Pneumococcal vaccination among U.S. Medicare beneficiaries aged ≥65 years, 2010-2019
Authors: Jessica Hoehner, MPH1; Hilda Razzaghi, PhD2; Walter W. Williams, MD, MPH2; Miwako Kobayashi, MD, MPH3; Tara C. Jatlaoui, MD, MPH2; Xiyuan Wu, MS4 Thomas E. MaCurdy, PhD4,5 Jeffrey A. Kelman, MD6
1 Leidos, Inc., Atlanta, GA; 2Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; 3Division of Bacterial Disease, National Center for Immunization and Respiratory Diseases, CDC; 4Acumen, LLC, Burlingame, California; 5 Department of Economics, Stanford University; 6Center for Medicare, Centers for Medicare & Medicaid Services
Summary
Since 1984, the Advisory Committee on Immunization Practices (ACIP) has recommended that adults aged ≥65 years and those with certain high-risk conditions receive a single dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23). Those who received the vaccine prior to age 65 years because of high-risk conditions were to get a single dose at age 65 years or later, with no additional doses to be administered (1). In 2000, introduction of 7-valent pneumococcal conjugate vaccine (PCV7) in children indirectly contributed to a decline in the incidence of invasive pneumococcal disease (IPD) among both vaccinated and unvaccinated adults aged ≥65 years (2). PCV7 was replaced by the 13-valent pneumococcal conjugate vaccine (PCV13) in 2010 (2). In 2011, PCV13 was approved for use among adults aged ≥50 years, and in June 2012, ACIP recommended routine use of PCV13 in series with PPSV23 for adults aged ≥19 years with immunocompromising conditions*, cerebrospinal fluid leak, or cochlear implant.
In 2014, results of a randomized placebo-controlled trial that demonstrated the efficacy of PCV13 against nonbacteremic pneumococcal pneumonia became available, and on August 13, 2014, ACIP recommended one dose of PCV13 in series with PPSV23 for all adults aged ≥65 years who have never received PCV13 or whose PCV13 history is unknown (3,4). At the time of the 2014 vote, ACIP acknowledged that the long-term public health benefits of this recommendation could be limited in the setting of continued indirect effects of pediatric PCV13 vaccination. On June 26, 2019, after having reviewed the evidence accrued during the preceding 3 years (6), ACIP voted to remove the recommendation for routine PCV13 use among adults aged ≥65 years and to recommend administration of PCV13 based on shared clinical decision-making for adults aged ≥65 years who do not have an immunocompromising condition, cerebrospinal fluid (CSF) leak, or cochlear implant, and who have not previously received PCV13. All adults aged ≥65 years should continue to receive 1 dose of PPSV23. If the decision is made to administer PCV13, it should be given at least 1 year before PPSV23. ACIP continues to recommend PCV13 in series with PPSV23 for adults aged ≥19 years with an immunocompromising condition, CSF leak, or cochlear implant.* If the decision is made to administer PCV13, it should be administered first, and PPSV23 should be administered at least one year later (7).
To estimate PCV13 and PPSV23 vaccination coverage among adults aged ≥65 years before and after implementation of the 2014 recommendations, the Centers for Disease Control and Prevention (CDC) analyzed claims for vaccination submitted for reimbursement to the Centers for Medicare & Medicaid Services (CMS) (8). This report provides proportions of claims for PPSV23 and PCV13 and the receipt of PPSV23 and PCV13, regardless of which vaccine was received first, from any time during a beneficiary’s enrollment in Medicare Parts A (hospital insurance) and B (medical insurance) since reaching age 65 years, among beneficiaries continuously enrolled in Medicare Parts A and B during annual periods from January 1, 2010 through December 31, 2019. For example, to be included in the 2019 estimates, beneficiaries needed to be continuously enrolled from Jan 1, 2019 to Dec 31, 2019, and any pneumococcal vaccination reported since the beneficiary’s enrollment in Medicare through the end of 2019 was considered.
Implementation of PCV13 and PPSV23 vaccination recommendations has not been equal across all adults aged ≥65 years. In 2019, 63.2% of Medicare beneficiaries aged ≥65 years had claims for any pneumococcal vaccination, 46.2% for PPSV23, 49.3% for PCV13, and 32.3% for both PCV13 and PPSV23. When examining claims for receipt of either PPSV23 or PCV13 at the end of the overall period of analysis, December 2019, claims varied by age, race/ethnicity, and presence of immunocompromising and chronic conditions.*†
Methods
CDC monitored PCV13 and PPSV23 claims submitted for reimbursement to CMS among beneficiaries aged ≥65 years who were continuously enrolled in Medicare Parts A and B§ during annual periods from January 1, 2010 through December 31, 2019. For example, to be included in the 2019 estimates, beneficiaries needed to be continuously enrolled from Jan 1, 2019 to Dec 31, 2019, and any pneumococcal vaccination reported since the beneficiary’s enrollment in Medicare through the end of 2019 were considered. The CMS database includes claims for vaccinations administered beginning in 1999 (8). Enrollment periods covered both the four years prior to and the four years following issuance of the recommendation for routine use of both PCV13 and PPSV23 for adults ages ≥65 years (7).§ The number of beneficiaries per annual enrollment period ranged from 24.2 million to 25.8 million. This reflects between 45% — 48% of adults aged ≥65 years in the United States in 2019 according to the American Community Survey (9). Beneficiaries were considered to be vaccinated with PPSV23 or PCV13 if a claim for vaccination was submitted at any time during a beneficiary’s history of enrollment in Medicare Parts A and B since reaching age 65 and before the end of the enrollment period of interest.§
PCV13 and PPSV23 were identified by current procedural terminology codes 90670 and 90732, respectively. Claims submitted from any hospital or outpatient setting (including pharmacies) were included. Claims submitted to CMS for at least one PCV13 dose (with or without PPSV23), at least one PPSV23 dose (with or without PCV13), at least one dose each of PCV13 and PPSV23, and at least one dose of either vaccine were stratified by age, race/ethnicity, state of residence, and the presence of immunocompromising or underlying medical conditions (10, 11).†¶
Race/ethnicity was designated in mutually exclusive categories as Hispanic or Latino, Black, White, Asian, American Indian/Alaska Native (AI/AN), and “other.” ¶ Chronic medical conditions were identified by the presence of International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes listed on any claim submitted to CMS during a beneficiary’s history of enrollment in Medicare Parts A and B through the end of the enrollment period of interest.§
Results
Vaccination reported overall
Claims for receipt of ≥1 dose of any pneumococcal vaccination (either PCV13 or PPSV23) among Medicare beneficiaries aged ≥65 years ranged from 41.0% in December 2010 to 63.2% in December 2019 (Figure). In that same period, claims for receipt of ≥1 dose of PPSV23 ranged from 41.0% to 46.2%. In the period following the ACIP recommendation for routine use of PCV13 in series with PPSV23 for adults aged ≥65 years, claims for receipt of ≥1 dose of PCV13 among Medicare beneficiaries aged ≥65 ranged from 24.1% in December 2015 to 49.3% in December 2019. Claims for receipt of both vaccines (i.e., at least one dose each of PCV13 and PPSV23) ranged from 14.1% in December 2015 to 32.3% in December 2019.
Vaccination by demographic characteristics, medical conditions, and state
Claims reported for beneficiaries who met inclusion criteria in 2019 varied between beneficiaries of differing demographic characteristics, between those with and without immunocompromising conditions, and between those with and without underlying conditions (Table 1) * †. The percentages of beneficiaries with claims for any pneumococcal vaccine types were lowest among beneficiaries aged 65–69 years (49.6%) and highest among beneficiaries aged ≥85 years (75.3%). Claims for PPSV23, PCV13, or both vaccines were higher among White beneficiaries than among beneficiaries of other racial/ethnic groups; the largest differences were between White and Hispanic beneficiaries (47.8% vs. 32.2% for any PPSV23 dose; 51.2% vs. 30.1% for any PCV13 dose; and 33.9% vs. 16.2% for both PCV13 and PPSV23).
The percentages of beneficiaries with claims were higher among those with immunocompromising conditions compared to those without these conditions (PPSV23: 53.8% vs. 32.1%, PCV13: 55.2% vs. 38.2%, and both vaccines: 37.8% vs. 21.9%) *. Similarly, the percentages of beneficiaries with claims for PPSV23, PCV13, and both vaccines among those with underlying medical conditions were higher than the percentage of reported claims among beneficiaries without these conditions (PPSV23: 50.2% vs. 25.4%, PCV13: 52.5% vs. 32.5%, and both vaccines: 35.0% vs. 18.0%, respectively).†
Claims for pneumococcal vaccination among beneficiaries aged ≥65 years in December 2019 also varied by state of residence (Table 2). The percentage of beneficiaries with claims for any PCV13 dose ranged from 40.8% in Mississippi to 64.8% in Wisconsin, claims for any PPSV23 dose ranged from 32.3% in Alaska to 56.8% in Wisconsin, and claims for both vaccines ranged from 23.2% in Alaska to 47.6% in Wisconsin.
Discussion
A little over four years after the ACIP recommendation for routine use of PCV13 in series with PPSV23 in adults aged ≥65 years, claims for PCV13 reached 49.3% in December 2019, surpassing the proportion of claims for PPSV23 (46.2%). However, claims for receipt of both vaccines remained lower, even during the period when both vaccines were recommended for use.
Vaccination by demographic characteristics, medical conditions, and state
Implementation of PCV13 and PPSV23 vaccination recommendations has not been equal across all adults aged ≥65 years. Beneficiaries aged 65–69 years had the lowest percentage of claims for receipt of either or both vaccines. White beneficiaries were more likely to have claims for receipt of either or both vaccines than beneficiaries of other racial/ethnic groups, especially Hispanic beneficiaries. Differences in coverage with pneumococcal vaccine, as well as other vaccines, among older adults by race/ethnicity are well documented, and might be attributable to differences in patient, provider, and system factors, including differences in attitudes toward vaccination and concerns about vaccination safety, in access to care, provider recommendation for vaccination, and in quality of care received by different racial/ethnic groups (14,15).
Although PCV13 and PPSV23 were routinely recommended for all adults aged ≥65 years, beneficiaries aged ≥65 years with underlying medical conditions were more likely to have been vaccinated than those without such conditions.* Adults with immunocompromising conditions have been recommended to receive PCV13 since 2012 (10); however, PCV13 coverage in this group did not increase until after 2014 when all adults aged ≥65 years were recommended to receive PCV13 in series with PPSV23. This implies that there are challenges with the implementation of risk-based vaccine recommendations, and this finding is consistent with the low pneumococcal vaccine coverage reported in adults aged 19–64 years with indications for vaccination.
Vaccination with both types of pneumococcal vaccine also varied by state, as has been previously reported for PPSV23. State variation in vaccination coverage has been attributed to differences in health care delivery infrastructure and vaccination programs, as well as differences in population characteristics between states (15).
Limitations
The findings in this report are subject to limitations related to the use of Medicare claims data to estimate vaccination coverage. The beneficiaries in this study population with claims for pneumococcal vaccination may not be representative of pneumococcal vaccination coverage among all adults aged ≥65 years in the United States for reasons listed below.
History of vaccination
The pneumococcal vaccination status (i.e., whether they had indications for and had received PPSV23 or PCV13 previously) of persons prior to their eligibility for Medicare was not known so could not be considered in the analysis. This might affect as many as 20% of the Medicare population (CDC, unpublished data.)
Bundled claims
Reports for claims of pneumococcal vaccination may have been combined with other claims and contributed to underrepresentation of vaccinated persons in the study population. Doses administered during hospitalization might have been processed among other claims during hospitalization leading to a potential underestimation of beneficiaries who had received pneumococcal vaccination. For those beneficiaries enrolled in Medicare Part C, claims for pneumococcal vaccination could also be under reported because Medicare is not billed separately for vaccinations. In addition, neither the proportion of beneficiaries who may have moved back-and-forth from Medicare Part C plans to Medicare Parts A and B during the study period nor whether Part C beneficiaries reported a claim for pneumococcal vaccination was known. In 2019, 37% of Medicare beneficiaries were enrolled in Part C, with enrollment by state ranging from 1% to 63% (7).§
Vaccination prior to CMS reports
The CMS database does not include claims for vaccinations administered before 1999 (8). Whereas not having information on pneumococcal vaccination claims before 1999 would not have affected estimates for PCV13 vaccination, the percentage of persons vaccinated with PPSV23 could have been underestimated, particularly among older beneficiaries who reached age 65 before 1999 and might have been vaccinated with PPSV23 after its licensure for use in the United States in 1983. This applies to 6,302,731 or 24.6% of all beneficiaries included in analysis.
Misclassification of race/ethnicity group
Race/ethnicity of Hispanic, Asian and American Indian/Alaska Native beneficiaries could also potentially have been misclassified because of the change in categorization of race/ethnicity information collected by the Social Security Administration in 1980 (16).¶ This may have led to an under or over classification of beneficiaries as Hispanic, Asian and American Indian/Alaska Native. No validation study of pneumococcal vaccination claims to medical records has been done to quantify the extent of possible bias due to errors in coding race/ethnicity.
Claims among those with immunocompromising conditions
Finally, estimates for beneficiaries with immunocompromising conditions may have been overestimated because claims among beneficiaries with immunocompromising conditions included those who had ever reported such conditions and may have recovered, and individuals who were tested for such conditions and did not have them.*
Conclusions
Increase in PCV13 coverage was observed during 2014–2019 when routine use of both PCV13 and PPSV23 was recommended in adults aged ≥65 years; however, the proportion of beneficiaries who received both vaccines remained lower, especially in certain subgroups. Higher-valent pneumococcal conjugate vaccines (15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine) were recently licensed for use in adults aged ≥18 years, and ACIP is currently considering the use of these new vaccines. Findings from this analysis will help identify policy options that reduce disparity in vaccine coverage and maximize pneumococcal disease prevention in adults.
Footnotes:
* Beneficiaries with immunocompromising conditions included those who had ever reported any of the conditions listed below and may have recovered as well as individuals who were tested for such conditions and did not have them. Conditions include underlying renal failure, nephrotic syndrome, immunodeficiency, iatrogenic immunosuppression, generalized malignancy, human immunodeficiency virus, Hodgkin disease, leukemia, lymphoma, multiple myeloma, solid organ transplants, congenital or acquired asplenia, sickle cell disease, or other hemoglobinopathies.
† Underlying conditions includes all immunocompromising conditions listed above plus chronic heart disease (including congestive heart failure and cardiomyopathies, excluding hypertension), chronic lung disease (including chronic obstructive pulmonary disease, emphysema, and asthma) and diabetes mellitus.
§ Each enrollment period is from January 1 through December 31 of the year. Denominators in each subgroup include all beneficiaries continuously enrolled in Medicare Parts A and B at least one year prior to December 31 of the respective year.
¶ Race/ethnicity was categorized as White, Black, Asian, American Indian/Alaska Native, Hispanic, and “other.” Beneficiaries identified as Hispanic might be of any race. “Other” includes persons of multiple race. Excludes 484,843 beneficiaries with unknown race/ethnicity.
FIGURE. Proportion of Medicare beneficiaries aged ≥65 years with claims submitted for pneumococcal vaccination, regardless of prior vaccination – United States, 2010–2019*
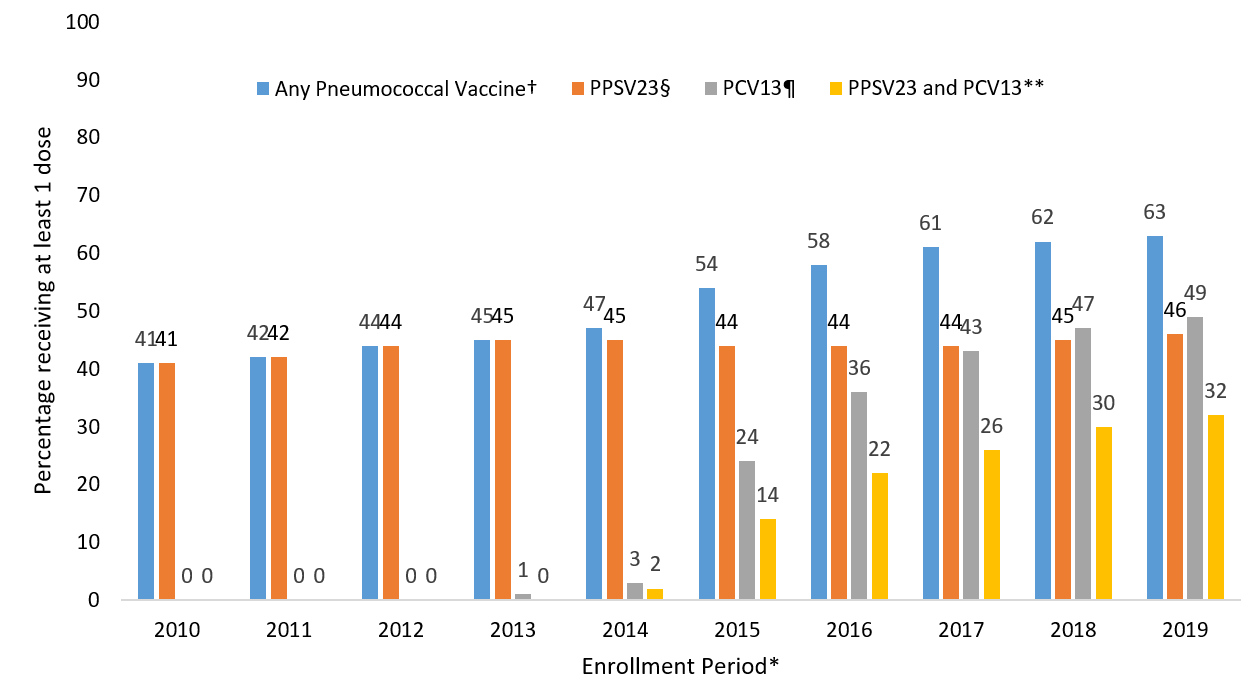
* Each enrollment period is from January 1 through December 31 of the year. Denominators in each subgroup include all beneficiaries continuously enrolled in Medicare Parts A and B at least one year prior to December 31 of the respective year.
†Percentage with at least one claim for PPSV23 or PCV13 since January 1, 1999 through the end of the enrollment period.
§Percentage with at least one claim for PPSV23 since January 1, 1999 through the end of the enrollment period.
¶ Percentage with at least one claim for PCV13 since January 1, 1999 through the end of the enrollment period.
** Percentage with at least one claim for PPSV23 and at least one claim for PCV13 since January 1, 1999 through the end of the enrollment period.
TABLE 1. Proportion of Medicare beneficiaries aged ≥65 years with claims submitted for pneumococcal vaccination regardless of prior vaccination, by age, race/ethnicity, and presence of chronic and immunocompromising medical conditions, United States, December 2019*
| Category | Total enrolled beneficiaries | ≥1 dose PPSV23† (%) | ≥1 dose PCV13 § (%) |
Both PPSV23 & PCV13¶ (%) | Any pneumococcal (%)** |
|---|---|---|---|---|---|
| Age (years) | |||||
| 65–69 | 7,957,095 | 29.7 | 40.4 | 20.5 | 49.6 |
| 70–74 | 6,796,985 | 44.8 | 51.2 | 33.2 | 62.8 |
| 75–79 | 4,743,679 | 55.0 | 55.4 | 39.4 | 71.0 |
| 80–84 | 3,150,261 | 62.1 | 55.4 | 42.5 | 75.1 |
| >85 | 3,110,957 | 62.2 | 52.4 | 39.3 | 75.3 |
| Race/ethnicity†† | |||||
| White | 21,911,116 | 47.8 | 51.2 | 33.9 | 65.0 |
| Black | 1,803,442 | 35.1 | 34.9 | 20.8 | 49.3 |
| Asian | 525,916 | 43.2 | 41.9 | 25.5 | 59.6 |
| Hispanic | 400,149 | 32.2 | 30.1 | 16.2 | 46.1 |
| American Indian/Alaska Native | 121,697 | 38.2 | 40.2 | 21.0 | 57.4 |
| Other Race | 452,338 | 43.8 | 45.6 | 29.1 | 60.3 |
| Immunocompromising Condition§§ | |||||
| Yes | 16,813,636 | 53.8 | 55.2 | 37.8 | 71.2 |
| No | 8,945,341 | 32.1 | 38.2 | 21.9 | 48.4 |
| Underlying conditions ¶¶ | |||||
| Yes | 21,665,388 | 50.2 | 52.5 | 35.0 | 67.7 |
| No | 4,093,589 | 25.4 | 32.5 | 18.0 | 39.9 |
* Denominator in each subgroup includes all beneficiaries continuously enrolled in Medicare Parts A and B at least one year prior to December 31, 2019.
† Percentage of beneficiaries with at least one claim for 23-valent pneumococcal polysaccharide vaccine (PPSV23) since January 1, 1999 through December 31, 2019.
§ Percentage of beneficiaries with at least one claim for 13-valent pneumococcal conjugate vaccine (PCV13) since January 1, 1999 through December 31, 2019.
¶ Percentage of beneficiaries with at least one claim for PPSV23 and at least one claim for PCV13 since January 1, 1999 through December 31, 2019.
** Percentage of beneficiaries with at least one claim for PPSV23 or PCV13 since January 1, 1999 through December 31, 2019.
†† Race/ethnicity was categorized as White, Black, Asian, American Indian/Alaska Native, Hispanic, and “other.” Beneficiaries identified as Hispanic might be of any race. “Other” includes persons of multiple race. Excludes 489,148 beneficiaries with unknown race/ethnicity.
§§ Beneficiaries with immunocompromising conditions included those who had ever reported any of the conditions listed below and may have recovered as well as individuals who were tested for such conditions and did not have them. Conditions include cerebrospinal fluid leak, cochlear implant, sickle cell disease or other hemoglobinopathy, congenital or acquired asplenia, congenital or acquired immunodeficiency (including B- or T-lymphocyte deficiency, complement deficiencies, and phagocytic disorders excluding chronic granulomatous disease), HIV infection, chronic renal failure, nephrotic syndrome, leukemia, lymphoma, Hodgkin disease, generalized malignancy, immunosuppression due to treatment with immunosuppressive drugs, including long-term corticosteroids and radiation therapy, solid organ transplant, and multiple myeloma. Use of ICD codes might be nonspecific in identifying generalized malignancies if providers use these codes for “rule-out” diagnoses.
¶¶Includes all immunocompromising conditions listed above plus chronic heart disease (including congestive heart failure and cardiomyopathies, excluding hypertension), chronic lung disease (including chronic obstructive pulmonary disease, emphysema, and asthma) and diabetes mellitus.
TABLE 2. Proportion of Medicare beneficiaries aged ≥65 years with claims submitted for pneumococcal vaccination regardless of prior vaccination, by state, United States, December 2019*
| State | Total enrolled beneficiaries | ≥1 dose PPSV23† (%) |
≥1 dose PCV13§ (%) |
Both PPSV23 & PCV13¶ (%) | Any pneumococcal** (%) |
|---|---|---|---|---|---|
| Alabama | 226,078 | 43.5 | 42.1 | 26.7 | 58.8 |
| Alaska | 36,314 | 32.3 | 42.5 | 23.2 | 51.6 |
| Arizona | 348,306 | 44.0 | 47.6 | 29.6 | 62.0 |
| Arkansas | 197,939 | 46.2 | 47.0 | 31.6 | 61.6 |
| California | 1,335,206 | 42.9 | 44.4 | 27.8 | 59.4 |
| Colorado | 232,525 | 47.3 | 52.6 | 34.4 | 65.4 |
| Connecticut | 172,772 | 48.3 | 53.2 | 34.7 | 66.9 |
| Delaware | 93,149 | 52.8 | 58.2 | 39.3 | 71.7 |
| District of Columbia | 25,508 | 40.6 | 42.6 | 26.1 | 57.2 |
| Florida | 1,082,931 | 45.6 | 45.9 | 29.7 | 61.7 |
| Georgia | 435,544 | 46.5 | 49.6 | 32.7 | 63.4 |
| Hawaii | 56,014 | 42.7 | 48.7 | 30.5 | 61.0 |
| Idaho | 89,888 | 42.2 | 45.3 | 29.0 | 58.6 |
| Illinois | 724,550 | 47.8 | 50.5 | 34.3 | 63.9 |
| Indiana | 410,690 | 52.3 | 55.7 | 38.8 | 69.2 |
| Iowa | 246,746 | 50.3 | 56.1 | 39.1 | 67.4 |
| Kansas | 200,849 | 46.4 | 50.6 | 33.7 | 63.3 |
| Kentucky | 236,925 | 45.6 | 46.3 | 30.7 | 61.2 |
| Louisiana | 203,674 | 44.1 | 42.0 | 27.2 | 58.9 |
| Maine | 88,055 | 44.2 | 52.2 | 32.8 | 63.6 |
| Maryland | 416,069 | 48.1 | 53.2 | 34.7 | 66.6 |
| Massachusetts | 467,168 | 47.4 | 58.9 | 35.6 | 70.7 |
| Michigan | 501,833 | 47.9 | 48.5 | 32.9 | 63.5 |
| Minnesota | 193,321 | 41.3 | 41.1 | 27.9 | 54.5 |
| Mississippi | 183,733 | 41.3 | 40.8 | 25.6 | 56.5 |
| Missouri | 321,573 | 46.0 | 49.5 | 33.1 | 62.4 |
| Montana | 80,768 | 41.9 | 47.4 | 30.6 | 58.7 |
| Nebraska | 138,453 | 47.7 | 51.0 | 35.1 | 63.6 |
| Nevada | 116,548 | 38.0 | 41.5 | 25.6 | 53.9 |
| New Hampshire | 119,340 | 50.3 | 61.0 | 39.8 | 71.4 |
| New Jersey | 483,541 | 45.7 | 46.8 | 30.0 | 62.5 |
| New Mexico | 101,484 | 41.7 | 41.2 | 25.6 | 57.3 |
| New York | 819,575 | 44.5 | 45.5 | 29.6 | 60.3 |
| North Carolina | 588,585 | 50.7 | 55.6 | 37.8 | 68.5 |
| North Dakota | 51,246 | 48.7 | 53.6 | 37.4 | 64.9 |
| Ohio | 588,945 | 48.4 | 52.0 | 34.4 | 66.0 |
| Oklahoma | 249,548 | 46.5 | 46.7 | 30.2 | 63.1 |
| Oregon | 188,393 | 44.2 | 48.5 | 31.9 | 60.8 |
| Pennsylvania | 721,502 | 49.7 | 57.9 | 38.4 | 69.1 |
| Rhode Island | 44,414 | 39.4 | 46.9 | 26.2 | 60.2 |
| South Carolina | 361,830 | 47.2 | 53.5 | 34.2 | 66.6 |
| South Dakota | 63,713 | 46.8 | 51.1 | 35.5 | 62.4 |
| Tennessee | 363,035 | 47.1 | 50.9 | 33.1 | 64.8 |
| Texas | 1,032,225 | 45.7 | 44.7 | 29.1 | 61.3 |
| Utah | 116,019 | 47.6 | 54.7 | 34.2 | 68.1 |
| Vermont | 59,321 | 42.1 | 53.8 | 31.7 | 64.2 |
| Virginia | 576,978 | 50.2 | 56.1 | 37.6 | 68.8 |
| Washington | 387,774 | 44.6 | 50.9 | 33.0 | 62.5 |
| West Virginia | 104,310 | 41.0 | 41.6 | 26.0 | 56.6 |
| Wisconsin | 334,250 | 56.8 | 64.8 | 47.6 | 74.0 |
| Wyoming | 45,548 | 40.0 | 45.3 | 27.8 | 57.4 |
| Median | – | 45.9 | 49.1 | 32.3 | 62.5 |
| Range across states | – | 32.3–56.8 | 40.8–64.8 | 23.2–47.6 | 51.6–74.0 |
* Denominator in each subgroup includes all beneficiaries continuously enrolled in Medicare Parts A and B at least one year prior to December 31, 2019. Excludes 120,567 beneficiaries with unknown state.
† Percentage of beneficiaries with at least one claim for 23-valent pneumococcal polysaccharide vaccine (PPSV23) since January 1, 1999 through December 31, 2019.
§ Percentage of beneficiaries with at least one claim for 13-valent pneumococcal conjugate vaccine (PCV13) since January 1, 1999 through December 31, 2019.
¶ Percentage of beneficiaries with at least one claim for PPSV23 and at least one claim for PCV13 since January 1, 1999 through December 31, 2019.
**Percentage of beneficiaries with at least one claim for PPSV23 or PCV13 since January 1, 1999 through December 31, 2019.
References
- Update: pneumococcal polysaccharide vaccine usage—United States. MMWR Morb Mortal Wkly Rep 1984;33:273–6, 281.
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained Reductions in Invasive Pneumococcal Disease in the Era of Conjugate Vaccine. J Infect Dis 2010 Jan;201(1):32–41.
- Bonten M, Bolkenbaas M, Huijts S, et al. Community Acquired Pneumonia Immunization Trial in Adults (CAPiTA). Abstract no. 0541. Pneumonia. 2014;3:95. Available from https://pneumonia.org.au/2020/01/31/new-pneumonia-study/.
- Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2014;63(37):822–5.
- Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, et al. Intervals Between PCV13 and PPSV23 Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64(34):944–7.
- Centers for Disease Control and Prevention. Grading of Recommendations Assessment, Development and Evaluation (GRADE) for use of PCV13 among adults ≥65 years old. 2018. Available from: www.cdc.gov/vaccines/acip/recs/grade/PCV13.html
- Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2019;68(46);1069–1075.
- Centers for Medicare and Medicaid Services. Medicare Enrollment Dashboard. U.S. Department of Health and Human Services. 2017. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/Dashboard
- American Community Survey. 2019: ACS 1-Year Estimates Selected Population Profiles
- Centers for Disease Control and Prevention. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2012;61(40):816–19.
- Pilishvili T, Bennett NM. Pneumococcal Disease Prevention Among Adults: Strategies for the Use of Pneumococcal Vaccines. Am J Prev Med 2015 Dec 1;49(6):S383–90.
- Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N Engl J Med 2015; 372(12):1114–25.
- Lu PJ, O’Halloran A, Williams WW, Lindley MC, Farrall S, Bridges CB. Racial and Ethnic Disparities in Vaccination Coverage Among Adult Populations in the U.S. Vaccine 2015; 33:D83–91.
- O’Halloran AC, Lu PJ, Pilishvili T. Pneumococcal Vaccination Coverage Among Persons ≥65 years-United States, 2013. Vaccine 2015; 33(42):5503–6.
- Hung M, Williams W, Lu PJ, Woods LO., Koppaka Ram, Lindley MC. Vaccination Coverage Among Adults in the United States, National Health Interview Survey, 2017. AdultVaxView. 201. Available from: www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2017.html
- Eicheldinger CR, Bonito A. More Accurate Racial and Ethnic codes for Medicare Administrative Data. Health Care Financ Rev 2008;29(3):27–42.