Key points
- One inactivated tick-borne encephalitis (TBE) vaccine (manufactured as TICOVAC) is available in the United States.
- TBE vaccine is approved for persons 1 year of age and older.
- TBE is a very low risk disease for most travelers.
- TBE vaccine is recommended or should be considered for some travelers who are at increased risk for infection based on travel season, location, activities, and duration.
TBE vaccine
One inactivated TBE vaccine (called TICOVAC and manufactured by Pfizer) is available in the United States. This vaccine was approved in August 2021 for individuals aged 1 year and older. This vaccine has been used for over 20 years in Europe. This vaccine and other TBE vaccines are available in many countries overseas where TBE virus is present.
The dose for persons aged 16 years and older is 0.5mL, and for children and adolescents up to 15 years of age is 0.25mL.
TICOVAC is given as a 3-dose series:
- Adults 16 years of age and older should have the first two doses spaced 14 days to 3 months apart and the third dose 5–12 months after the second dose.
- Children 1–15 years old should have the first two doses spaced 1–3 months apart and the third dose 5–12 months after the second dose.
A booster dose (fourth dose) may be given at least 3 years after completion of the primary vaccination series if ongoing exposure or re-exposure to TBE virus is expected.
More information about TBE vaccine can be found in the Tick-borne Encephalitis Vaccine Information Statement and in the TICOVAC product information available at the FDA TICOVAC webpage.

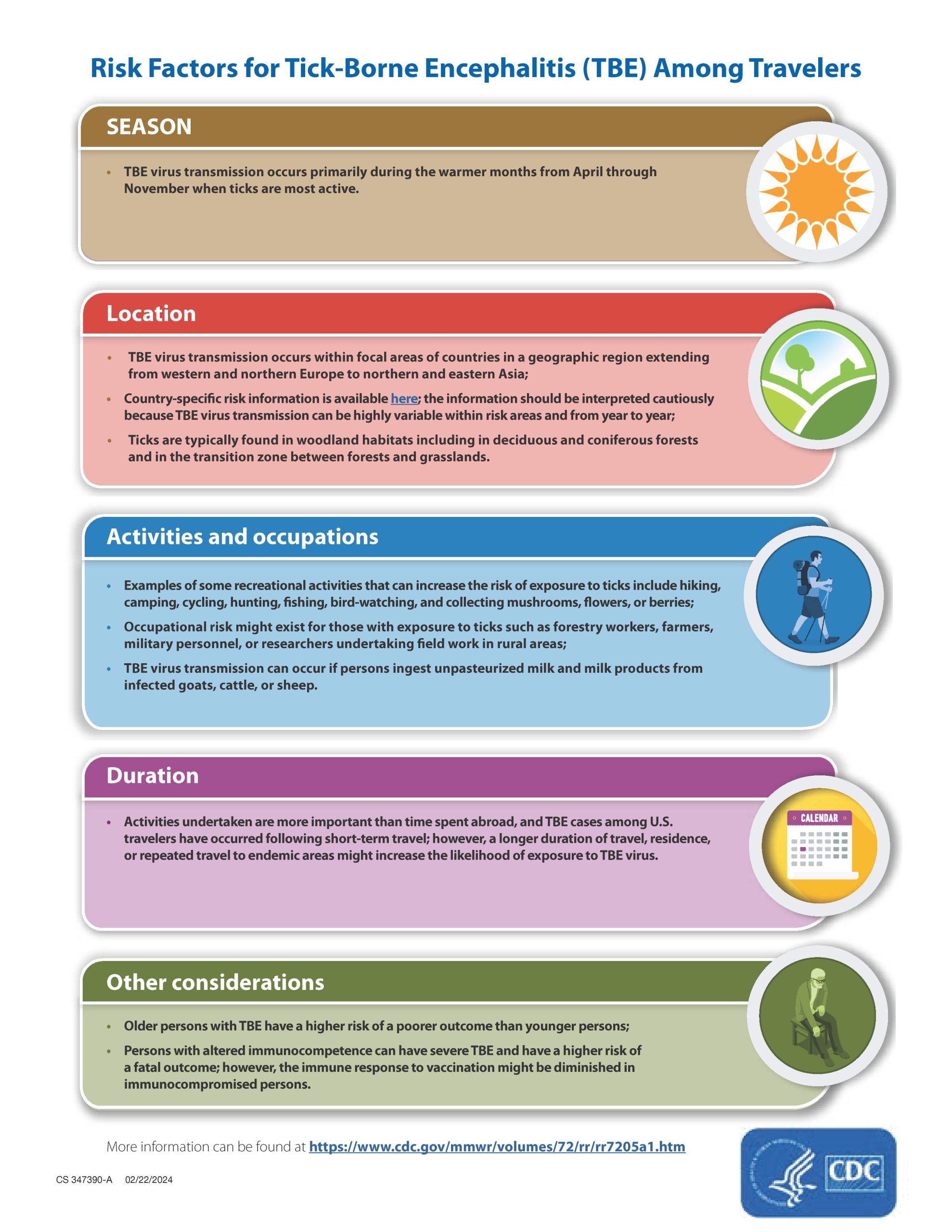
Risk factors for TBE among travelers
The risk for TBE for most U.S. travelers visiting TBE-endemic areas is very low. However, some people who travel abroad are at increased risk for infection based on travel season, location, activities, and duration. Key factors include:
- Traveling during the warmer spring and summer months.
- Participating in certain recreational outdoor activities (e.g., hiking, camping, hunting, fishing) in tick habitats in or on the edges of forests.
- Working in outdoor settings where there is an increased risk of coming into contact with infected ticks (e.g., forestry workers, farmers, military personnel, researcher undertaking field work).
- Staying for longer periods or undertaking repeated travel to endemic areas, which might increase the likelihood of exposure to TBE virus. However, activities undertaken are more important to determine risk than time spent abroad.
Risk factors for TBE among travelers
Considerations for TBE vaccination for travelers
Healthcare providers should advise all travelers to TBE-endemic areas to take steps to avoid tick bites. TBE vaccine can further reduce the risk for infection and might be indicated for some persons at increased risk for TBE. The risk-benefit assessment for vaccination should include several factors:
- Likelihood of exposure to TBE virus-infected ticks based on activities and itinerary (e.g., location, rurality, season, and duration of travel or residence). Persons with extensive exposure to ticks are likely to be at highest risk and could include shorter-term (e.g., <1 month) travelers with daily or frequent exposure, or longer-term travelers with regular (e.g., a few times a month) exposure, to environments that might harbor infected ticks.
- Rare occurrence of TBE but its potentially high morbidity and mortality;
- Higher risk of severe disease among certain individuals (e.g., older persons);
- Availability of a vaccine with a good long-term immunogenicity and safety profile;
- Possibility but low probability of serious adverse events after vaccination, like any vaccine;
- Likelihood of future travel to TBE-endemic areas;
- Individual's personal perception and tolerance of risk for a potentially severe disease.
Available data from human and animal studies, and the genetic and antigenic similarity between the three subtypes of TBE virus, suggest that the TBE vaccine (based on a European TBE virus) provides protection against the other main subtypes, but data are limited, and vaccine effectiveness has not been demonstrated.
TBE vaccine recommendations
The U.S. Advisory Committee on Immunization Practices approved recommendations for use of TBE vaccine among U.S. persons who travel abroad in February 2022.
- TBE vaccine is recommended for persons who are moving or traveling to a TBE-endemic area and will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
- TBE vaccine also may be considered for persons traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas ticks are likely to be found. The decision to vaccinate should be based on an assessment of their planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Healthcare providers can use CDC's decisions tree to help determine when TBE vaccination for travelers is appropriate.

Click here for a downloadable PDF of the TBE vaccine decision tree.
Contraindications and precautions
- Contraindications: A severe allergic reaction (e.g., anaphylaxis) to any component of the TBE vaccine, including substances remaining from the manufacturing process (e.g., protamine sulfate, neomycin, gentamicin, and chick protein).
- Precautions: Immunocompromising conditions or being immunosuppressed.
Side effects of TBE vaccine
- TBE vaccine is considered to have a good safety profile, with adverse events reported more commonly after the primary series doses than after a booster dose.
- The most common local reactions are injection site tenderness and pain.
- The most common systemic reactions in adults are fatigue, headache, and myalgia.
- The most common systemic reactions in children are headache and fever.
Healthcare providers are encouraged to report all adverse events that might be caused by vaccination to the CDC/FDA Vaccine Adverse Events Reporting System (VAERS)by one of the following methods.
If you need further assistance with reporting to VAERS, please email info@VAERS.org or call 1-800-822-7967.
For more information
- MMWR Recommendations and Reports: Advisory Committee on Immunization Practices (ACIP) recommendations for use of TBE vaccine.
- CDC's Health Information for International Travel (Yellow Book): Information about TBE, risk for travelers, and recommendations for prevention by vaccination.
- Hills SL, Broussard KR, Broyhill JC, Shastry LG, Cossaboom CM, White JL, et al. Tick-borne encephalitis among US travellers, 2010-20. J Travel Med. 2022;29(2):taab167. doi: 10.1093/jtm/taab167
- Loew-Baselli A, Poellabauer EM, Pavlova BG, Fritsch S, Firth C, Petermann R, et al. Prevention of tick-borne encephalitis by FSME-IMMUN vaccines: review of a clinical development programme. Vaccine. 2011;29(43):7307-7319. doi: 10.1016/j.vaccine.2011.07.089
