 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Malaria Surveillance --- United States, 2000Louise M. Causer, M.B.B.S.1,2 Abstract Problem/Condition: Malaria is caused by four species of intraerythrocytic protozoa of the genus Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, or P. malariae). Malaria is transmitted by the bite of an infective female Anopheles sp. mosquito. The majority of malaria infections in the United States occur among persons who have traveled to areas with ongoing transmission. In the United States, cases can occur through exposure to infected blood products, by congenital transmission, or locally through mosquitoborne transmission. Malaria surveillance is conducted to identify episodes of local transmission and to guide prevention recommendations for travelers. Period Covered: Cases with onset of illness during 2000. Description of System: Malaria cases confirmed by blood smear are reported to local and state health departments by health-care providers or laboratory staff. Case investigations are conducted by local and state health departments, and reports are transmitted to CDC through the National Malaria Surveillance System (NMSS). Data from NMSS serve as the basis for this report. Results: CDC received reports of 1,402 cases of malaria with an onset of symptoms during 2000 among persons in the United States or one of its territories. This number represents a decrease of 9.0% from the 1,540 cases reported for 1999. P. falciparum, P. vivax, P. malariae, and P. ovale were identified in 43.6%, 37.2%, 4.8%, and 2.3% of cases, respectively. Nine patients (0.6% of total) were infected by >2 species. The infecting species was unreported or undetermined in 161 (11.5%) cases. Compared with 1999, the number of reported malaria cases acquired in Africa decreased by 13.1% (n = 783), and a decrease of 3.3% (n = 238) occurred in cases acquired in Asia. Cases from the Americas decreased by 1.1% (n = 271) from 1999. Of 825 U.S. civilians who acquired malaria abroad, 190 (23.0%) reported that they had followed a chemoprophylactic drug regimen recommended by CDC for the area to which they had traveled. Four patients became infected in the United States, two through congenital transmission and two through probable induced transmission. Six deaths were attributed to malaria, four caused by P. falciparum, one caused by P. malariae, and one by P. ovale. Interpretation: The 9.0% decrease in malaria cases in 2000, compared with 1999, resulted primarily from decreases in cases acquired in Africa and Asia. This decrease could have resulted from local changes in disease transmission, decreased travel to these regions, fluctuation in reporting to state and local health departments, or an increased use of effective antimalarial chemoprophylaxis. In the majority of reported cases, U.S. civilians who acquired infection abroad were not on an appropriate chemoprophylaxis regimen for the country in which they acquired malaria. Public Health Actions: Additional information was obtained concerning the six fatal cases and the four infections acquired in the United States. Persons traveling to a malarious area should take one of the recommended chemoprophylaxis regimens appropriate for the region of travel, and travelers should use personal protection measures to prevent mosquito bites. Any person who has been to a malarious area and who subsequently develops a fever or influenza-like symptoms should seek medical care immediately and report their travel history to the clinician; investigation should include a blood-film test for malaria. Malaria infections can be fatal if not diagnosed and treated promptly. Recommendations concerning malaria prevention can be obtained from CDC by calling the Malaria Hotline at 770-488-7788 or by accessing CDC's Internet site at http://www.cdc.gov/travel. Introduction Malaria is caused by infection with one or more of four species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, and P. malariae) that can infect humans. The infection is transmitted by the bite of an infective female Anopheles sp. mosquito. Malaria infection remains a devastating global problem, with an estimated 300--500 million cases occurring annually (1). Forty-one percent of the world's population lives in areas where malaria is transmitted (e.g., parts of Africa, Asia, the Middle East, Central and South America, Hispaniola, and Oceania) (1), and 700,000--2.7 million persons die of malaria each year, 75% of them African children (2). In previous years, malaria was endemic throughout much of the continental United States; an estimated 600,000 cases occurred during 1914 (3). During the late 1940s, a combination of improved socioeconomic conditions, water management, vector-control efforts, and case management was successful at interrupting malaria transmission in the United States. Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate the reintroduction of transmission and to monitor patterns of antimalarial drug resistance that guide prevention recommendations for U.S. travelers. Anopheline mosquitos remain seasonally present in all states except Hawaii. Through 2000, the majority of cases of malaria diagnosed in the United States have been imported from regions of the world where malaria transmission is known to occur, although congenital infections and infections resulting from exposure to blood or blood products are reported in the United States also. In addition, a limited number of cases are reported that might have been acquired through local mosquitoborne transmission (4). State and local health departments and CDC investigate malaria cases acquired in the United States, and CDC analyzes data from imported cases to detect acquisition trends. This information is used to guide malaria prevention recommendations for travelers abroad. For example, an increase in P. falciparum malaria among U.S. travelers to Africa, an area with increasing chloroquine resistance, prompted CDC to change the recommended chemoprophylaxis regimen from chloroquine to mefloquine in 1990 (5). The signs and symptoms of malaria illness are varied, but the majority of patients experience fever. Other common symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. The diagnosis of malaria should be considered for persons who experience these symptoms and who have traveled to an area with known malaria transmission. Malaria should also be considered in the differential diagnoses of persons who experience fevers of unknown origin, regardless of their travel history. Untreated P. falciparum infections can rapidly progress to coma, renal failure, pulmonary edema, and death. Asymptomatic parasitemia can occur among persons who have been long-term residents of malarious areas. This report summarizes malaria cases reported to CDC with onset of symptoms in 2000. Methods Data Sources Malaria case data are reported to the National Malaria Surveillance System (NMSS) and the National Notifiable Diseases Surveillance System (NNDSS) (6). Although both systems rely on passive reporting, the numbers of reported cases might differ between the two systems because of differences in collection and transmission of data. A major difference in the data collected in these two systems is that NMSS receives more detailed clinical and epidemiologic data regarding each case (e.g., information concerning the area to which the infected person has traveled). This report presents only data regarding cases reported to NMSS. Cases of blood-film--confirmed malaria among civilians and military personnel are identified by health-care providers or laboratories. Each slide-confirmed case is reported to local or state health departments and to CDC on a uniform case report form that contains clinical, laboratory, and epidemiologic information. CDC staff review all report forms when received and request additional information, if necessary (e.g., when no recent travel to a malarious country is reported). Reports of other cases are telephoned directly by health-care providers to CDC, usually when assistance with diagnosis or treatment is requested. All cases that have been acquired in the United States are investigated, including all induced and congenital cases and possible introduced or cryptic cases. Information derived from uniform case report forms is entered into a database and analyzed annually. Definitions The following definitions are used in this report:
This report also uses terminology derived from the recommendations of the World Health Organization; (7). Definitions of the following terms are included for reference:
Microscopic Diagnosis of Malaria The early diagnosis of malaria requires that physicians consider malaria in the differential diagnosis of every patient who is experiencing fever; the evaluation of such a patient should include taking a comprehensive travel history. If malaria is suspected, a Giemsa-stained smear of the patient's peripheral blood should be examined for parasites. Thick and thin blood films must be prepared correctly because diagnostic accuracy depends on blood-film quality and examination by experienced laboratory personnel* (see Appendix for procedures for accurately diagnosing malaria). Results General Surveillance During 2000, CDC received 1,402 malaria case reports occurring among persons in the United States and its territories, representing a 9.0% decrease from the 1,540 cases reported for 1999 (8). This incidence is the third highest number of reported cases since 1980 and represents the second highest number of U.S. civilian cases reported in the past 30 years (Table 1). In 2000, a total of 827 cases occurred among U.S. civilians, compared with 833 cases reported for 1999, and the number of cases among foreign civilians also decreased from 381 cases to 354 (Figure 1). Cases among U.S. military personnel decreased from 55 to 46 in 2000. In 175 cases, information was insufficient to determine civilian or military status. Plasmodium Species The infecting species of Plasmodium was identified in 1,241 (88.5%) of the cases reported in 2000. P. falciparum and P. vivax were identified in blood smears from 43.6% and 37.2% of infected persons, respectively (Table 2). The 611 P. falciparum cases reported for 2000 represented a 13.7% decrease from the 708 cases in 1999, whereas the number of P. vivax infections increased by 10.6% (from 472 in 1999 to 522 in 2000). Among 1,181 cases in which both the region of acquisition and the infecting species were known, 75.6% of infections acquired in Africa were attributed to P. falciparum; 13.2% were attributed to P. vivax. The converse was true of infections acquired in the Americas and Asia: 86.5% and 85.9% were attributed to P. vivax, and only 7.3% and 8.3% were attributed to P. falciparum, respectively. Region of Acquisition and Diagnosis Approximately 99% (n = 1,398) of reported cases were imported. Of 1,330 imported cases in which the region of acquisition was known, the majority (58.9%; n = 783) were acquired in Africa; 20.1% (n = 271) and 17.9% (n = 238) were acquired in the Americas and Asia, respectively (Table 3). The highest concentration of cases acquired in Africa came from countries in West Africa (66.7%; n = 522), whereas the majority of cases acquired in Asia came from the Indian subcontinent (67.2%; n = 160). The other regions where imported cases of malaria were acquired were Central America and the Caribbean (15.0%; n = 200), South America (4.3%; n = 57), and Oceania (1.7%; n = 22). Information regarding region of acquisition was missing for 68 (4.9%) of the imported cases. The number of reported malaria cases acquired in Africa decreased by 13.1% (n = 783), compared with 1999. The same number of cases acquired in the Americas (n = 271) were reported for 2000 as compared with 1999. Cases from Asia decreased by 3.3% (n = 238), compared with 1999. In the United States, the five health departments reporting the highest number of malaria cases were New York City (n = 220), California (n = 194), New York State (n = 77), Texas (n = 70), and Illinois (n = 67) (Figure 2). All of these health departments reported a decrease in cases compared with 1999. This overall decrease in reported number of cases might reflect a decreased rate of international travel, a reduced risk for malaria among travelers, poorer access to health care, or fluctuation in surveillance by state and local health departments. Interval Between Arrival and Illness The interval between date of arrival in the United States and onset of illness and the infecting Plasmodium species were known for 623 (44.6%) of the imported cases of malaria (Table 4). Symptoms began before arrival in the United States for 63 (10.1%) persons, whereas symptoms began after arrival in the United States for 560 (89.9%) of these patients. Clinical malaria developed within 1 month after arrival in 313 (80.9%) of the 387 P. falciparum cases and in 75 (38.9%) of the 193 P. vivax cases (Table 4). Only 5 (0.8%) of the 560 persons became ill >1 year after returning to the United States. Imported Malaria Cases Imported Malaria Among U.S. Military Personnel In 2000, a total of 46 cases of imported malaria were reported among U.S. military personnel. Of the 45 cases for whom information regarding chemoprophylaxis use was available, seven patients were not using any prophylaxis. Imported Malaria Among Civilians A total of 1,179 imported malaria cases were reported among civilians. Of these, 825 (70.0%) cases occurred among U.S. residents, and 354 (30.0%) cases occurred among residents of other countries (Table 5). Of the 825 imported malaria cases among U.S. civilians, 555 (67.3%) had been acquired in Africa, an increase of 1.1% from cases reported in 1999. Asia accounted for 106 (12.8%) cases of imported malaria among U.S. civilians, whereas travel to the Central American and Caribbean regions accounted for an additional 90 (10.9%) cases. Of the 354 imported cases among foreign civilians, the majority of cases were acquired in either Africa (n = 150; 42.4%) or Asia (n = 90; 25.4%). Antimalarial Chemoprophylaxis Use Chemoprophylaxis Use Among U.S. Civilians Information concerning chemoprophylaxis use and travel area was known for 758 (91.9%) of the 825 U.S. civilians who had imported malaria. Of these 758 persons, 452 (59.6%) had not taken any chemoprophylaxis, and 94 (12.4%) had not taken a CDC-recommended drug for the area visited (9). Only 190 (25.1%) U.S. civilians had taken a CDC-recommended medication (9). Data for the specific drug taken were missing for the remaining 22 (2.9%) travelers. A total of 128 (67.4%) patients on CDC-recommended prophylaxis had taken mefloquine weekly; 22 (11.6%) had taken doxycycline daily; and 21 (11.1%) who had traveled only in areas where chloroquine-resistant malaria has not been documented, had taken chloroquine weekly. Nineteen patients (10.0%) had taken combinations of drugs that included >1 CDC-recommended drugs for the travel region. Of the 94 patients taking a nonrecommended drug, 54 (57.4%) reported taking chloroquine either alone or in combination with another ineffective drug during travel to an area where chloroquine resistance has been documented. Malaria Infection After Recommended Prophylaxis Use A total of 236 patients (i.e., 190 U.S. civilians, 32 persons in the U.S. military, 6 foreign civilians, and 8 persons whose information regarding their status was missing) experienced malaria after taking a recommended antimalarial drug for chemoprophylaxis. Information regarding infecting species was available for 209 (88.6%) patients taking a recommended antimalarial drug; the infecting species was undetermined for the remaining 27. Cases of P. vivax or P. ovale After Recommended Prophylaxis Use. Of the 236 patients who experienced malaria after recommended chemoprophylaxis use, 121 cases (51.3%) were caused by P. vivax and 10 (4.2%) by P. ovale. Notes on the malaria case surveillance reports indicated that 25 (19.1%) of these 131 patients were noncompliant with antimalarial prophylaxis. A total of 39 (29.8%) cases of P. vivax or P. ovale occurred >45 days after arrival in the United States. These cases were consistent with relapsing infections and, thus, do not indicate prophylaxis failures. Information was insufficient, because of missing data regarding symptom onset or return date, to assess whether 77 cases were relapsing infections. Fifteen cases, all caused by P. vivax, occurred <45 days after the patient returned (n = 12) or before return (n = 3) to the United States. Of these 15 patients, five were known to be noncompliant with their antimalarial chemoprophylaxis. Region of acquisition varied for the 10 remaining case patients who were not known to be noncompliant (one from West Africa, one from East Africa, one from Central Africa,† five from Central America, and two from Papua New Guinea). Blood samples were not available; therefore, serum drug levels were not measured for any of these patients. The probable explanations for these cases are either inappropriate dosing or noncompliance that was not reported. Evidence is lacking that would indicate any new area of chloroquine-resistant P. vivax. Cases of P. falciparum and P. malariae After Recommended Prophylaxis Use. The remaining 105 cases of malaria reported among persons who had taken a recommended antimalarial drug for chemoprophylaxis include 61 cases of P. falciparum, 16 cases of P. malariae, one case of mixed infection, and 27 cases in which the infecting species was unidentified. A total of 59 of the 61 P. falciparum cases among those who reported taking a recommended antimalarial drug were acquired in Africa, one in the Caribbean, and one in South America. In 24 (39.3%) of these 61 cases, noncompliance with antimalarials was reported. Of the remaining 37 cases of P. falciparum for which patient compliance was unknown, the majority were acquired in Africa (n = 35): 24 in West Africa, seven in East Africa, one in Central Africa, one in Southern Africa,§ and two in an unspecified African region. Two cases were acquired outside Africa: one in the Caribbean (Haiti) and one in South America (Brazil). Serum drug levels were not available for any of these 37 patients. Eight of the 16 P. malariae cases among those who reported taking a recommended antimalarial drug were acquired in Africa. In three (18.8%) of these 16 cases, noncompliance with antimalarials was reported. In the 13 remaining cases, whether the patient complied with prophylaxis was unknown; four had traveled in the Americas, five in Africa, three in Asia, and one in Oceania. Purpose of Travel Purpose of travel to malaria-endemic areas was reported for 635 (77.0%) of the 825 U.S. civilians with imported malaria (Table 6). Of the U.S. civilians with malaria, the largest percentage (35.9%) were persons who had visited friends or relatives in malarious areas; the second and third largest percentages, 10.3% and 10.2%, had traveled for tourism and to do missionary work, respectively. Malaria During Pregnancy A total of 35 cases of malaria were reported among pregnant women in 2000, representing 7.8% of cases among women. Fifteen (42.9%) were among U.S. civilians. Eleven of these 15 women (73.3%) had traveled to visit friends and relatives, of whom 72.7% traveled in Africa. Only 1 pregnant woman (6.9%) reported taking prophylaxis, compared with 31.1% of nonpregnant women. Twelve women (86.7%) were hospitalized, compared with 56.3% of nonpregnant women.
Malaria Acquired in the United States Two cases of congenital malaria were reported in 2000 and are described in the following case reports:
Induced Malaria Two cases of induced malaria were reported in 2000 and are described in the following case reports:
Deaths Attributed to Malaria Six deaths attributable to malaria were reported during 2000 and are described in the following case reports:
A total of 1,402 cases of malaria were reported to CDC for 2000, representing a 9.0% decrease from the 1,540 cases reported for 1999. This change primarily resulted from a decrease in cases acquired in Africa and the Americas. Beginning in 2000, CDC routinely contacts state health departments to ask for outstanding reports from the previous reporting year or for a statement that reporting is complete. The decrease in cases in 2000, compared with 1999, possibly is a result of expected variation in the reporting system, although other possibilities include decreased international travel, changing patterns of travel (e.g., immigration from malarious areas or adventure tourism), or an increased use of effective antimalarial chemoprophylaxis. One reason for conducting malaria surveillance is to monitor for prophylaxis failures that might indicate emergence of drug resistance; however, >70% of imported malaria among U.S. civilians occurred among persons who were either not taking prophylaxis or were taking nonrecommended prophylaxis for the region to which they were traveling. Among the 117 cases where appropriate prophylaxis was reported, 60 (i.e., 37 P. falciparum, 10 P. vivax, and 13 P. malariae) had insufficient information to determine whether these represented problems with adherence while using correct antimalarial chemoprophylaxis or emerging drug resistance. No conclusive evidence existed to indicate a single national or regional source of infection among this group of patients or the failure of a particular chemoprophylactic regimen. Health-care providers are encouraged to contact CDC rapidly whenever they suspect chemoprophylaxis failure, thus enabling measurement of serum drug levels of the antimalarial drugs in question. In 2001, to be better able to evaluate chemoprophylaxis failures, CDC revised the NMSS case report form to facilitate collection of more thorough data regarding chemoprophylaxis. The current form solicits more detailed information regarding the prescribed regimen, the degree of compliance with the regimen, and the reasons for noncompliance, if any. Data gathered from the responses will be useful in generating public health messages to improve use of antimalarial chemoprophylaxis, and therefore decrease malaria-associated morbidity and mortality among U.S. civilians. The importance of taking correct precautions and chemoprophylaxis is underscored by the six fatal cases of malaria that occurred in the United States in 2000. An earlier review of deaths attributed to malaria in the United States identified certain risk factors for fatal malaria, including failure to take recommended antimalarial chemoprophylaxis, refusal of or delay in seeking medical care, and misdiagnosis (11). The occurrence of 15 cases of malaria among pregnant U.S. civilians is cause for concern also. Malaria during pregnancy among nonimmune women is more likely to result in severe disease or contribute to an adverse outcome for the woman than malaria in a nonpregnant woman (12); the fetus might be adversely affected as well (13). Pregnant travelers should be counseled to avoid travel to malarious areas, if possible. If deferral of travel is impossible, pregnant women should be informed that the risks for malaria outweigh those associated with prophylaxis and that safe chemoprophylaxis regimens are available. Specific guidance for pregnant travelers is available from CDC's website at http://www.cdc.gov/travel/mal_preg_pub.htm. Signs and symptoms of malaria are often nonspecific, but fever is usually present. Other symptoms include headache, chills, increased sweating, back pain, myalgia, diarrhea, nausea, vomiting, and cough. Prompt diagnosis requires that malaria be included in the differential diagnosis of illness in a febrile person with a history of travel to a malarious area. Clinicians should ask all febrile patients for a travel history, including when evaluating febrile illnesses among international visitors, immigrants, refugees, migrant laborers, and international travelers. Treatment for malaria should be initiated immediately after the diagnosis has been confirmed by a positive blood film. Treatment should be determined on the basis of the infecting Plasmodium species, the probable geographic origin of the parasite, the parasite density, and the patient's clinical status (14). Although nonfalciparum malaria rarely causes complications, persons with P. falciparum infection are at risk for developing life-threatening complications. Health-care workers should be familiar with prevention, recognition, and treatment of malaria and are encouraged to consult appropriate sources (Table 7) for malaria treatment recommendations or call CDC's National Center for Infectious Diseases, Division of Parasitic Diseases at 770-488-7788. Detailed recommendations for preventing malaria are available 24 hours/day from CDC by telephone at 877-394-8747 (toll-free voice information system) or 888-232-3299 (toll-free facsimile request line), or on the Internet at http://www.cdc.gov/travel/diseases.htm#malaria. In addition, CDC biannually publishes recommendations in the Health Information for International Travel (9), which is available for purchase from the Public Health Foundation at 877-252-1200 or 301-645-7773; it is also available and updated more frequently on CDC's Internet site at http://www.cdc.gov/travel. CDC provides support for the diagnosis of malaria through DPDx, a program that enhances diagnosis of parasitic diseases throughout the world. It includes an Internet site, http://www.dpd.cdc.gov/dpdx, that contains information regarding laboratory diagnosis, geographic distribution, clinical features, treatment, and life cycles of >100 parasite species. The DPDx Internet site is also a portal for diagnostic assistance for health-care providers through telediagnosis. Digital images captured from diagnostic specimens are submitted for diagnostic consultation through electronic mail. Because laboratories can transmit images to CDC and rapidly obtain answers to their inquiries, this system allows more efficient diagnosis of difficult cases and more rapid dissemination of information. Approximately 36 laboratories in 34 states have or are in the process of acquiring the hardware to perform telediagnosis. Acknowledgments The authors acknowledge the state, territorial, and local health departments, health-care providers, and laboratories for reporting this information to CDC. References
* To obtain confirmation diagnosis of blood films from questionable cases and to obtain appropriate treatment recommendations, contact either your state or local health department or CDC's National Center for Infectious Diseases, Division of Parasitic Diseases, Malaria Epidemiology Branch at 770-488-7788. † East, West, and Central Africa: Angola, Benin, Burkina Faso, Burundi, Cameroon, Cape Verde Islands, Central African Republic, Chad, Comoros, Congo, Côte d'Ivoire, Democratic Republic of the Congo, Djibouti, Equatorial Guinea, Eritrea, Ethiopia, Gabon, The Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Niger, Nigeria, Réunion, Rwanda, São Tomé and Príncipe, Senegal, Seychelles, Sierra Leone, Somalia, Sudan, Togo, Uganda, Tanzania, Zambia, and Zimbabwe. § Southern Africa: Botswana, Lesotho, Namibia, Saint Helena, South Africa, and Swaziland. * In Figures A-1 and A-2, the hands are illustrated ungloved to better indicate their placement during the procedures. However, wearing gloves while processing blood specimens is recommended to prevent transmission of bloodborne pathogens (MMWR 1988;37:377--82, 387--8 and MMWR 1987;36[No. S2]). Table 1 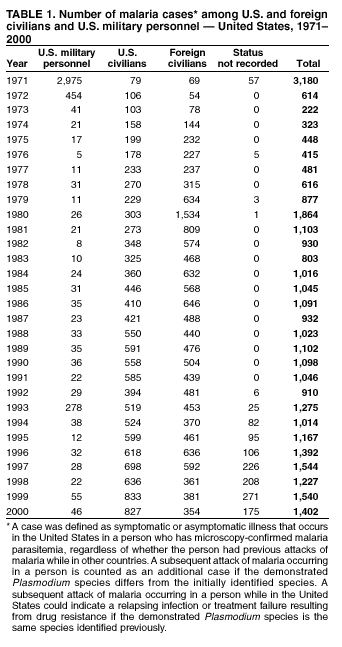 Return to top. Figure 1 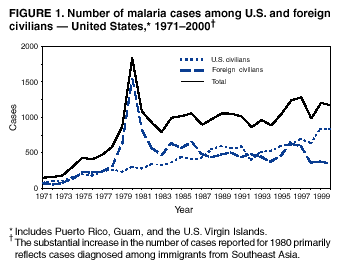 Return to top. Table 2 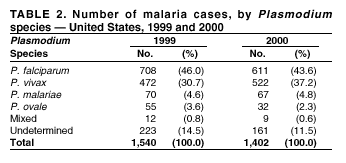 Return to top. Figure 2 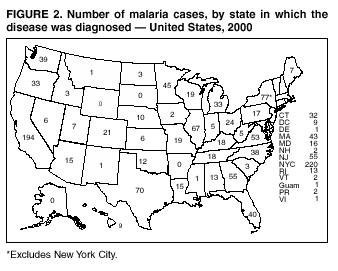 Return to top. Table 3 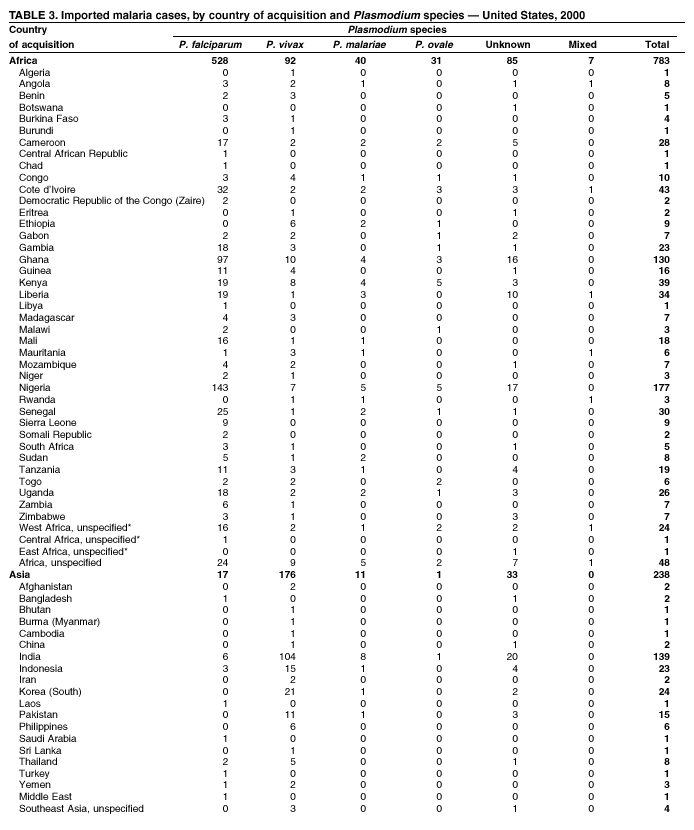 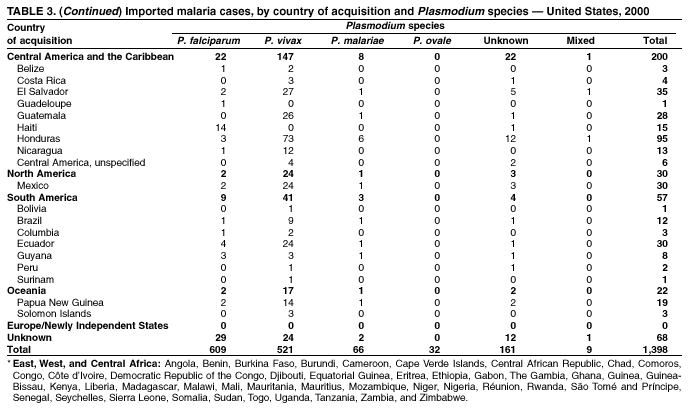 Return to top. Table 4 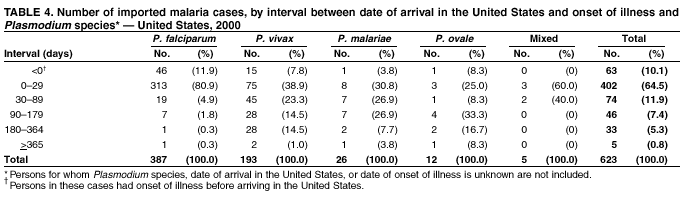 Return to top. Table 5 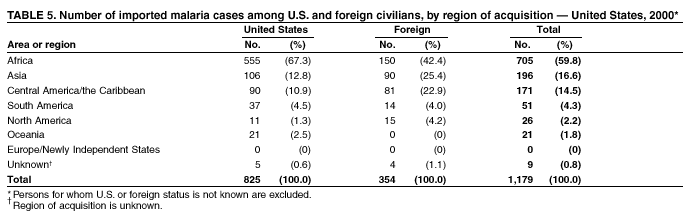 Return to top. Table 6 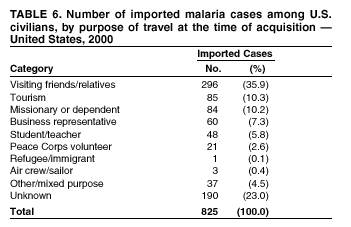 Return to top. Table 7  Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/2/2002 |
|||||||||
This page last reviewed 7/2/2002
|