Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Progress Toward Interruption of Wild Poliovirus Transmission --- Worldwide, 2009
In 1988, an estimated 350,000 cases of poliomyelitis were occurring annually worldwide. By 2005, because of global vaccination efforts, indigenous transmission of wild poliovirus (WPV) types 1 and 3 (WPV1 and WPV3) had been eliminated from all but four countries (Afghanistan, India, Nigeria, and Pakistan). No cases of WPV type 2 have been reported since 1999. This report describes progress toward global WPV eradication during 2009 and updates previous reports (1--6). During 2009 a total of 1,606 cases of WPV infection were reported, compared with 1,651 in 2008. WPV3 incidence increased 67%, to 1,124 cases, compared with 675 in 2008. However, WPV1 incidence decreased 51%, to 482 cases in 2009, compared with 976 cases in 2008. In India, nearly all polio cases in 2009 were reported in high-risk districts in western Uttar Pradesh and central Bihar. In Afghanistan and Pakistan, WPV circulation in high-risk districts continued because of difficulties vaccinating children in conflict-affected areas and operational limitations in parts of Pakistan (5). In Nigeria, cases decreased by 51%, to 388 cases in 2009, compared with 798 in 2008. During 2009, outbreaks from importation of WPV affected 19 previously polio-free African countries (2). Two key steps are needed to make further progress in polio eradication: 1) addressing local barriers to interrupting transmission, and 2) using bivalent oral poliovirus vaccine (bOPV) broadly for WPV 1 and 3 in supplemental immunization activities (SIAs).
Routine Vaccination
Global routine vaccination coverage of infants with 3 doses of trivalent oral poliovirus vaccine (tOPV) by age 12 months was estimated at 83% in 2008,* and coverage varied by World Health Organization (WHO) region: African (72%), South-East Asian (73%), Eastern Mediterranean (84%), Americas (92%), European (96%), and Western Pacific (97%). Estimated national 3-dose tOPV coverage for 2008 was 85% in Afghanistan, 81% in Pakistan, 67% in India, and 61% in Nigeria. However, routine 3-dose tOPV coverage of <40% was reported from the Indian states of Bihar and Uttar Pradesh, parts of Afghanistan and Pakistan, and the northern Nigerian states.†
Supplementary Immunization Activities
In 2009, a total of 270 oral polio vaccine (OPV) SIAs§ were conducted in 40 countries (101 national immunization days, 120 subnational immunization days, 21 child health days, and 28 mop-up rounds). An estimated 2.21 billion OPV doses were administered to approximately 360 million children aged <5 years. Of those doses, 39% were tOPV, 51% were monovalent OPV type 1 (mOPV1), 10% were monovalent OPV type 3, and <1% were bOPV. Of the 270 SIAs, 85 (32%) were conducted in the four polio-endemic countries (34 in India, 23 in Pakistan, 13 in Afghanistan, and 15 in Nigeria), 136 (50%) in countries where WPV was reintroduced in 2009 (15) or earlier (five), and 49 (18%) in 16 countries without confirmed WPV cases in 2009.
Acute Flaccid Paralysis Surveillance
The acute flaccid paralysis (AFP) surveillance system is fundamental to monitoring progress toward polio eradication. The system tracks all AFP cases in children aged <15 years and all paralytic illness cases in persons of any age when polio is suspected. The quality of AFP surveillance is monitored by WHO performance indicators.¶ In 2009, each WHO region (except for the European Region) maintained the overall sensitivity of AFP surveillance at certification-standard levels (Table). Since 2005, an operational target for all countries reporting WPV and for neighboring countries has been to achieve a nonpolio AFP rate of >2 cases per 100,000 children aged <15 years. In 2009, all four polio-endemic countries and the 19 other countries with WPV circulation reached this target nationally, although subnational surveillance quality varied substantially.
Wild Poliovirus Incidence
Of 1,606 WPV cases with onset of paralysis reported worldwide during 2009 (Table, Figure), 1,256 (78%) were from the four polio-endemic countries, 207(13%) were from 15 previously polio-free countries after WPV importation, and 143 (9%) were from four countries with reestablished transmission (transmission for >12 months after importation). WPV1 cases decreased from 976 in 2008 to 482 in 2009, whereas WPV3 cases increased from 675 in 2008 to 1,124 in 2009. The number of polio-affected districts decreased 3%, from 496 in 2008 to 481 in 2009.
India. India reported 741 WPV cases in 2009 (79 WPV1, 661 WPV3, and one mixed WPV1/WPV3), an increase compared with 559 cases in 2008. WPV transmission mainly occurred in the northern states of Uttar Pradesh (33 WPV1, 568 WPV3, and one mixed WPV1/WPV3) and Bihar (38 WPV1 and 79 WPV3). The remaining cases in six states and Delhi (eight WPV1 and 14 WPV3) resulted from importation from these two states. Environmental sampling in Mumbai detected one WPV1-positive sample in January 2009 and one WPV3-positive sample in December 2009, whereas sampling in 2008 detected two WPV1-positive samples and 31 WPV3-positive samples. All positive samples in 2008--2009 were of Bihar origin.
Afghanistan and Pakistan. Afghanistan reported 38 WPV cases in 2009 (15 WPV1 and 23 WPV3), compared with 31 WPV cases in 2008, and Pakistan reported 89 WPV cases (60 WPV1, 28 WPV3, and one mixed WPV1/WPV3), compared with 117 cases in 2008. WPV transmission was restricted primarily to previously affected districts in both countries (5). In Afghanistan, 34 (90%) WPV cases occurred in 12 high-risk districts in the conflict-affected southern region. Pakistan experienced continued WPV transmission in security-compromised areas of the Northwest Frontier Province, and in accessible areas of Balochistan and Sindh provinces, where managerial and operational limitations continued to affect vaccination coverage. During 2009, both countries continued to conduct coordinated SIAs and used multiple strategies to reach previously unvaccinated children.
Nigeria. Reported WPV cases in Nigeria decreased from 798 in 2008 (721 WPV1, 76 WPV3, and one mixed WPV1/WPV3) to 388 in 2009 (75 WPV1 and 313 WPV3). After increased involvement of state and local authorities and traditional leaders in 2008--2009, community acceptance and indicators of SIA quality improved in some previously high-incidence states in northern Nigeria. In addition, a sustained decrease in the weekly incidence of cases (particularly WPV1) occurred in the second half of 2009, especially in the northern states (4). However, surveillance monitoring for 2009 indicated that among children aged 6--35 months, up to 50% received <3 doses OPV and up to 20% received no doses in previously high-incidence northern states.
Importations. In 2009, as a consequence of importations that occurred in 2008 or earlier, WPV transmission was confirmed to be reestablished in Angola and Chad and suspected to be reestablished, based on virologic data, in the Democratic Republic of the Congo (DRC) and southern Sudan (2). During August 2008--December 2009, WPV endemic to Nigeria was exported, mostly through intermediate countries, to 10 countries in west Africa and two countries in central Africa and resulted in 178 cases in 2009.** In 2009, WPV3 transmission occurred in the Central Africa Republic through importations from Chad (transmission since 2007, originating from Nigeria) and from DRC (after transmission in Angola in 2008, originating from India) (2). WPV1 outbreaks in Kenya and Uganda in 2009 resulted from importations from southern Sudan (genetic linkage to WPV1 isolated during the outbreak in Sudan during 2004--2005, originating from Nigeria). In Burundi, two WPV1 cases were detected with genetic linkage to WPV1 isolated in DRC in 2008 (after transmission in Angola in 2008, originating from India).
Vaccine-Derived Polioviruses
In 2009, 175 circulating vaccine-derived polioviruses (cVDPVs) were detected from persons with AFP in six countries, including northern Nigeria (153 type 2 cVDPVs), where transmission of cVDPVs has continued since 2005; Guinea (one type 2 cVDPV, imported from Nigeria) (4,6); DRC (four type 2 cVDPVs); Ethiopia (one type 2 cVDPV, one type 3 cVDPV); Somalia (four type 2 cVDPVs); and India (11 type 2 cVDPVs).
Reported by
Polio Eradication Dept, World Health Organization, Geneva, Switzerland. Div of Viral Diseases and Global Immunization Div, National Center for Immunization and Respiratory Diseases, CDC.
Editorial Note
The 1,606 WPV cases reported in 2009 were within the range of cases reported annually since 2005 (1,315 to 1,997 cases). The predominant use of mOPV1 in SIAs since 2006 resulted in reduced numbers of WPV1 cases in 2007 (321) and 2009 (482) but were accompanied by an increase in WPV3 cases, from 994 in 2007 to 1,124 in 2009. These cyclic alternating increases in WPV1 and WPV3 incidence, combined with stagnation in the level of total annual reported cases, prompted development of bOPV in 2007, which became available at the end of 2009. This vaccine is designed to be used for SIAs in countries or areas where both serotypes are circulating, and as supplies allow, currently is in large-scale use in most SIAs in all endemic countries.
In 2009, in response to ongoing WPV1 and WPV3 transmission in all endemic countries and recognition of reestablished transmission in some previously polio-free countries, WHO requested an independent, external evaluation to identify and evaluate barriers to interrupting WPV transmission (7). This evaluation showed that improvements in SIA operations will be required in local, high-risk areas of each country to achieve further progress toward polio eradication. The evaluation also found that the greatest challenges to further progress include funding shortages that limit implementation of SIAs, complacency or continued nonengagement by local health or political authorities, surveillance weaknesses (especially at the subnational level), and continued inability to access children in insecure areas.
The Global Polio Eradication Initiative (GPEI) is using a new strategic plan for 2010--2012, which incorporates lessons learned since GPEI began in 1988, and introduces specific new strategies, milestones for monitoring progress, enhanced oversight, and defined mechanisms for taking corrective actions, with the objective of interrupting poliovirus transmission by the end of 2012 (Box) (8).
GPEI and national authorities are trying to improve the accountability of local leaders, increase the reliability of SIA quality monitoring, better address the needs of migrant and other underserved populations, and strengthen routine immunization systems. The justification for further financing of GPEI to complete polio eradication is sound, both from a humanitarian and economic perspective. A decision to change course from eradication to polio control has been shown by mathematical modeling to be a more costly option over a 20-year period and also will lead to an upsurge to as many as 200,000 polio cases per year in low-income countries (9).
Despite persistence of WPV transmission and importation outbreaks during 2009, as of May 5, 2010, the reported number of WPV cases has declined since the latter part of 2009 in historically high-risk areas of many affected countries. During October--April, when occurrences are seasonally lower, no WPV1 cases were reported from either of the two endemic areas of India (last case in November 2009), and only three WPV3 cases and two WPV1 cases were reported from Nigeria. Also, no WPV cases have been reported since November 2009 from 11 of the 15 African countries affected by new importations in 2009. As of May 5, a total of 115 WPV cases had been reported globally in 2010, compared with 396 in 2009 in this same period, a 71% decline in large part accounted for by the decrease in cases in Nigeria. These trends should be interpreted with caution because of the expected decreased incidence during the low season for poliovirus transmission and occasional delays in confirmation of WPV cases. The notably low WPV incidence in Nigeria has highlighted the opportunity to interrupt WPV transmission in that country in the near future if recent improvements in vaccinating children are maintained and further strengthened.
References
- CDC. Progress toward interruption of wild poliovirus transmission---worldwide, 2008. MMWR 2009;58:308--12.
- CDC. Wild poliovirus type 1 and type 3 importations---15 countries, Africa, 2008--2009. MMWR 2009;58:357--62.
- CDC. Progress toward poliomyelitis eradication---India, January 2007--May 2009. MMWR 2009;58:719--23.
- CDC. Progress toward poliomyelitis eradication---Nigeria, January 2008--July 2009. MMWR 2009;58:1150--4.
- CDC. Progress toward poliomyelitis eradication---Afghanistan and Pakistan, 2009. MMWR 2010;59:268--72.
- CDC. Update on vaccine-derived polioviruses---worldwide, January 2008--June 2009. MMWR 2009;58:1002--6.
- Global Polio Eradication Initiative. Independent evaluation of major barriers to interrupting poliovirus transmission. Geneva, Switzerland: World Health Organization; 2009. Available at http://www.polioeradication.org/content/general/polio_evaluation_report.asp. Accessed May 11, 2010.
- Global Polio Eradication Initiative. Strategic plan 2010--2012: final text for World Health Assembly. Geneva, Switzerland: World Health Organization; 2010. Available at http://www.polioeradication.org/content/publications/stratplan.2010-12.asp. Accessed May 11, 2010.
- Thompson KM, Duintjer-Tebbens RJ. Eradication versus control for poliomyelitis: an economic analysis. Lancet 2007;369:1363--71.
* The most recent year with data available; World Health Organization/UNICEF estimates; coverage data available at http://www.who.int/immunization_monitoring/en/globalsummary/countryprofileselect.cfm.
† Measure DHS (Demographic and Health Surveys) Project, Key Indicators Survey. Calverton, MD:ICF Macro; available at http://www.measuredhs.com; and unpublished data from National Polio Surveillance Project, India.
§ Mass campaigns conducted for a brief period (days to weeks) in which 1 dose of OPV is administered to all children aged <5 years, regardless of vaccination history. Campaigns can be conducted nationally or in portions of the country.
¶ AFP surveillance quality is monitored by performance indicators that measure the sensitivity and specificity of detecting WPV transmission. Certification standard WHO targets are a nonpolio AFP detection rate of >1 case per 100,000 population aged <15 years and adequate stool specimen collection from >80% of AFP cases, in which two specimens are collected ≥24 hours apart, both within 14 days of paralysis onset, shipped on ice or frozen ice packs, and arriving in good condition to a WHO-accredited laboratory. National data might mask surveillance system weaknesses at subnational levels.
** Benin, Burkina Faso, Cameroon, Central African Republic, Cote d'Ivoire, Guinea, Liberia, Mali, Mauritania, Niger, Sierra Leone, Togo.
What is already known on this topic?
The Global Polio Eradication Initiative (GPEI) has reduced poliomyelitis >99% worldwide, from an estimated 350,000 cases of polio in 125 countries in 1988, to 1,606 cases in 23 countries in 2009.
What is added by this report?
The 1,606 WPV cases reported in 2009 were within the range of cases reported annually since 2005 (1,315 to 1,997 cases); 78% were from the four polio-endemic countries, 13% were from 15 previously polio-free countries after WPV importation, and 9% were from four countries with reestablished transmission after importation.
What are the implications for public health practice?
A new GPEI strategic plan for 2010--2012 is being implemented, with the objective of interrupting poliovirus transmission by the end of 2012.
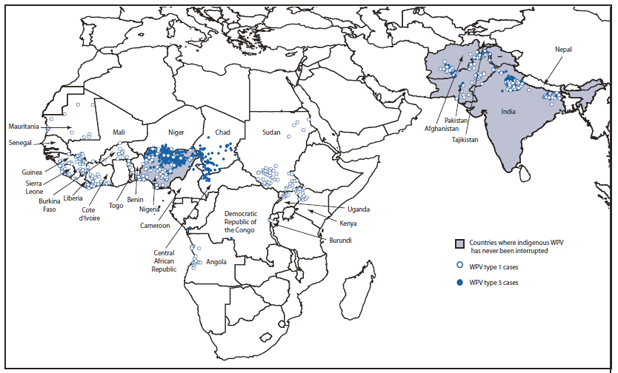
* Data reported to the World Health Organization as of May 5, 2010, excluding polioviruses detected by environmental surveillance and vaccine-derived polioviruses.
Alternate Text: The figure above shows distribution and location of wild poliovirus (WPV) cases (N = 1,606) worldwide in 2009. Of 1,606 WPV cases with onset of paralysis reported worldwide during 2009, a total of 1,256 (78%) were from the four polio-endemic countries (Afghanistan, India, Nigeria, and Pakistan), 207(13%) were from 15 previously polio-free countries after WPV importation, and 143 (9%) were from four countries with reestablished transmission (transmission for >12 months after importation).
BOX. Main points from the World Health Organization (WHO) Global Polio Eradication Initiative (GPEI) Strategic Plan 2010--2012*
|
Major lessons |
What's different in 2010--2012? |
|---|---|
|
Population immunity thresholds needed to stop poliovirus transmission differ and are higher in Asia than in Africa |
WHO will use a new "geographic" strategy and tailor oral polio vaccine (OPV) campaign strategy and monitoring activities more closely to local circumstances than previously, thereby increasing program efficiency. |
|
Immunity gaps allow virus to persist in smaller areas and population subgroups than previously thought |
WHO systematically is developing district- and population-specific strategies and capacity, and special tactics for underserved populations, to address heterogeneity in OPV coverage. Improved real-time and independent monitoring of supplemental immunization activities (SIAs) has been developed where needed, and results of monitoring will be posted internationally within 2 weeks of each campaign. |
|
Routes of poliovirus spread and risks for outbreaks are now largely predictable |
WHO will target immunization systems strengthening and preplanned, synchronized SIAs to reduce the risk for outbreaks after wild poliovirus (WPV) importation, and use a two-pronged approach to enhance the speed, quality, and effectiveness of response activities reported, should an outbreak occur. |
|
Optimizing the balance of use of monovalent OPVs is much more difficult than anticipated |
WHO will use bivalent OPV in those areas where WPV types 1 and 3 are circulating, and implement a balance of monovalent, bivalent, and trivalent OPV SIAs to interrupt WPV transmission and maintain population immunity. |
* Adapted from the WHO GPEI 2010--2012 strategic plan, available at http://www.polioeradication.org/content/publications/stratplan.2010-12.asp.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


