Reported Tuberculosis in the United States, 2021
Drug-Resistant TB
Resistance to anti-TB drugs is a concern in the United States and globally.
- Resistance to isoniazid, one of the most common anti-TB drugs, can be a precursor to multidrug-resistant (MDR) TB, which is defined as resistance to at least isoniazid and rifampin.
- In 2021, CDC adopted new definitions of pre-extensively drug-resistant (pre-XDR) and XDR TB.
- Pre-XDR TB is caused by an organism that is resistant to isoniazid, rifampin, and a fluoroquinolone or by an organism that is resistant to isoniazid, rifampin, and a second-line injectable (amikacin, capreomycin, and kanamycin).
- XDR TB is caused by an organism that is resistant to isoniazid, rifampin, a fluroquinolone, and a second-line injectable (amikacin, capreomycin, and kanamycin) or by an organism that is resistant to isoniazid, rifampin, a fluoroquinolone, and bedaquiline or linezolid.
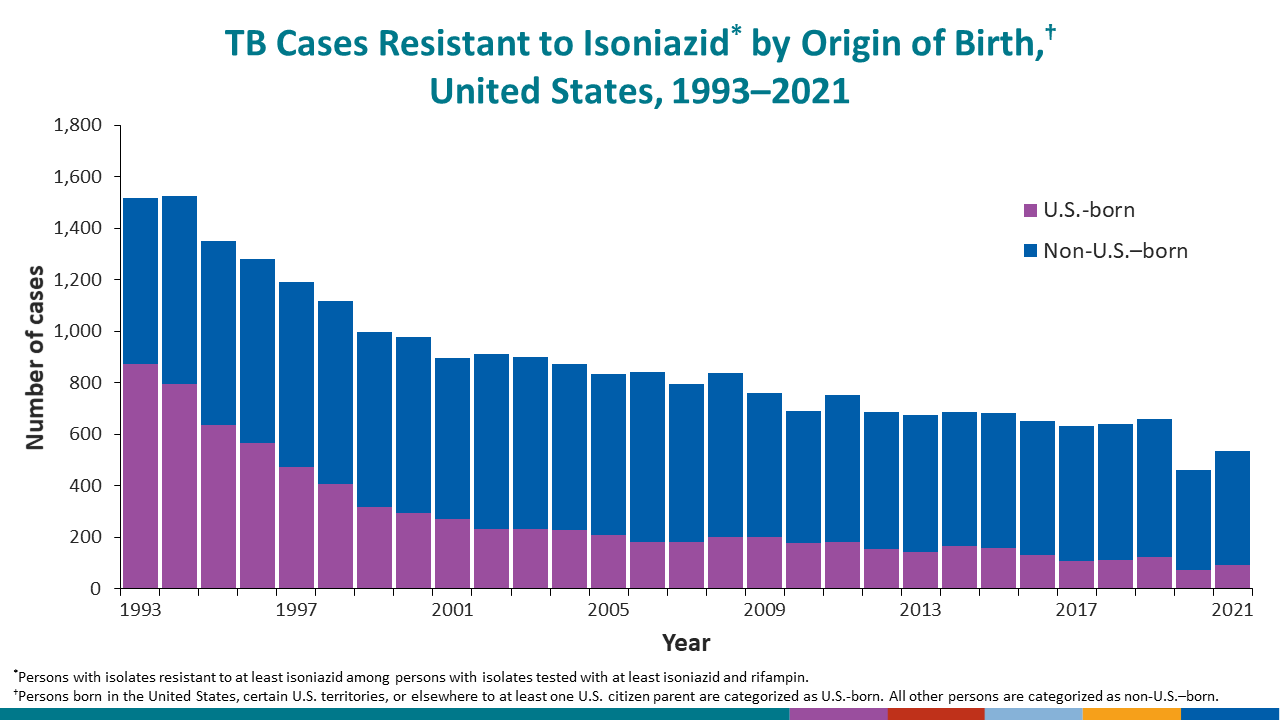
During 2021, isoniazid resistance at initial diagnosis was reported for 536 (8.9%) cases in the United States, including 5.8% of cases among U.S.-born persons and 10.0% of cases among non-U.S.–born persons (Drug Resistance: 1993–2021, Drug Resistance Among U.S.-Born Persons: 1993–2021, Drug Resistance Among Non-U.S.–Born Persons: 1993–2021).
MDR TB at initial diagnosis was reported for 77 (1.3%) cases, including 11 (0.7%) cases among U.S.-born persons and 66 (1.5%) of cases among non-U.S.–born persons (Drug Resistance: 1993–2021, Drug Resistance Among U.S.-Born Persons: 1993–2021, Drug Resistance Among Non-U.S.–Born Persons: 1993–2021).
Pre-XDR and XDR TB continue to be rare in the United States, with four cases of pre-XDR TB and two additional cases of XDR TB in 2021. Stringent guidelines for TB treatment in the United States, including diligent follow-up efforts by public health personnel, greatly reduce the risk of developing drug-resistant TB.
Learn more in the Executive Commentary.