Wastewater Surveillance Testing Methods
Use this guidance to implement wastewater-based disease surveillance. Wastewater-based disease surveillance is a rapidly developing science, and CDC will continue to update guidance and information as it becomes available.
Testing methods overview
Multiple testing methods and laboratory workflows are used to quantify SARS-CoV-2 in wastewater across the United States. Laboratory controls can ensure that results are comparable by accounting for method performance and data quality. Based on the levels of SARS-CoV-2 in wastewater, methods can be adapted to higher or lower detection limits as needed. For example, if levels of SARS-CoV-2 RNA are sufficiently high in wastewater, small volumes of wastewater (e.g., 1 ml) may be tested without additional concentration processes. Testing methods include sample processing steps, use of laboratory controls, and implementation of biosafety measures to ensure that data can be interpreted for public health use.
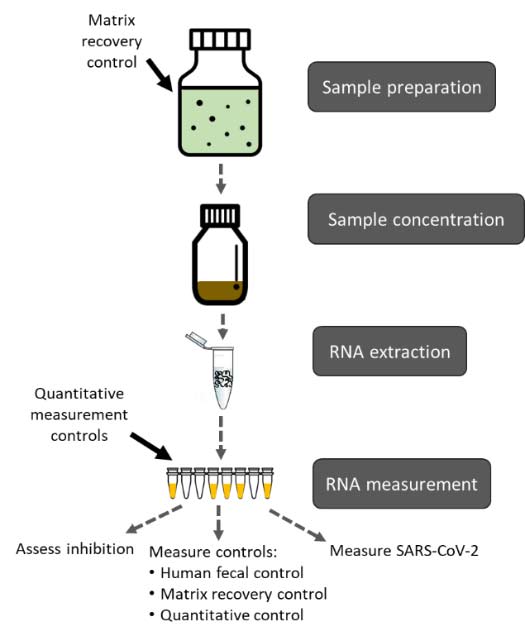
After sample collection:
Sample preparation is the first step in SARS-CoV-2 wastewater testing. A matrix recovery control should be spiked into the sample during this step.
Sample concentration is the second step.
RNA extraction from the concentrated wastewater sample is the third step.
RNA measurement is the final step. Along with measurement of SARS-CoV-2 RNA in this step, several laboratory controls should also be measured, including matrix recovery controls, human fecal normalization, quantitative measurement controls, and controls to assess molecular method inhibition.
Sample processing
Sample processing for measuring SARS-CoV-2 RNA in wastewater involves sample preparation, sample concentration, RNA extraction, and RNA measurement methods. Methods selected at each step must be tailored for use with wastewater, which is a chemically and biologically complex and variable mixture. Evaluate the performance of these wastewater sample processing procedures using appropriate laboratory controls. Proper biosafety protocols for processing wastewater samples that may contain SARS-CoV-2 should be followed and are described later on this web page.
Sample preparation
Properly storing and preparing wastewater samples help ensure that SARS-CoV-2 RNA wastewater measurements are accurate.
- Storage: Refrigerate samples at 4°C immediately after collection and, if possible, process them within 24 hours to reduce SARS-CoV-2 RNA degradation and increase surveillance utility. If you cannot process samples within 24 hours after collection, you should spike a matrix recovery control into the sample prior to refrigerating it at 4°C or freezing it at -20°C or -70°C.
- Homogenization: Both liquid wastewater and primary sludge samples should be well-mixed prior to removing portions of collected wastewater for downstream processing. Mix by inverting samples several times (for liquid samples) or by mechanical mixing. Homogenizing samples can also include procedures to break up wastewater solids and disaggregate virus particles, such as by sonication.
- Sample clarification: Clarifying liquid wastewater samples by removing large solids can aid subsequent filtration-based concentration steps if the samples are used for sample concentration. However, removing solids will also remove SARS-CoV-2 RNA adhered to those solids. You can clarify samples using filters with a large pore size (5 µm or larger) or centrifugation.
Sample concentration
Concentrating wastewater samples can improve detection of SARS-CoV-2 RNA. Concentration may be more important for untreated wastewater samples than primary sludge samples. See What to Sample for more information on selecting a sample type.
Concentration approaches evaluated to date that yield adequate recovery for SARS-CoV-2 detection in wastewater include:
- Ultrafiltration
- Filtration through an electronegative membrane with sample pre-treatment by addition of MgCl2 or acidification
- Polyethylene glycol (PEG) precipitation
- Skim milk flocculation
- Ultracentrifugation
Consider the following factors when selecting a virus concentration method:
- Sample type: For untreated wastewater samples, several filtration and precipitation methods, listed above, are available. For primary sludge samples, centrifugation is the most effective way to concentrate solids.
- Sample volume: Large untreated wastewater sample volumes may require dividing the sample prior to membrane filtration (due to slow filtration rate) or PEG precipitation (due to centrifuge volume constraints). Sample volumes greater than 5 L may require pre-concentration by methods designed to concentrate a large volume, such as large cartridge ultrafiltration.
- Potential supply chain issues: Methods that require commercial filtration products, such as membrane filters or ultrafiltration cartridges, may be more sensitive to supply chain issues than other methods.
- Sample processing time: Concentration method selection will be constrained by method processing time and availability of laboratory personnel. Membrane filtration of turbid wastewater samples may take several hours.
- Availability of laboratory equipment: Centrifuge volumes and force capacity, as well as availability of membrane filtration units, will also constrain method selection.
RNA extraction
Nucleic acid extraction and purification is an essential step in isolating SARS-CoV-2 RNA from the sewage mixture. Sewage is a complex mixture with materials known to interfere with molecular viral quantification methods, so consider the following when selecting an extraction method:
- Select an extraction protocol designed to produce highly purified nucleic acid extracts from environmental samples. Commercial kits are available for environmental sample extraction.
- Use an extraction kit or a protocol that is designed specifically to purify RNA and includes RNase denaturants prior to lysis.
- Avoid degradation of extracted RNA due to multiple freeze-thaw cycles by aliquoting extracts into separate tubes and storing them at -70°C or below.
RNA measurement
Detection methods: Quantify SARS-CoV-2 RNA in wastewater using either RT-qPCR (reverse transcription-quantitative polymerase chain reaction) or RT-ddPCR (RT-droplet digital PCR; other forms of digital PCR are also possible but less common). Each method can be performed as either a 1-step reaction, in which RT and PCR occur in the same reaction mixture, or a 2-step reaction, in which RT and PCR are performed in separate, sequential reactions. A 1-step RT-ddPCR protocol is advantageous for wastewater because RT is performed in individual droplets, which can reduce RT inhibition compared to RT in bulk solution, as in a 2-step process and in RT-qPCR.
Genetic targets: Primers and probes targeting regions of the SARS-CoV-2 N (N1 and N2, published by CDC) and E genes (E_sarbeco, Corman et al., 2020 EuroSurveillance) have been reported to be sensitive and specific for quantifying SARS-CoV-2 RNA in wastewater. When possible, compare wastewater measurements using the same target genes.
Laboratory controls
Laboratory controls are essential for comparing SARS-CoV-2 RNA wastewater concentrations over time and across wastewater sources, especially when you use different testing methods. CDC recommends the following types of measurement laboratory controls for SARS-CoV-2 wastewater surveillance:
- Matrix recovery control
- Human fecal normalization
- Quantitative measurement controls
- Inhibition assessment
- Negative controls
Matrix recovery controls
Use a matrix recovery control (also called a process control) to understand the amount of virus lost during sample processing. This control is important for comparing concentrations resulting from different testing methods and over time. It is important to quantitatively assess recovery because wastewater is chemically and biologically complex and variable, and often contains constituents that can interfere with sample concentration, nucleic acid extraction, or molecular quantification methods. You must include a matrix recovery control in method validation and, if possible, include it with each sample to account for unexpected changes in wastewater composition. Always include a matrix recovery control when wastewater conditions (such as from rainwater inflows) or laboratory methods change.
A matrix recovery control that is more biologically similar to SARS-CoV-2 may more accurately represent the recovery of SARS-CoV-2 from a wastewater sample. Candidates for matrix recovery controls are enveloped viruses with single-stranded RNA genomes, including murine coronavirus (also called murine hepatitis virus), bovine coronavirus, and bovine respiratory syncytial virus.
Human fecal normalization
Normalizing SARS-CoV-2 wastewater concentrations prior to calculating trends accounts for changes in wastewater dilution and differences in relative human waste input over time. If the number of people contributing to the sewershed is expected to change over the surveillance period (due to tourism, weekday commuters, temporary workers, etc.), normalizing SARS-CoV-2 concentrations by the amount of human feces in wastewater can be important for interpreting SARS-CoV-2 concentrations and comparing concentrations between sewage samples over time. Human fecal normalization controls are organisms or compounds specific to human feces that can be measured in wastewater to estimate its human fecal content.
Human normalization controls include, but are not limited to:
- Fecal indicator viral molecular targets: Pepper Mild Mottle virus, crAssphage
- Fecal indicator bacterial molecular targets: Bacteroides HF183, Lachnospiraceae Lachno3
Normalizing SARS-CoV-2 concentrations using human fecal controls (e.g., the ratio of SARS-CoV-2 to human fecal control concentrations) can also account for viral losses that occur anywhere between fecal input into the wastewater system and quantification at the laboratory. However, human fecal normalization cannot replace matrix recovery controls for method performance evaluation.
Quantitative measurement controls
You must include quantitative measurement controls for all SARS-CoV-2 RNA quantification methods. For RT-qPCR, derive a calibration curve from a control of known concentration. For RT-ddPCR, include a control of known quantity with each instrument run. RNA controls are preferable to DNA controls for accurate RNA target quantification. Aliquot quantitative measurement controls to avoid freeze-thaw cycles and store them at -70°C or below.
Inhibition assessment
Use inhibition testing to determine whether RNA quantification processes (RT and PCR) are performing as expected. Wastewater is a complex and variable mixture, and often contains compounds that can impede accurate measurement by interfering with RNA quantification methods.
Inhibition can be assessed using several approaches:
- When SARS-CoV-2 RNA concentrations are high, assess inhibition by evaluating whether the concentrations measured in the extracted RNA diluted to different levels scale with the dilution as expected. This method is preferred because it enables direct evaluation of inhibition in the same reaction used to quantify SARS-CoV-2 in the sample.
- When SARS-CoV-2 RNA concentrations are too low to be quantified after dilution, assess inhibition by spiking viral RNA (for example, synthetic SARS-CoV-2 RNA or purified RNA from a non-human coronavirus, as described in Matrix Recovery Controls) into wastewater extracts, and comparing the measured concentration to either viral RNA spiked into molecular negatives (no template controls) or to a dilution of the spiked extract.
If you encounter inhibition, it can often be eliminated by diluting extracts. If you frequently encounter inhibition, further optimize sample processing or quantification methods.
Negative controls
Extraction blanks are made by extracting RNA without the addition of a wastewater sample. These controls are used to detect extraction reagent contamination. Include them with each set of samples extracted.
“No template controls” are molecular reaction reagents without added wastewater sample nucleic acid extract. Use these controls to detect molecular reagent contamination and include them with all PCR instrument runs.
Biosafety
Concentration of SARS-CoV-2 from wastewater requires bioaerosol-generating processes. CDC recommends conducting these processes in a Biosafety Level 2 (BSL2) facility with unidirectional airflow and BSL-3 precautions, including respiratory protection and a designated area to don and doff personal protective equipment. Laboratory waste from wastewater samples that may contain SARS-CoV-2 should be autoclaved and managed in accordance with BSL2 biosafety guidelines.
Pasteurization
Heat pasteurization of wastewater samples has been conducted to reduce biosafety risk from bioaerosol-generating procedures during wastewater sample processing. Consider the following when deciding whether to include pasteurization:
- The extent to which heat pasteurization will damage the short RNA fragments targeted by PCR is unknown in wastewater.
- Peer-reviewed reports have found that heat-treating respiratory specimens at 56ºC for 30 minutes causes a negligible change to the RNA measurement.
- Some researchers have reported that heat-treating wastewater at 60ºC can improve SARS-CoV-2 RNA measurement, but more data are needed to confirm this effect.