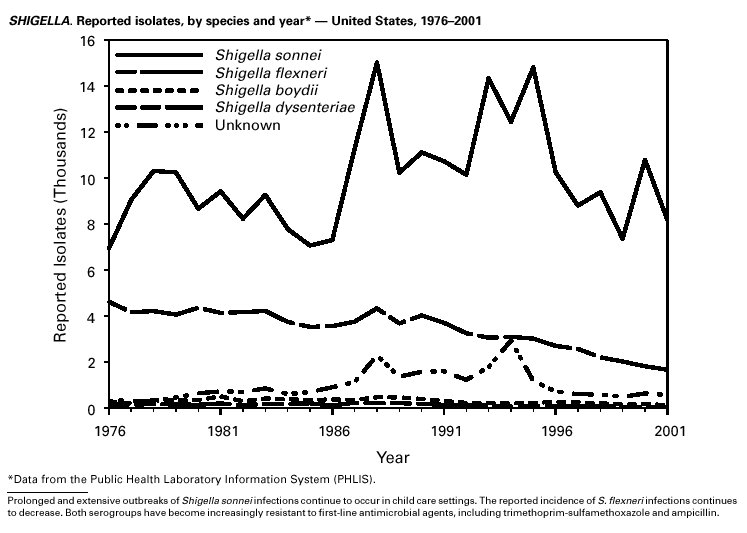
Shigella sonnei infections continue to account for approximately 75% of shigellosis in the United States. Prolonged, communitywide outbreaks of S. sonnei infections that are transmitted in child care centers and other settings where maintenance of good hygienic conditions requires special care account for much of the problem (1). In 2001, one such outbreak in Ohio and Kentucky accounted for several hundred laboratory-confirmed infections. S. sonnei can also be transmitted through contaminated foods and through water used for drinking or recreational purposes (2,3). Recent evidence suggests that S. sonnei infections are increasing among men who have sex with men (4).
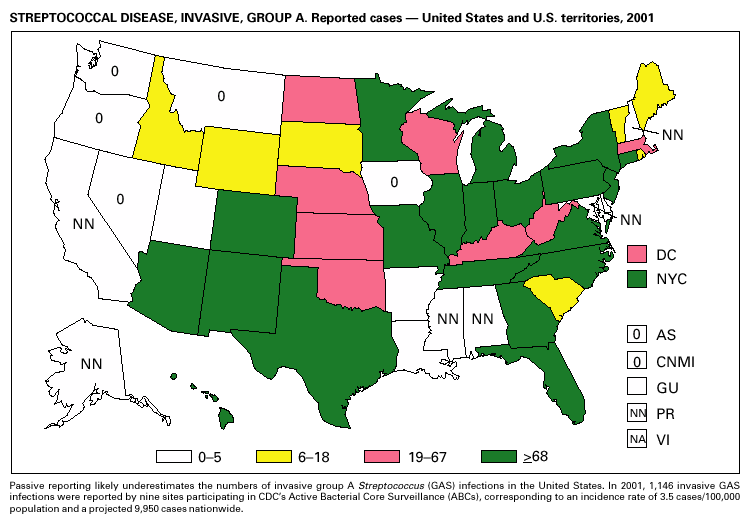
During 2001, 1,147 cases of invasive group A streptococcal (GAS) disease were reported from nine states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee) through the Active Bacterial Core Surveillance (ABCs) project under CDC’s Emerging Infections Program (1). Based on these 1,147 cases, CDC estimates that approximately 9,930 cases of invasive GAS disease (rate: 3.5/100,000) and 1,350 deaths occurred nationally during 2001. Disease incidence was highest among children aged <1 year (5.5/100,000) and adults aged >65 years (9.9/ 100,000). Streptococcal toxic-shock syndrome and necrotizing fasciitis accounted for approximately 5.9% and 6.7% of invasive cases, respectively. The overall case-fatality rate among persons with invasive GAS disease was 13.2%. In 2002, CDC published recommendations for the control of invasive group A streptococcal disease among household contacts of persons with invasive GAS infections and for responding to postpartum and postsurgical infections. These recommendations are based on routine surveillance data, studies of the epidemiology of subsequent invasive GAS infections among household contacts of case-patients and postpartum and postsurgical GAS clusters, and studies of the effectiveness of chemoprophylactic regimens for eradicating carriage (2).
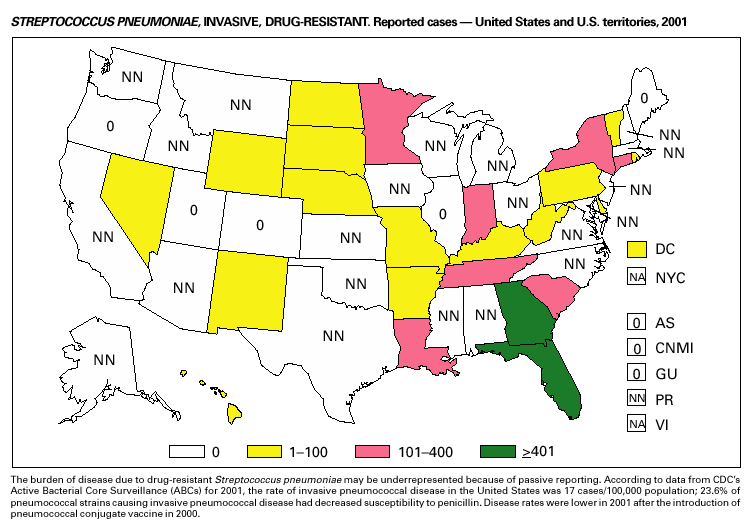
In 2001, the ABCs project of CDC’s Emerging Infections Program(1) collected information on invasive pneumococcal disease, including drug-resistant Streptococcus pneumoniae, in nine states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee). For the first time, the proportion of pneumococcal isolates that were drug resistant was lower in the current year than reported in the previous year. Of the 3,418 S. pneumoniae isolates collected in 2001, 9.7% exhibited intermediate resistance to penicillin (minimum inhibitory concentration [MIC] 0.1–1 µg/mL), and 15.6% were fully resistant (MIC >2 µg/mL); in 2000, 9.8% were intermediate and 17.1% were fully resistant (2). For cefotaxime, 10.5% of all isolates had intermediate resistance and 5.7% were fully resistant in 2001, compared with 9.8% of all isolates with intermediate resistance and 7.5% fully resistant in 2000. For erythromycin, 19.4% were resistant in 2001 versus 21.3% in 2000. Approximately one in six (16.9%) isolates had reduced susceptibility to at least three classes of drugs commonly used to treat pneumococcal infections, a decline from approximately one fifth (18.9%) of isolates in 2000. In February 2000, the Food and Drug Administration licensed a pneumococcal conjugate vaccine for use in infants and young children. In October 2000, the Advisory Committee on Immunization Practices issued recommendations for use of the vaccine in children aged <5 years (3). Among isolates from children aged <5 years reported to ABCs during 2001, 63.9% of all strains (n = 587) and 75.9% of strains not susceptible to penicillin (n = 199) were serotypes included in this 7-valent vaccine.
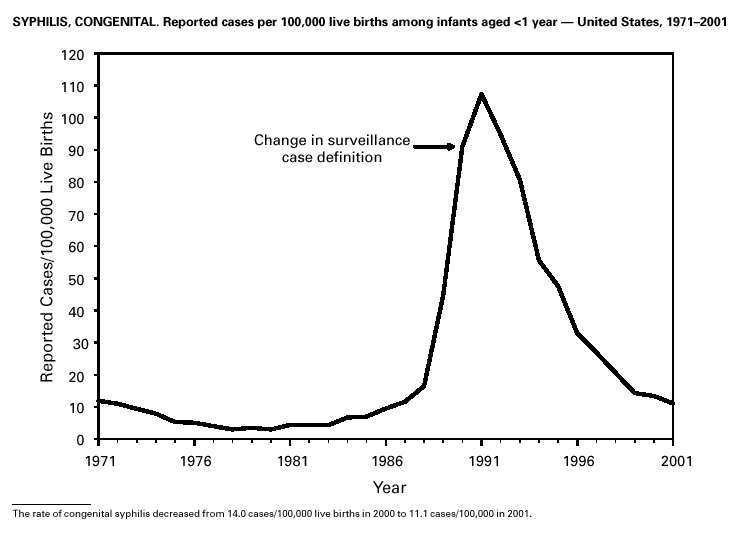
Invasive Streptococcus pneumoniae infection in children aged <5 years was reportable in 28 states and the District of Columbia in 2001. Of these 29 jurisdictions with mandated reporting, only 11 states and the District of Columbia reported cases. The incidence rate in these reporting areas was 13.3/100,000, which is lower than the rate of 39.7 cases/100,000 population estimated from data collected through the Active Bacterial Core Surveillance (CDC, unpublished data). Syphilis, CongenitalDuring 2001, a total of 441 cases of congenital syphilis were reported (rate: 11.1/ 100,000 live births). Like primary and secondary syphilis, the rate of congenital syphilis has declined sharply in recent years, from a peak of 107.3/100,000 in 1991 (1). The continuing decrease in the rate of congenital syphilis likely reflects the substantial reduction in the rate of primary and secondary syphilis among women that has occurred in the last decade. Congenital syphilis persists in the United States because a substantial number of women do not receive syphilis serologic testing until late in their pregnancy or not at all. This lack of screening is often related to absent or late prenatal care (2).
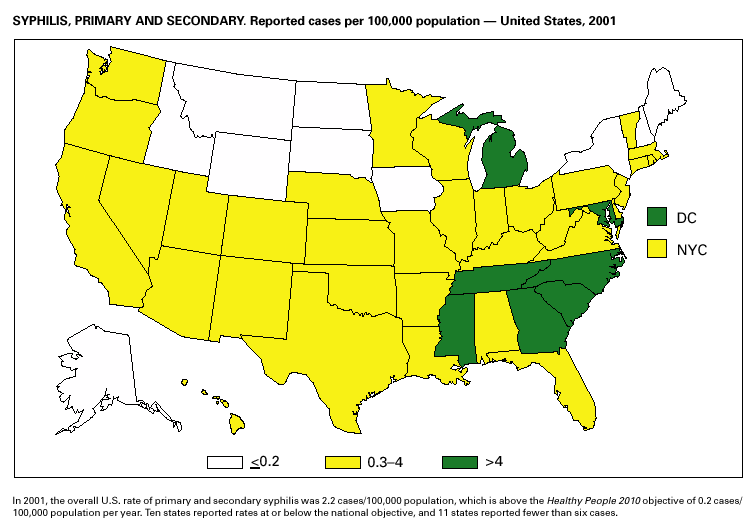
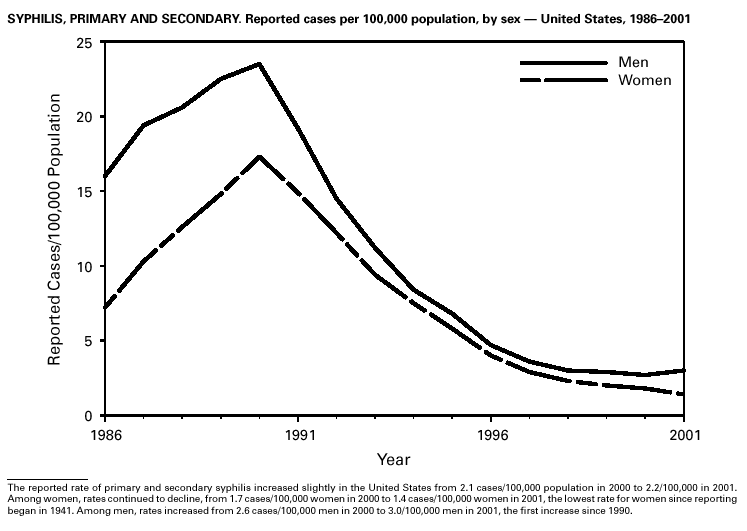
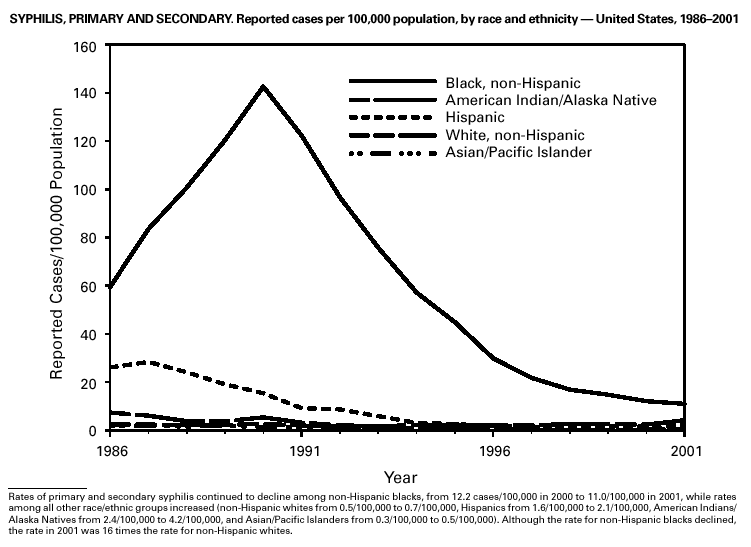
During 2001, a total of 6,103 primary and secondary syphilis cases were reported. From 1990 to 2000, the primary and secondary syphilis rate declined 90%, from 20.34/ 100,000 to 2.12/100,000. The overall 2001 rate (2.17/100,000) is a 2% increase from the 2000 rate, which was the lowest since reporting began in 1941 (1) and the first annual increase since 1990. The 2001 primary and secondary syphilis rate reflects a 15.4% increase among men but a 17.7% decrease among women. This disparity between men and women, observed across all racial and ethnic groups, along with reported outbreaks of syphilis among men who have sex with men (MSM) in large urban areas, suggests that increases in syphilis are occurring among MSM. Rates also remain disproportionately high in the South and among non-Hispanic blacks. (2,3).
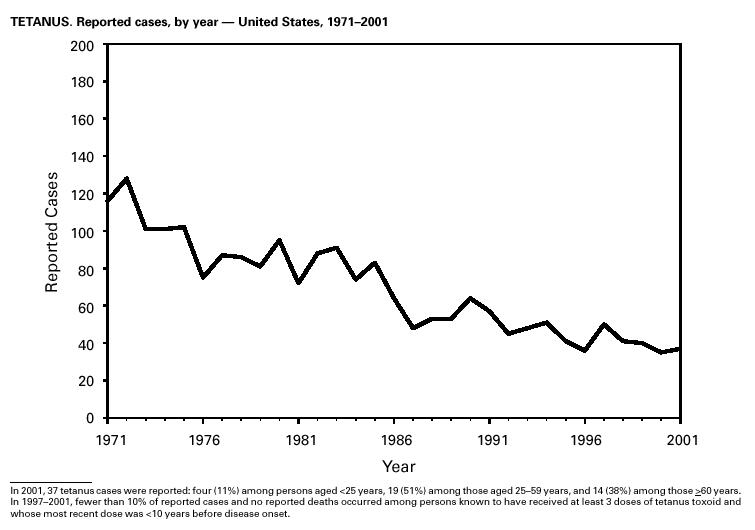
In 2001, 37 cases of tetanus were reported from 15 states. Four (10.8%) cases were among persons aged <25 years, 19 (51.4%) cases were among persons aged 25–59 years, and 14 (37.8%) cases were among persons aged >60 years. The percentage of cases among persons aged 25–59 years has increased during the last decade; previously, most cases were among persons aged >60 years (1). One neonatal case with an atypical presentation of tetanus was reported from California. The mother of the infant was foreign born and had an unknown vaccination status. The infant recovered after 30 days of hospitalization. Six (16.7%) of the non-neonatal cases were fatal. TuberculosisDuring 2001, a total of 15,989 cases (rate: 5.6/100,000 population) of tuberculosis (TB) were reported to CDC from the 50 states and the District of Columbia, representing a 2% decrease from 2000 and a 40% decrease from 1992, when the number of cases and the case rate most recently peaked in the United States (1). In 1991, 73% of reported cases were among U.S.-born persons (rate: 8.2/100,000), and 27% were among foreign-born persons (33.9/100,000). In comparison in 2001, there was an equal distribution (50%) in the number of TB cases among these two groups (case rates: 3.1/100,000 for U.S.-born persons and 26.6/100,000 for foreign-born persons) (1). Despite the decrease in case rate among foreign-born persons during the past decade, half of the TB cases in the United States in 2001 occurred in this population, and the case rate was eight times greater in this population than among U.S.-born persons. To address the high rate, CDC is collaborating with public health partners to implement TB control initiatives among recent international arrivals and residents along the border between the United States and Mexico and to strengthen TB programs in countries with a high incidence of TB disease (2). CDC has recently updated its comprehensive national action plan to reflect the alignment of its priorities with the Institute of Medicine report (3) and to ensure that priority prevention activities are undertaken with optimal collaboration and coordination among national and international public health partners (4).
In 2001, typhoid fever was diagnosed in 368 persons in the United States. Despite the availability of two effective vaccines, NNDSS reports 350–450 cases each year. Approximately 80% of these cases occur among persons who report international travel during the 6 weeks before illness. Persons visiting friends and relatives in their country of origin appear to be at high risk (1). In many areas of the world, Salmonella Typhi strains have acquired resistance to multiple antimicrobial agents, including ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole (1). S. Typhi outbreaks in the United States are generally small in size, but they can cause significant morbidity and are often foodborne, warranting thorough investigation (2).
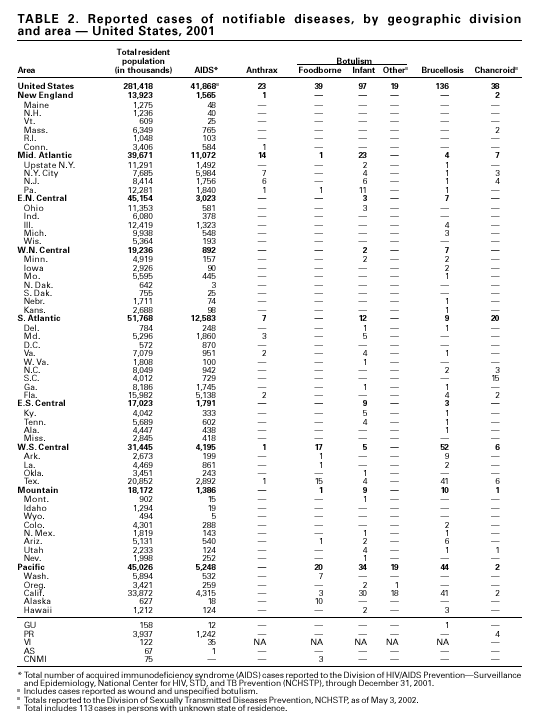
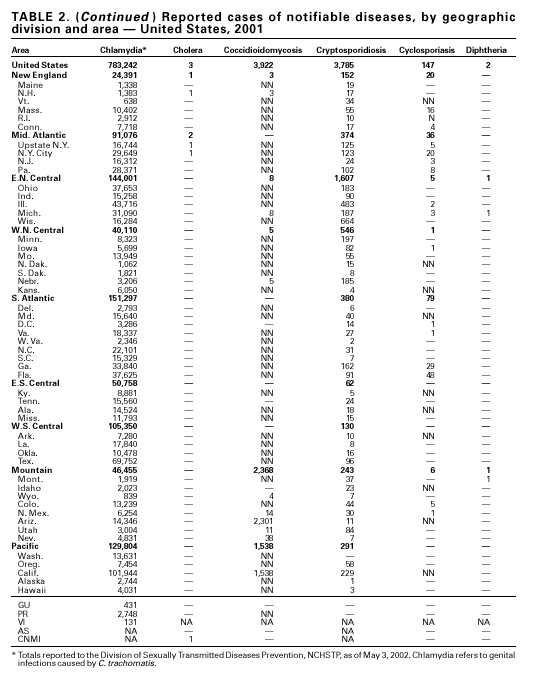
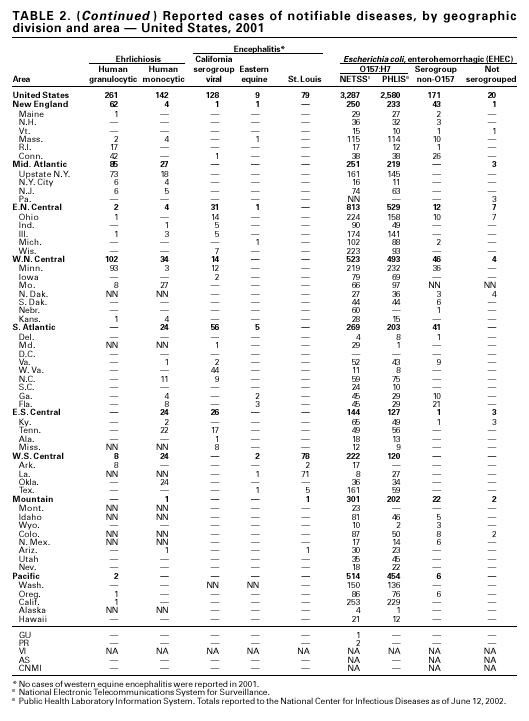
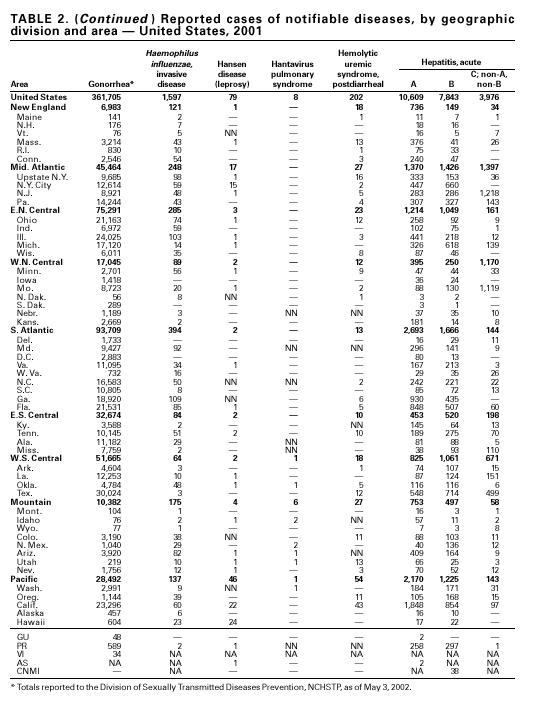
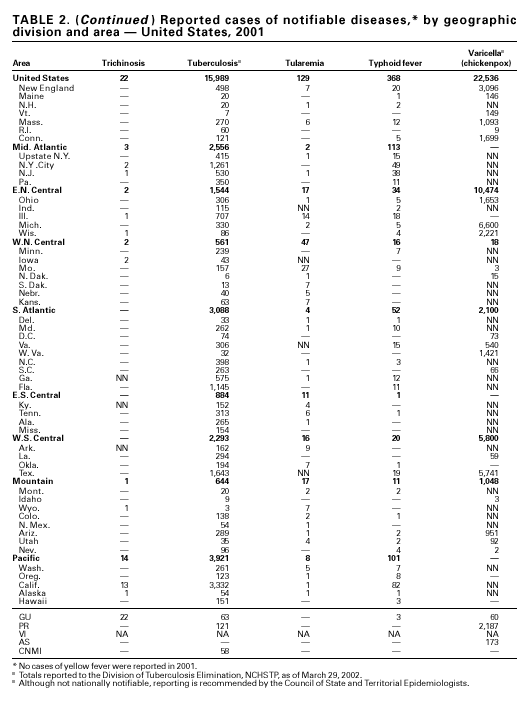
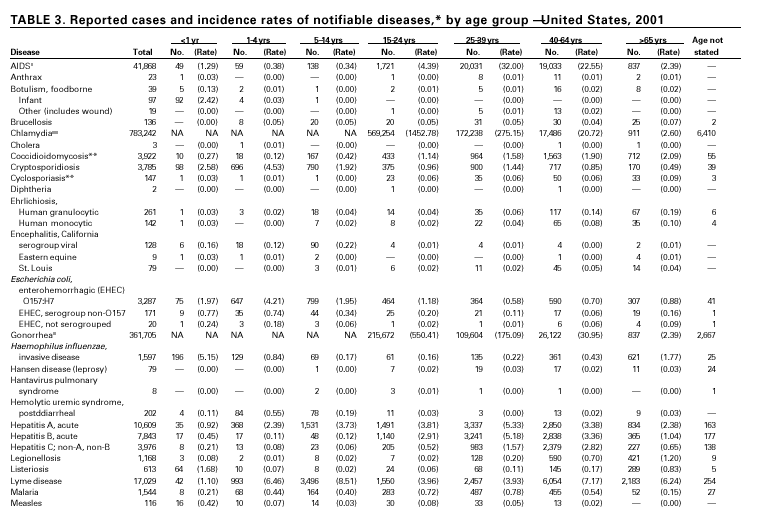
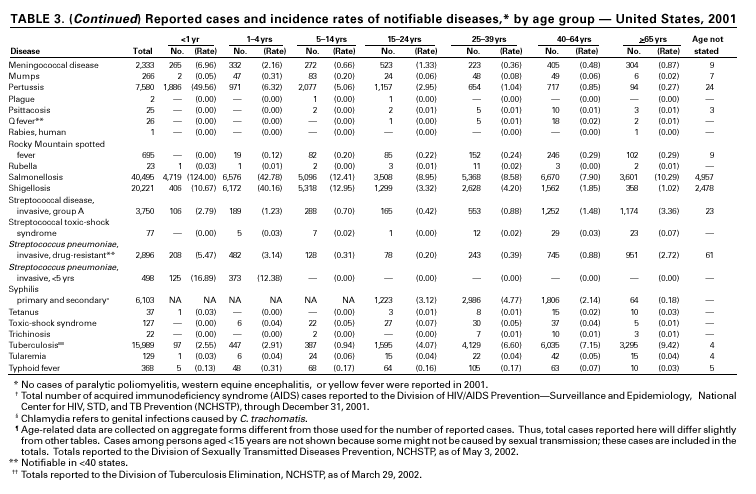
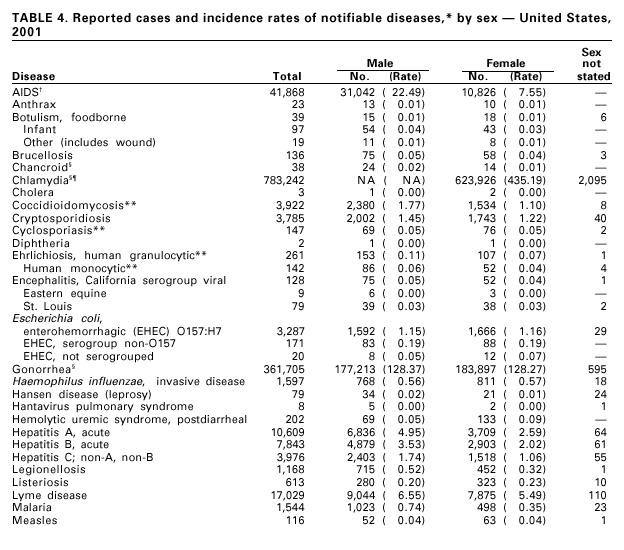
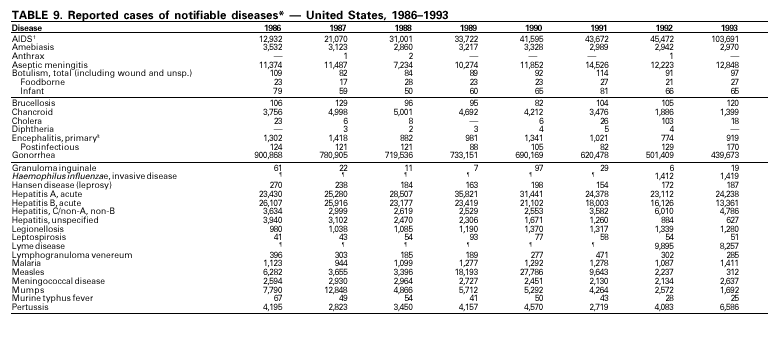
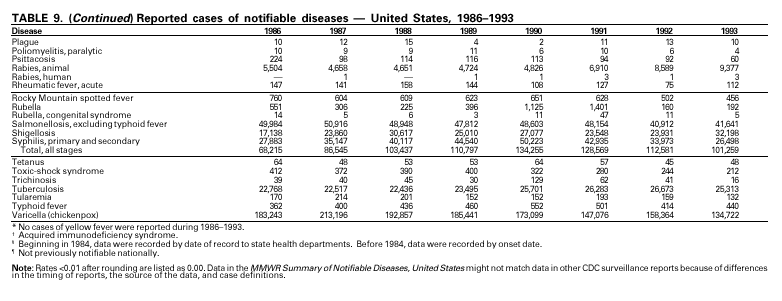
PART 1Summaries of Notifiable Diseases in the United States, 2001
PART 2Graphs and Maps for Selected Notifiable Diseases in the United States
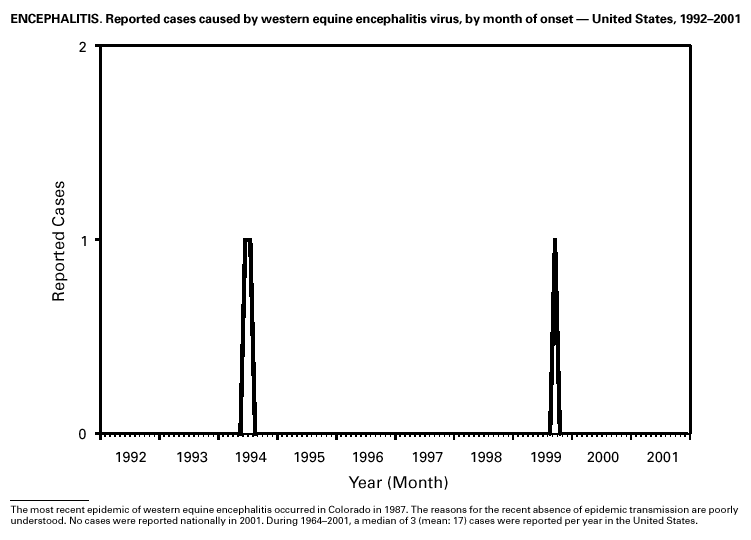
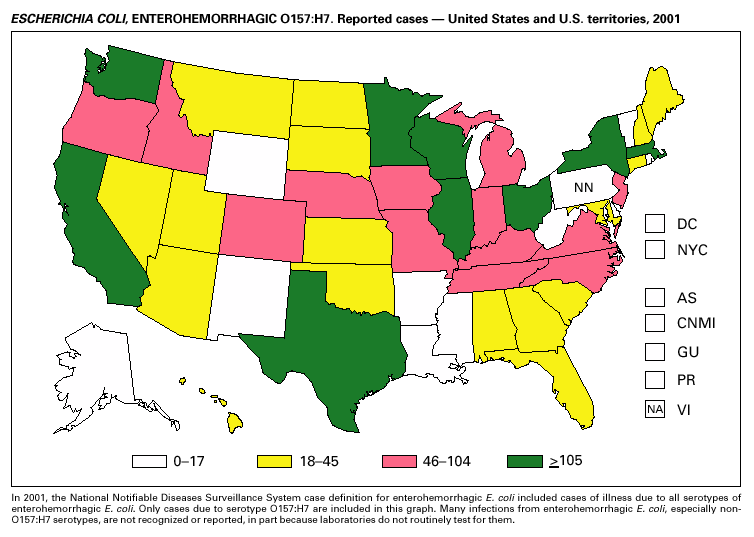
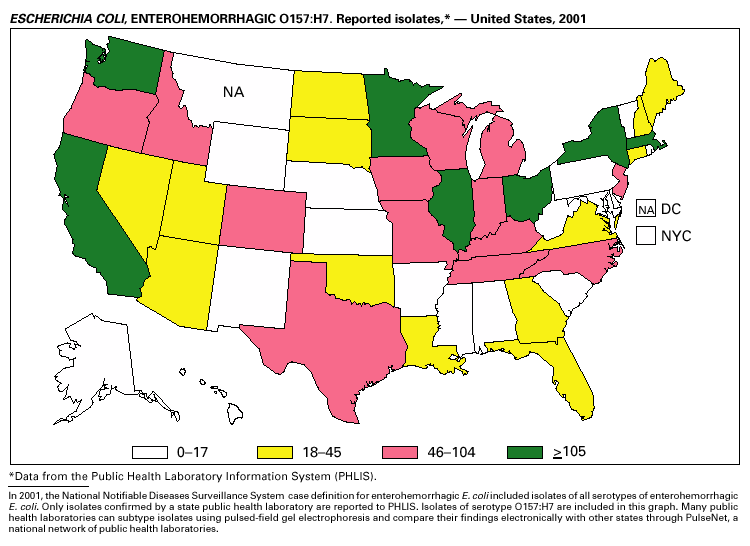
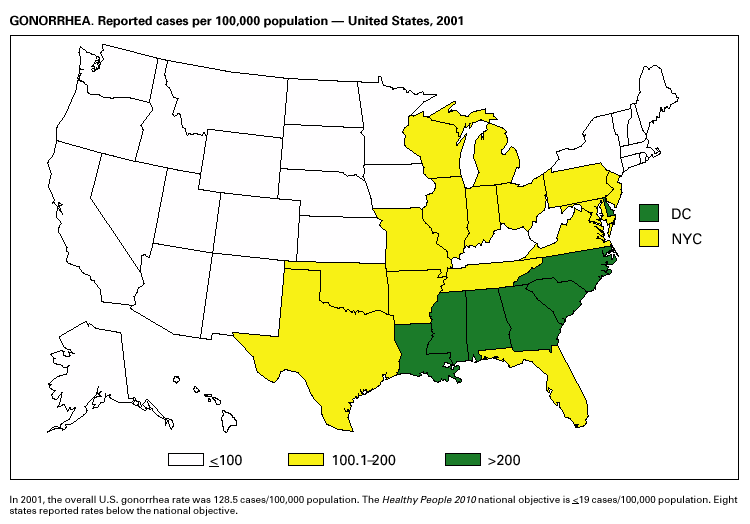
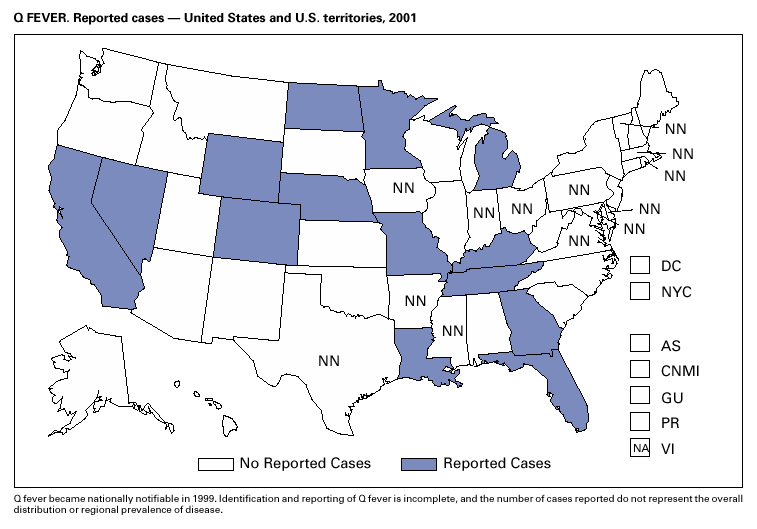
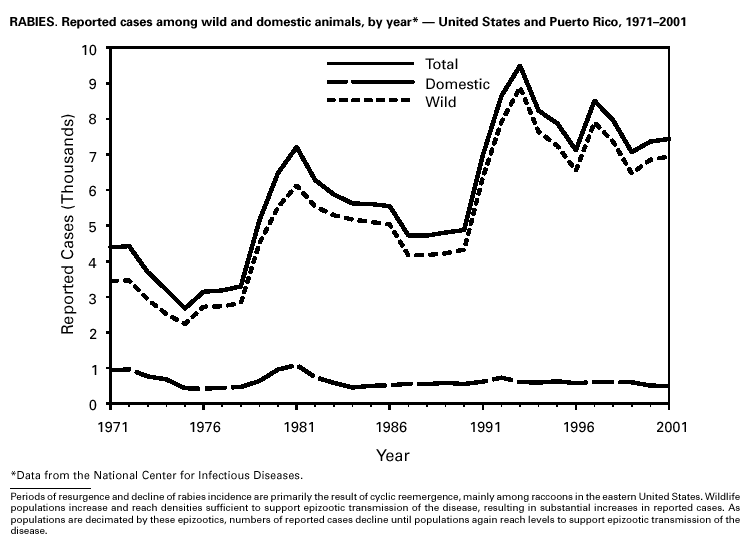
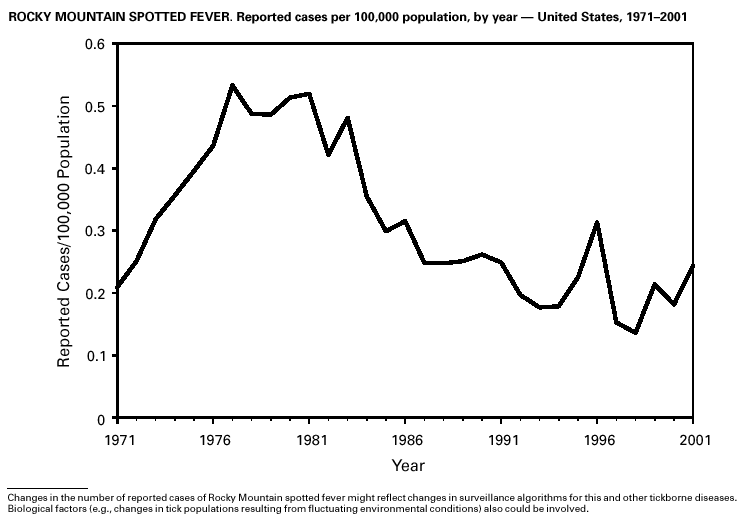
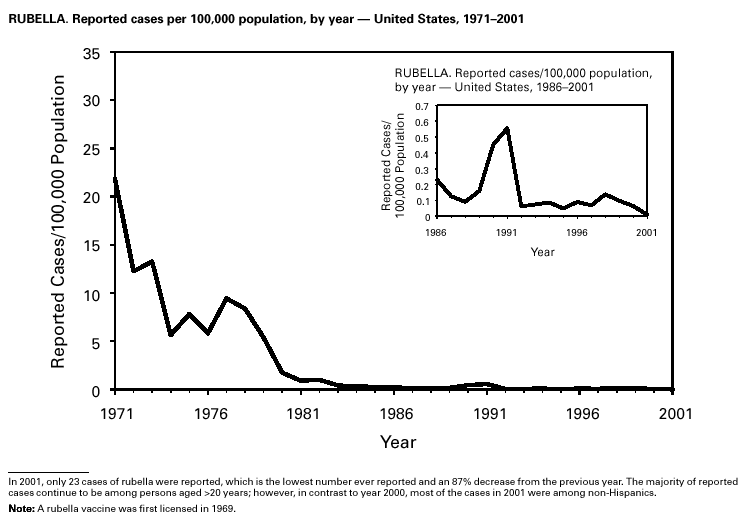
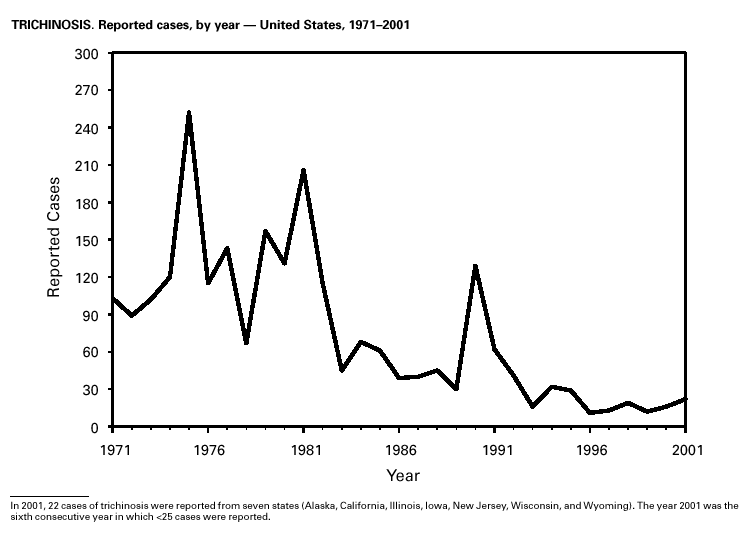
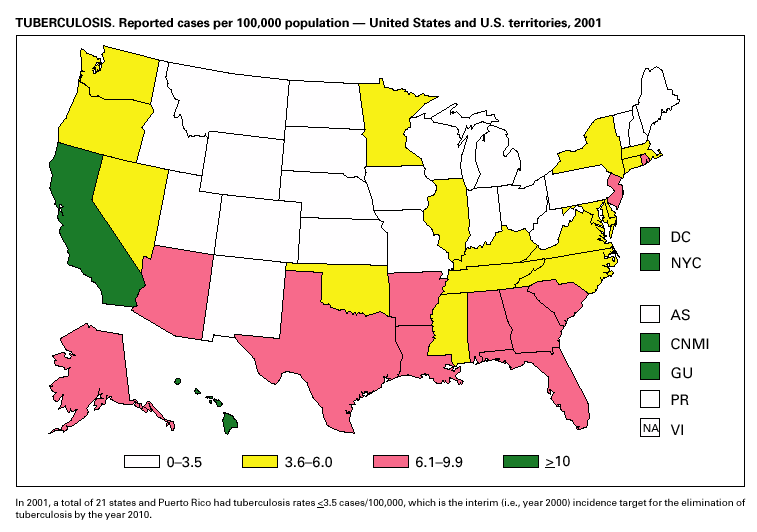
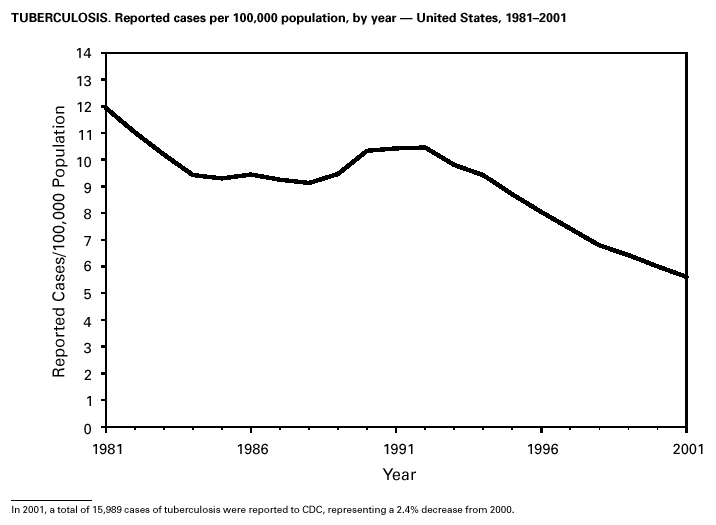
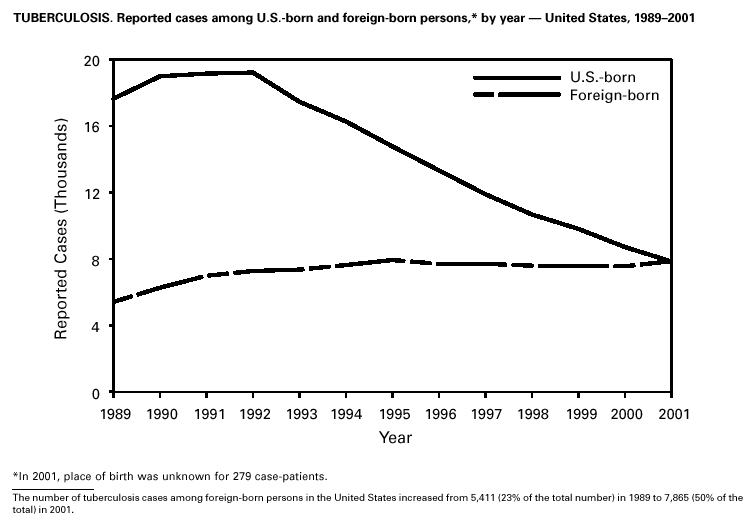
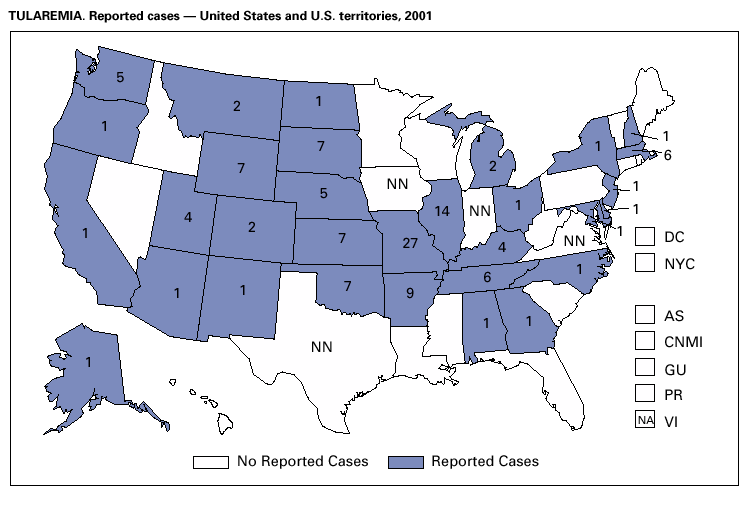
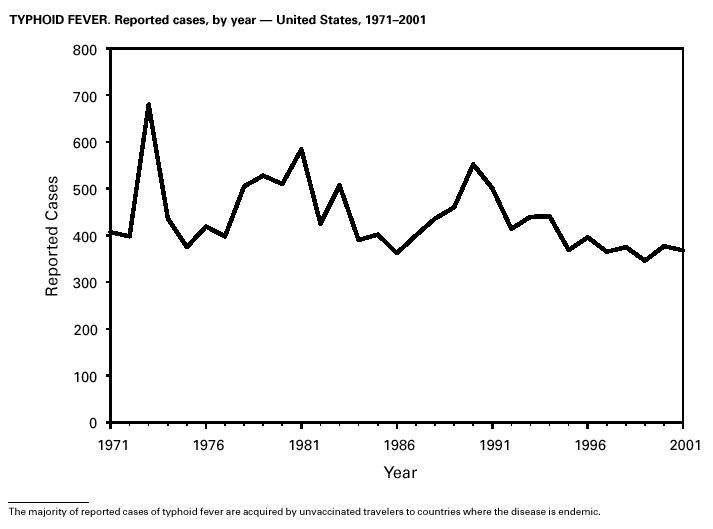
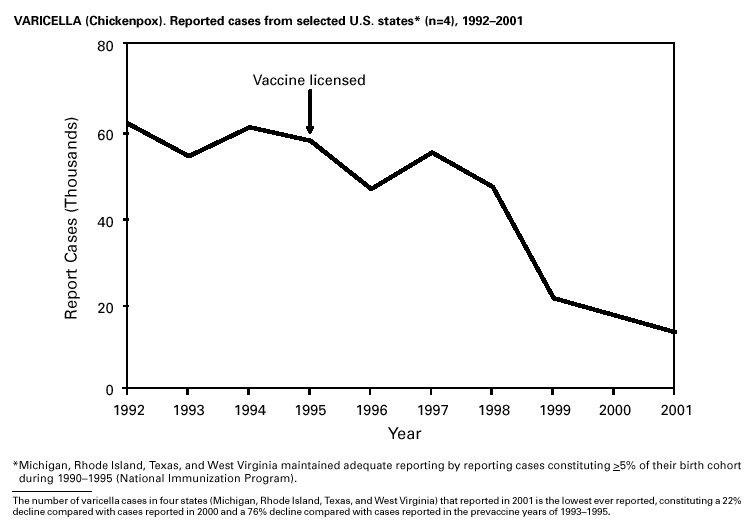
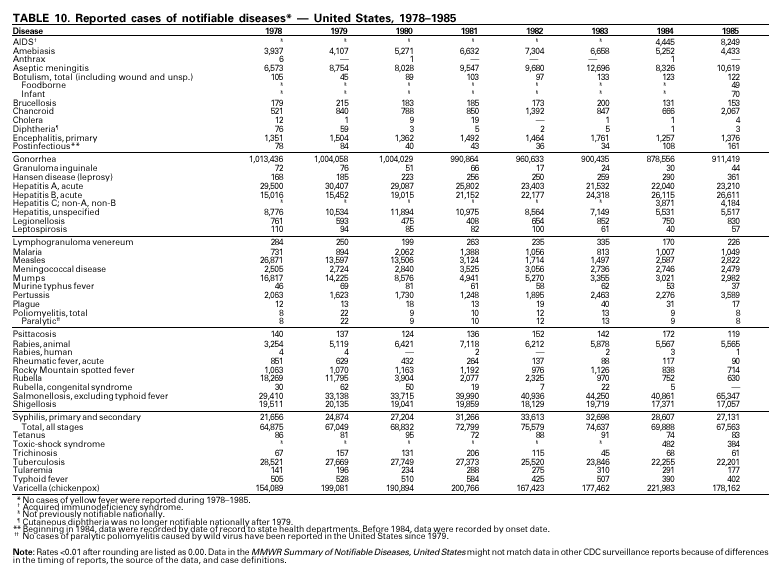
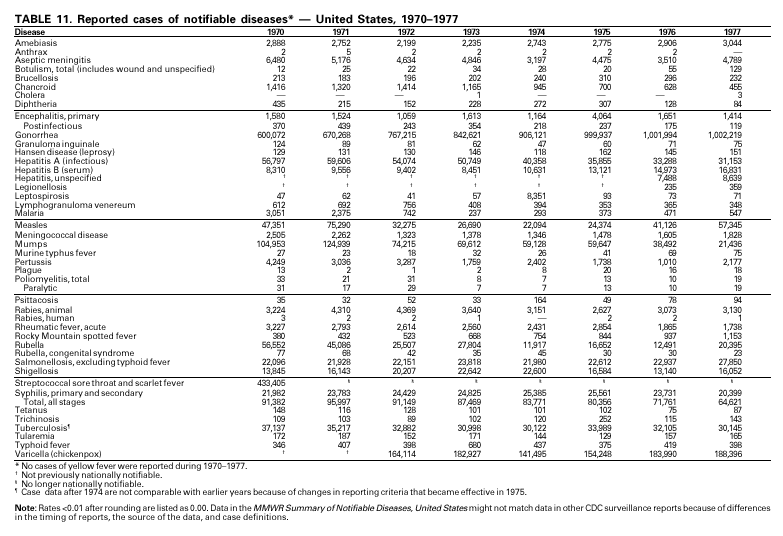
PART 3Historical Summaries of Notifiable Diseases in the United States, 1970–2001
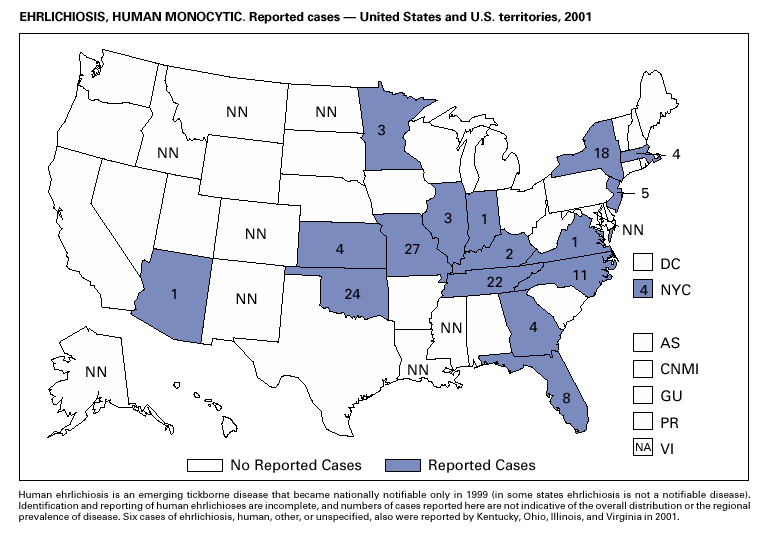
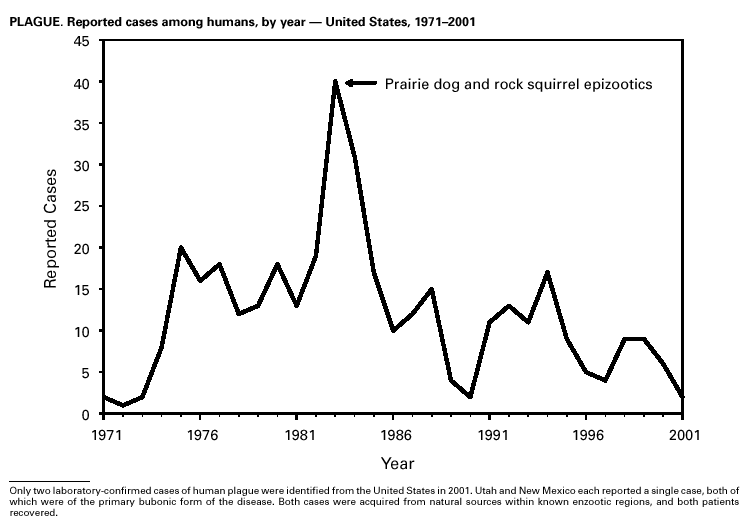
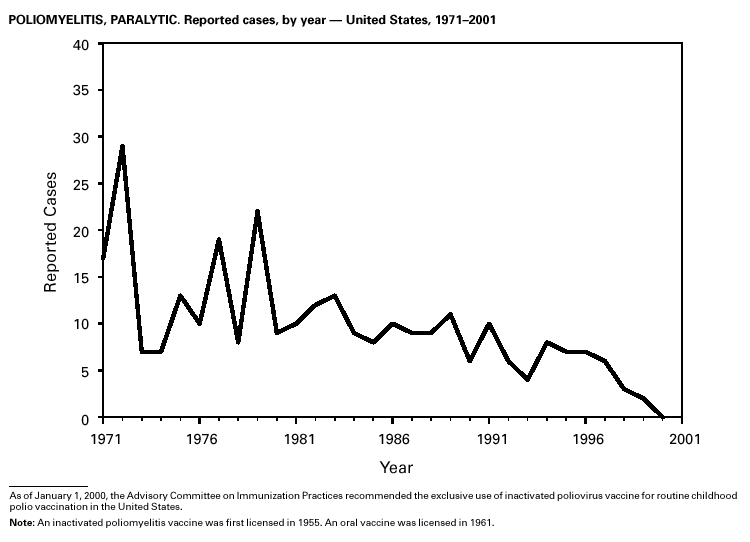
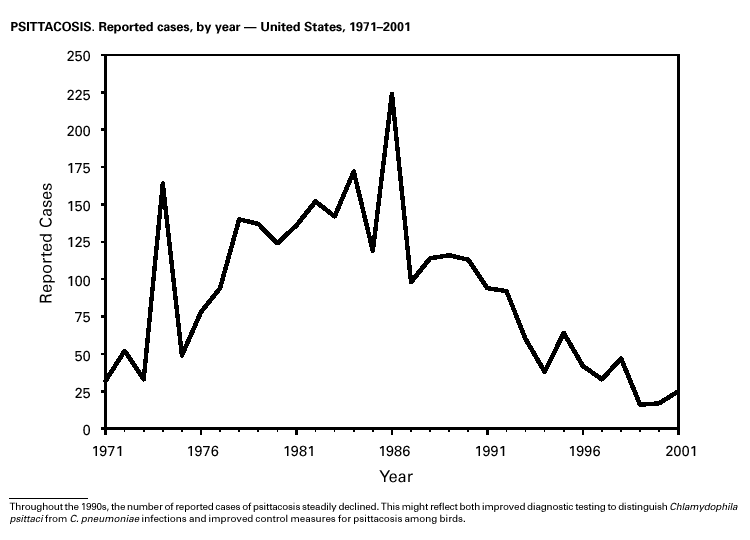
Selected ReadingGeneral Bayer R, Fairchild AL. Public health: surveillance and privacy. Science 2000;290:1898--9. CDC. Framework for program evaluation in public health. MMWR 1999;48(No. RR-11). CDC. Manual of procedures for the reporting of nationally notifiable diseases to CDC. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC, 1995. CDC. Manual for the surveillance of vaccine-preventable diseases. Atlanta: US Department of Health and Human Services, Public Health Service, CDC, 1999. Available at http://www.cdc.gov/nip/publications/surv-manual/begin.pdf. CDC. National Electronic Disease Surveillance System (NEDSS): a standards-based approach to connect public health and clinical medicine. J Public Health Management Practice 2001;7:43--50. CDC. Proceedings of the 1992 International Symposium on Public Health Surveillance. MMWR 1992;41(suppl). CDC. Sexually transmitted disease surveillance 1998. Atlanta: US Department of Health and Human Services, Public Health Service, CDC, 1999. Chin JE, ed. Control of communicable diseases manual. 17th ed. Washington, DC: American Public Health Association, 2000. Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the analytical literature review. Am J Epidemiol 2002;155:866--74. Effler P, Ching-Lee M, Bogard A, Ieong M-C, Nekomoto T, Jernigan D. Statewide system of electronic notifiable disease reporting from clinical laboratories: comparing automated reporting with conventional methods. JAMA 1999;282;1845--50. Freimuth V, Linnan HW, Potter P. Communicating the threat of emerging infections to the public. Emerg Infect Dis 2000;6:337--47. Koo D, Caldwell B. The role of providers and health plans in infectious disease surveillance. Eff Clin Pract 1999;2:247--52. Available at http://www.acponline.org/journals/ecp/sepoct99/koo.htm. Koo D, Wetterhall S. History and current status of the National Notifiable Diseases Surveillance System. J Public Health Management Practice 1996;2:4--10. Lin SS, Kelsey JL. Use of race and ethnicity in epidemiologic research: concepts, methodological issues, and suggestions for research. Epidemiol Rev 2000;22:187--202. Martin SM, Bean NH. Data management issues for emerging diseases and new tools for managing surveillance and laboratory data. Emerg Infect Dis 1995;1:124--8. Available at http://www.cdc.gov/ncidod/eid/vol1no4/martin2.htm#top. Niskar AS, Koo D. Differences in notifiable infectious disease morbidity among adult women---United States, 1992--1994. J Womens Health 1998;7:451--8. Panackal AA, M'ikanatha NM , Tsui FC, et al. Automatic electronic laboratory-based reporting of notifiable infectious diseases at a large health system. Emerg Infect Dis 2002;8:685--91. Pinner RW, Koo D, Berkelman RL. Surveillance of infectious diseases. In: Lederberg J, Alexander M, Bloom RB, eds. Encyclopedia of microbiology. 2nd ed. San Diego, CA: Academic Press, 2000;4:506--25. Pinner RW, Jernigan DB, Sutliff SM. Electronic laboratory-based reporting for public health. Military Medicine 2000;165(suppl 2):20--4. Roush S, Birkhead G, Koo D, Cobb A, Fleming D. Mandatory reporting of diseases and conditions by health care professionals and laboratories. JAMA 1999;282:164--70. Available at http://jama.ama-assn.org/issues/v282n2/abs/joc90413.html. Teutsch SM, Churchill RE, eds. Principles and practice of public health surveillance. 2nd ed. New York, NY: Oxford University Press, 2000. Thacker SB, Choi K, Brachman PS. The surveillance of infectious diseases. JAMA 1983;249: 1181--5. Thacker SB, Stroup DF. Future directions for comprehensive public health surveillance and health information systems in the United States. Am J Epidemiol 1994;140:383--97. AIDS CDC. HIV/AIDS Surveillance Report, 2002.Vol. 13, No. 2. Atlanta: Centers for Disease Control and Prevention. Available at http://www.cdc.gov/hiv/stats/hasrlink.htm. Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use incidence estimates and for clinical and prevention purposes. JAMA 1998;280:42--48. Klevens RM, Fleming PL, Li J, et al. The completeness, validity, and timeliness of AIDS surveillance data. Ann Epidemiol 2001;11:443--9. Botulism CDC. Botulism in the United States, 1899--1996: handbook for epidemiologists, clinicians, and laboratory workers. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC, 1998. Available at http://www.cdc.gov/ncidod/dbmd/diseaseinfo/botulism.pdf. Shapiro RHC, Swerdlow DL, Botulism in the United States: a clinical and epidemiologic review. Ann Intern Med 1998;129:221--8. Brucellosis Yagupsky P. Detection of brucellae in blood cultures. J Clin Microbiol 1999;37:3437--42. CDC. Human exposure to Brucella abortus strain RB51---Kansas, 1997. MMWR 1998;47:172--5. Chomel BB, DeBess EE, Mangiamele DM, et al. Changing trends in the epidemiology of human brucellosis in California from 1973 to 1992: a shift toward foodborne transmission. J Infect Dis 1994;170:1216--23. Taylor JP, Perdue JN. The changing epidemiology of human brucellosis in Texas, 1977--1986. Am J Epidemiol 1989;130:160--5. Chancroid DiCarlo RP, Armentor BS, Martin DH. Chancroid epidemiology in New Orleans men. J Infect Dis 1995;172:446--52. Mertz KJ, Weiss JB, Webb RM, et al. An investigation of genital ulcers in Jackson, Mississippi, with use of a multiplex polymerase chain reaction assay: high prevalence of chancroid and human immunodeficiency virus infection. J Infect Dis 1998;178:1060--6. Mertz KJ, Trees D, Levine WC, et al. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis 1998;178:1795--8. Chlamydia trachomatis, Genital infection CDC. Chlamydia trachomatis genital infections---United States, 1995. MMWR 1997;46:193--8. CDC. Sexually transmitted disease surveillance 1999 supplement: Chlamydia Prevalence Monitoring Project. Atlanta: US Department of Health and Human Services, CDC, 2000. Available at http://www.cdc.gov/nchstp/dstd/Stats_Trends/99Chlamydia.htm. Gaydos CA, Howell MR, Pare B, et al. Chlamydia trachomatis infections in female military recruits. N Engl J Med 1998;339:739--44. Mertz KJ, McQuillan GM, Levine WC, et al. A pilot study of chlamydial infection in a national household survey. Sex Transm Dis 1998;25:225--8. Cholera Mahon BE, Mintz ED, Greene KD, Wells JG, Tauxe RV. Reported cholera in the United States, 1992--1994: a reflection of global changes in cholera epidemiology. JAMA 1996;276:307--12. Mintz ED, Tauxe RV, Levine MM. The global resurgence of cholera. In: Noah ND, O'Mahony M, eds. Communicable disease epidemiology and control. Chichester, England: John Wiley & Sons, 1998:63--104. Steinberg EB, Greene KD, Bopp CA, Cameron DN, Wells JG, Mintz ED. Cholera in the United States, 1995--2000: trends at the end of the millennium. J Infect Dis 2001;184:799--802. World Health Organization. Guidelines for cholera control. Geneva, Switzerland: World Health Organization, 1993. Cyclosporiasis Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis 2000;31:1040--57. Ho AY, Lopez AS, Eberhart MG, et al. Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000. Emerg Infect Dis 2002;8:783--8. Lopez AS, Dodson DR, Arrowood MJ, et al. Outbreak of cyclosporiasis associated with basil in Missouri in 1999. Clin Infect Dis 2001;32:1010--7. Diphtheria Bisgard KM, Hardy IR, Popovic T, et al. Respiratory diphtheria in the United States, 1980 through 1995. Am J Public Health 1998;88:787--91. Dittmann S, Wharton M, Vitek C, et al. Successful control of epidemic diphtheria in the states of the former Union of Soviet Socialist Republics: lessons learned. J Infect Dis 2000;181(suppl 1):S10--S22. Galazka AM, Robertson SE. Diphtheria: changing patterns in the developing world and the industrialized world. Eur J Epidemiol 1995;11:107--17. Ehrlichiosis (Human Granulocytic and Human Monocytic) Belongia EA, Reed KD, Mitchell PD, et al. Tickborne infections as a cause of nonspecific febrile illness in Wisconsin. Clin Infect Dis 2001;32:1434--9. Childs JE, Sumner JW, Nicholson WL, Massung RF, Standaert SM, Paddock CD. Outcome of diagnostic tests using samples from patients with culture-proven human monocytic ehrlichiosis: implications for surveillance. J Clin Microbiol 1999;37:2997--3000. IJdo JW, Meek JI, Cartter ML, et al. The emergence of another tickborne infection in the 12-town area around Lyme, Connecticut: human granulocytic ehrlichiosis. J Infect Dis 2000;181:1388--93. McQuiston JH, Paddock CD, Holman RC, Childs JE. The human ehrlichioses in the United States [Review]. Emerg Infect Dis 1999;5:635--42. Available at http://www.cdc.gov/ncidod/eid/vol5no5/mcquiston.htm Encephalitis, Arboviral (California Serogroup Viral, Eastern Equine, St. Louis, Western Equine, West Nile Virus) Campbell GL, Marfin AM, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infectious Diseases 2002;2:519--29. Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area. N Eng J Med 2001;344:1807--14. Gonorrhea CDC. Gonorrhea---United States, 1998. MMWR 2000;49:538--42. CDC. Sexually transmitted diseases surveillance 2000 supplement: Gonococcal Isolate Surveillance Project (GISP) annual report---2000. Atlanta, GA: US Department of Health and Human Services, CDC, October 2001. Fox KK, del Rio C, Holmes KK, et al. Gonorrhea in the HIV era: a reversal in trends among men who have sex with men. Am J Public Health 2001;91:959--64. Haemophilus influenzae, Invasive Disease Fry AM, Lurie P, Gidley M, Schmink S, Lingappa J, Rosenstein NE. Haemophilus influenzae type b (Hib) disease among Amish children in Pennsylvania: reasons for persistent disease. Pediatrics 2001;108:1--6. Hantavirus Pulmonary Syndrome Douglass RJ, Wilson T, Semmens WJ, et al. Longitudinal studies of Sin Nombre virus in deer mouse-dominated ecosystems of Montana. Am J Trop Med Hyg 2001;65:33--41. Yates TL, Mills JN, Parmenter CA, et al. The ecology and evolutionary history of an emergent disease: hantavirus pulmonary syndrome. BioScience 2002;52:989. Hemolytic Uremic Syndrome, Postdiarrheal Banatvala N, Griffin PM, Greene KD, et al. The United States National Prospective Hemolytic Uremic Syndrome Study: microbiologic, serologic, clinical, and epidemiologic findings. J Infect Dis 2001;183:1063--70. CDC. Escherichia coli O111:H8 outbreak among teenage campers---Texas, 1999. MMWR 2000;49:321--4. Mahon BE, Griffin PM, Mead PS, Tauxe RV. Hemolytic uremic syndrome surveillance to monitor trends in infection with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli [Letter]. Emerg Infect Dis 1997;3:409--12. Available at http://www.cdc.gov/ncidod/eid/vol3no3/mahon.htm. Siegler RL, Pavia AT, Christofferson RD, Milligan MK. A 20-year population based study of postdiarrheal hemolytic uremic syndrome in Utah. Pediatrics 1994;94:35--40. Hepatitis A Armstrong GL, Bell BP. Hepatitis A virus infections in the United States: model-based estimates and implications for childhood immunization. Pediatrics 2002;109:839--45. Bell BP, Shapiro CN, Alter MJ, et al. The diverse patterns of hepatitis A epidemiology in the United States---implications for vaccination strategies. J Infect Dis 1998;178:1579--84. Lemon SM, Shapiro CN. The value of immunization against hepatitis A. Infect Agents Dis 1994;3:38--49. Shapiro CN, Coleman PJ, McQuillan GM, Alter MJ, Margolis HS. Epidemiology of hepatitis A: seroepidemiology and risk groups in the USA. Vaccine 1992;10(suppl 1):S59--S62. Hepatitis B Coleman PJ, McQuillan GM, Moyer LA, Lambert SB, Margolis HS. Incidence of hepatitis B virus infection in the United States, 1976--1994: estimates from the National Health and Nutrition Examination Surveys. J Infect Dis 1998;178:954--9. Goldstein ST, Alter MJ, Williams IT, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982--1998: implications for vaccination programs. J Infect Dis 2002;185:713--9. McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: The National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health 1999;89:14--8. Margolis HS, Alter MJ, Hadler SC. Hepatitis B: evolving epidemiology and implications for control [Review]. Semin Liver Dis 1991;11:84--92. Hepatitis C; Non-A, Non-B Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556--62. Armstrong GA, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777--82. CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR 1998;47(No. RR-19). HIV Infection, Pediatric Lansky A, Jones JL, Frey RL, Lindegren ML. Trends in HIV testing among pregnant women---United States, 1994--1997. Am J Public Health 2001;91:1291--3. Lindegren ML, Steinberg S, Byers RH, Jr. Epidemiology of HIV/AIDS in children. Pediatr Clin North Am 2000; 47(1):1--20. HIV Infection, Adult CDC. HIV/AIDS Surveillance Report, 2002 13, (No. 2). Atlanta: Centers for Disease Control and and Prevention. Vol. 13, No. 2. In press. Available at http://www.cdc.gov/hiv/stats/hasrlink.htm. Lee LM, Fleming PL. Trends in human immundeficiency virus diagnoses among women in the United States, 1994--1998. J Am Med Womens Assoc 2001;56:94--99. Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984--1997. JAMA 2001; 285:1308--15. Legionellosis American Society of Heating, Refrigerating, and Air-Conditioning Engineers. ASHRAE Guideline 12-2000. Minimizing the Risk of Legionellosis Associated With Building Water Systems. Atlanta: American Society for Heating, Refrigerating, and Air-Conditioning Engineers, Inc., 2000:1--17. Available at: www.baltimoreaircoil.com. CDC. Guidelines for prevention of nosocomial pneumonia. MMWR 1997;46(No. RR-1). Lyme Disease CDC. Lyme disease---United States, 2000. MMWR 2002;51:29--31. Hayes EB, Maupin GO, Mount GA, Piesman J. Assessing the prevention effectiveness of local Lyme disease control. J Public Health Management Practice 1999;5:84--92. Malaria Lobel HO, Kozarsky PE. Update on prevention of malaria for travelers. JAMA 1997;278:1767--71. MacArthur JR, Holtz TH, Jenkins J, et al Probable locally acquired mosquito-transmitted malaria in Georgia, 1999. Clin Infect Dis 2001;32(8):E124-8. Zucker JR. Changing patterns of autochthonous malaria transmission in the United States: a review of recent outbreaks. Emerg Infect Dis 1996;2:37--43. Available at http://www.cdc.gov/ncidod/eid/vol2no1/zuckerei.htm. Zucker JR, Campbell CC. Malaria. Principles of prevention and treatment [Review]. Infect Dis Clin North Am 1993;7:547--67. Measles CDC. Epidemiology of measles---United States, 1998. MMWR 1999;48:749--53. CDC. Measles---United States, 2000. MMWR 2002;51;120--3. CDC. Measles---United States, 1999. MMWR 2000;49:557--60. Meningococcal Disease Diermayer M, Hedberg K, Hoesly FC, et al. Epidemic serogroup B meningococcal disease in Oregon: the evolving epidemiology of the ET-5 strain. JAMA 1999;281:1493--7. Available at http://jama.ama-assn.org/issues/v281n16/rfull/joc81215.html. Lingappa JR, Rosenstein N, Zell E, Shutt K, Schuchat A, Perkins B, and the Active Bacterial Core surveillance (ABCs) Team of the Emerging Infections Program. Surveillance for meningococcal disease and strategies for use of conjugate meningococcal vaccines in the United States. Vaccine 2001;19:4566--75. Rosenstein NE, Perkins BA, Stephens DS Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001;344:1378--88. Mumps Briss PA, Fehrs LJ, Parker RA, et al. Sustained transmission of mumps in a highly vaccinated population: assessment of primary vaccine failure and waning vaccine-induced immunity. J Infect Dis 1994;169:77--82. CDC. Mumps surveillance United States, 1988--1993. MMWR 1995;44(No. SS-3). Hersh BS, Fine PE, Kent WK, et al. Mumps outbreak in a highly vaccinated population. J Pediatr 1991;119:187--93. Pertussis Bisgard KM, Christie CDC, Reising SF, et al. Molecular epidemiology of Bordetella pertussis by pulsed-field gel electrophoresis profile: Cincinnati, 1989--1996. J Infect Dis 2001;183:1360--7. CDC. Guidelines for the control of pertussis outbreaks. Atlanta: Centers for Disease Control and Prevention, 2000. Available at http://www.cdc.gov/nip/publications/pertussis/guide.htm. CDC. Pertussis deaths---United States, 2000. MMWR 2002;51:616--8. CDC. Pertussis---United States, 1997--2000. MMWR 2002;51:73--6. Plague Inglesby TV, Dennis DT, Henderson DA, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense [Review]. JAMA 2000;283:2281--90. Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E. Plague manual: epidemiology, distribution, surveillance and control. Geneva, Switzerland: World Health Organization, 1999. Poland JD, Dennis DT. Plague. In: Evans AS, Brachman PS, eds. Bacterial infections of humans: epidemiology and control. 3rd ed. New York, NY: Plenum Medical Book Company, 1998:545--58. Poliomyelitis, Paralytic Prevots DR, Khetsuriani N, Wharton M. Evidence for a decline in the number of vaccine-associated paralytic poliomyelitis cases in the United States following implementation of a sequential poliovirus vaccination schedule, 1997--1998. Presented at the 36th annual meeting of the Infectious Disease Society of America, November 12--15, 1998. Prevots DR, Sutter RW, Strebel PM, Weibel RE, Cochi SL. Completeness of reporting for paralytic poliomyelitis, United States, 1980 through 1991. Implications for estimating the risk of vaccine-associated disease. Arch Pediatr Adolesc Med 1994;148:479--85. Psittacosis Moroney JF, Guevara R, Iverson C, et al. Detection of chlamydiosis in a shipment of pet birds, leading to recognition of an outbreak of clinically mild psittacosis in humans. Clin Infect Dis 1998;26:1425--9. Q Fever Bernard KW, Parham GL, Winkler WG, Helmick CG. Q fever control measures: recommendations for research facilities using sheep. Infect Control 1982;3:461--5. Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985--1998. Clinical and epidemiologic features of 1,383 infections. Medicine 2000;79:109--23. Tissot-Dupont H, Torres S, Nezri M, Raoult D. Hyperendemic focus of Q fever related to sheep and wind. Am J Epidemiol 1999;150:67--74. Rabies, Animal and Human Krebs JW, Mondul, AM, Rupprecht CE, Childs JE. Rabies surveillance in the United States during 2000. J Am Vet Med Assoc 2001;219:1687--99. Available at http://www.cdc.gov/ncidod/dvrd/rabies/professional/Surveillance00/text00.htm National Association of State and Territorial Public Health Veterinarians, Inc. Compendium of animal rabies prevention and control, 2002. Available at http://www.cdc.gov/ncidod/dvrd/rabies/professional/publications/compendium/comp_02.htm. Noah DL, Drenzek CL, Smith JS, et al. Epidemiology of human rabies in the United States, 1980 to 1996 [Review]. Ann Intern Med 1998;128:922--30. Rocky Mountain Spotted Fever Childs JE, Paddock CD. Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? Am J Trop Med Hyg 2002;66:450--7. Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis 2001;184:1437--44. Treadwell TA, Holman RC, Clarke MA, Krebs JW, Paddock CD, Childs JE. Rocky Mountain spotted fever in the United States, 1993--1996. Am J Trop Med Hyg 2000;63:21--6. Rubella and Congenital Rubella Syndrome Danovaro-Holliday MC, LeBaron CW, Allensworth C, et al. A large rubella outbreak with spread from the workplace to the community. JAMA 2000;284:2733--9. Reef SE, Frey TK, Theall K, et al. The changing epidemiology of rubella in the 1990s: on the verge of elimination and new challenges for control and prevention. JAMA 2002;287:464--72. Reef S, Plotkin S, Cordero J, et al. Preparing for congenital rubella syndrome elimination: summary of the Workshop on Congenital Rubella Syndrome Elimination in the United States. Clin Infect Dis 2000;31:85--95. Salmonella Mahon BE, Slutsker L, Hutwagner L, et al. Consequences in Georgia of a nationwide outbreak of Salmonella infections: what you don't know might hurt you. Am J Public Health 1999;89:31--5. Olsen SJ, Bishop R, Brenner FW, et al. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987--1997. J Infect Dis 2001;183:753--61. Shigellosis Mohle-Boetani JC, Stapleton M, Finger R, et al. Communitywide shigellosis: control of an outbreak and risk factors in child day-care centers. Am J Public Health 1995;85:812--6. Sobel J, Cameron DN, Ismail J, et al. A prolonged outbreak of Shigella sonnei infections in traditionally observant Jewish communities in North America caused by a molecularly distinct bacterial subtype. J Infect Dis 1998;177:1405--8. Streptococcal Disease, Invasive, Group A The Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002;35:950--9. CDC. Active Bacterial Core Surveillance (ABCs) report. Emerging Infections Program Network. Group A streptococcus, 2001. Atlanta, GA: US Department of Health and Human Services, CDC, 2002. Available at http://www.cdc.gov/ncidod/dbmd/abcs/survreports/gas01_provis.pdf. Facklam RF, Martin DR, Lovgren M, et al. Extension of the Lancefield classification for group A streptococci by addition of 22 new M protein gene sequence types from clinical isolates: emm103 to emm124. Clin Infect Dis 2002;34:28--38. O'Brien KL, Beall B, Barrett NL, et al. Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network. Epidemiology of invasive group A streptococcus disease in the United States, 1995--1999.Clin Infect Dis 2002;35:268--76. Streptococcus pneumoniae, Drug-Resistant, Invasive Disease Robinson KA, Baughman W, Rothrock G, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995--1998: opportunities for prevention in the conjugate vaccine era. JAMA 2001;285:1729--35. Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 2000;343:1917--24. Syphilis, Congenital CDC. Congenital syphilis---United States, 2000. MMWR 2001;50:573--7. CDC. Guidelines for the prevention and control of congenital syphilis. MMWR 1988;37(No. S-1). Southwick KL, Guidry HM, Weldon MM, Mertz KJ, Berman SM, Levine WC. An epidemic of congenital syphilis in Jefferson County, Texas, 1994--1995: inadequate prenatal syphilis testing after an outbreak in adults. Am J Public Health 1999;89:557--60. Syphilis, Primary and Secondary CDC. The national plan to eliminate syphilis from the United States. Atlanta: US Department of Health and Human Services, CDC, October 1999. CDC. Primary and secondary syphilis---United States, 1999. MMWR 2001;50:113--7. CDC. Sexually transmitted disease surveillance supplement 2000: syphilis surveillance report. Atlanta, GA: US Department of Health and Human Services, CDC, December 2001. Tetanus CDC. Tetanus---Puerto Rico, 2002. MMWR 2002;51:613--15. Fair E, Murphy T, Golaz A, Wharton M. Philosophic objection to vaccination as a risk for tetanus among children <15 years of age. Pediatrics 2002;109:E2. McQuillan GM, Kruszon-Moran D, Deforest A, Chu SY, Wharton M. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med 2002;136:660--6. Toxic-Shock Syndrome Hajjeh RA, Reingold R, Weil A, Shutt K, Schuchat A, Perkins BA. Toxic shock syndrome in the United States: surveillance update, 1979--1996. Emerg Infec Dis 1999;5:807--10. Available at <http://www.cdc.gov/ncidod/eid/vol5no6/hajjeh.htm>. Schuchat A, Broome CV. Toxic shock syndrome and tampons. Epidemiol Rev 1991;13:99--112. Gaventa S, Reingold AL, Hightower AW, et al. Active surveillance for toxic shock syndrome in the United States, 1986. Rev Infect Dis 1989;11(suppl):S28--S34. Tuberculosis CDC. Reported tuberculosis in the United States, 2001. Atlanta, GA: US Department of Health and Human Services, CDC, September 2002. Available at http://www.cdc.gov/tb. Saraiya M, Cookson ST, Tribble P, et al. Tuberculosis screening among foreign-born persons applying for permanent US residence. Am J Public Health 2002;92:826--829. Talbot EA, Moore M, McCray E, Binkin NJ. Tuberculosis among foreign-born persons in the United States, 1993--1998. JAMA 2000;284:2894--900. Tularemia CDC. Tularemia---United States, 1990--2000. MMWR 2002;51:181--4. Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA 2001;285:2763--73. Feldman KA, Enscore R, Lathrop S, et al. Outbreak of primary pneumonic tularemia on Martha's Vineyard. N Engl J Med 2001:345:1219--26. Trichinosis Moorhead A, Grunenwald PE, Dietz VJ, Schantz PM. Trichinellosis in the United States, 1991--1996: declining but not gone. Am J Trop Med Hyg 1999;60:66--9. McAuley JB, Michelson MK, Schantz PM. Trichinosis surveillance, United States, 1987--1990. In: CDC surveillance summaries, December 1991. MMWR 1991;40(No. SS-3):35--42. Typhoid Fever Ackers ML, Puhr ND, Tauxe RV, Mintz ED. Laboratory-based surveillance of Salmonella Serotype Typhi infections in the United States: antimicrobial resistance on the rise. JAMA 2000;283:2668--73. Mermin JH, Townes JM, Gerber M, Dolan N, Mintz ED, Tauxe RV. Typhoid fever in the United States, 1985--1994: changing risks of international travel and increasing antimicrobial resistance. Arch Intern Med 1998;158:633--8. Olsen SJ, Bleasdale SC, Magnano AR, et al. Outbreaks of typhoid fever in the United States, 1960--1999. Epidemiol Infect; 2002: In Press. Varicella CDC. Varicella-related deaths---Florida, 1998. MMWR 1999;48:379--81. 
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/17/2003 |
|||||||||
This page last reviewed 4/17/2003
|