 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Interruption of Wild Poliovirus Transmission --- Worldwide, 2008Since 1988, when the Global Polio Eradication Initiative was established, the incidence of polio has decreased from an estimated 350,000 cases annually to 1,655 reported in 2008.* Cases of wild poliovirus (WPV) type 2 were last reported in October 1999, and indigenous WPV types 1 and 3 (WPV1 and WPV3) have been eliminated from all but four countries worldwide (Afghanistan, India, Nigeria, and Pakistan). This report updates previous reports (1,2) and describes overall progress toward global eradication in 2008. Despite accelerated efforts, polio cases increased 26%, from 1,315 cases in 2007 to 1,655 in 2008. This increase primarily resulted from an increase in Nigeria from 285 cases in 2007 to 801 cases in 2008. Resurgent WPV1 transmission in northern states of Nigeria spread to polio-free southern states and eight neighboring countries in 2008. In India, repeated use of monovalent oral poliovirus vaccine (OPV) type 1 (mOPV1) during 2005--2008 interrupted WPV1 transmission in the western districts of the northern state of Uttar Pradesh for >12 months during 2007--2008; however, in mid-2008, WPV1 imported from the neighboring state of Bihar caused renewed transmission. In Afghanistan and Pakistan, problems in accessing children in conflict-affected areas increased, and an upsurge in WPV1 and WPV3 cases occurred, including an outbreak of WPV1 in Punjab Province, Pakistan. In Africa, during 2008, sustained WPV transmission for >12 months after importation continued in Angola, Chad, the Democratic Republic of the Congo (DRC), Niger, and southern Sudan. Increased political oversight and accountability and improved vaccination outreach to insecure areas are needed to achieve the eradication goal. Wild Poliovirus IncidenceA total of 1,655 WPV cases with onset of paralysis during 2008 were reported worldwide (Table, Figure), a 26% increase from 1,315 cases reported in 2007.† Of these, 1,509 (91%) were reported from the four polio-endemic countries, and 146 were reported from the 14 countries with cases after WPV importation. The number of WPV1 cases increased from 321 in 2007 to 984 in 2008, whereas the number of WPV3 cases decreased from 994 in 2007 to 671 in 2008. Nigeria. Nigeria reported 801 WPV cases in 2008 (729 WPV1, 71 WPV3, and one WPV1/WPV3 coinfection). Ongoing WPV1 transmission in the northern states increased and spread to polio-free southern states. Surveillance monitoring indicates that in high-incidence states, approximately 60% of children in the target group remain underimmunized (i.e., they received <3 doses); approximately 20% of children had received no doses. After the establishment in mid-2008 of a national polio eradication task force and enhanced engagement of local authorities, indicators of the quality of supplementary immunization activities (SIAs)§ and community acceptance improved in some previously high-risk states in northern Nigeria, such as Kebbi and Jigawa, where the accountability of local government officials for SIA implementation was increased. India. India reported 559 WPV cases in 2008 (75 WPV1 and 484 WPV3), mainly from the northern states of Uttar Pradesh and Bihar. Occasional importations of WPV from these states into other Indian states did not lead to further cases because of 1) greater vaccine effectiveness and higher routine vaccination coverage than in Uttar Pradesh and Bihar and 2) large-scale, rapid response SIAs. Western Uttar Pradesh, previously an endemic area with the highest rates of WPV transmission in the world, had been free of indigenous WPV1 for >12 months before re-importation of WPV1 from Bihar in mid-2008. This triggered a new outbreak of 62 cases in Uttar Pradesh in 2008 (accounting for 82.6% of the cases reported from India), which continues into 2009. Afghanistan and Pakistan. Afghanistan reported 31 WPV cases in 2008 (25 WPV1 and six WPV3), and Pakistan reported 118 cases (81 WPV1 and 37 WPV3). Most of the cases in Afghanistan (90%) occurred in the conflict-affected southern and eastern regions. Pakistan experienced a resurgence of WPV transmission in all areas, compounded when WPV1 was imported from polio-endemic areas of Pakistan in the second half of the year into Punjab Province, an area that had been polio-free for approximately 18 months (3). In both countries, serious security problems in areas along the common border allowed continued WPV transmission by limiting access to large numbers of children. In accessible areas of Pakistan, continued managerial and operational problems impeded full implementation of SIAs and adversely affected vaccination coverage; however, recent management innovations by local political authorities have shown promise of improved SIA implementation in Punjab and Sindh (3). ImportationsDuring the second half of 2008, WPV1 originating from northern Nigeria spread to eight neighboring African countries, including six¶ that had been polio-free since having cases during 2003--2005 (i.e., during the resurgence in WPV transmission in Nigeria [4]), and Niger and Chad, two countries that have repeatedly experienced new cases resulting from imported WPV from Nigeria since 2006. In addition, WPV3 from Nigeria spread to two countries, and WPV3 from India spread to two, and WPV3 from Chad spread to Sudan.** In five previously polio-free countries, transmission of WPV originally imported from Nigeria (Chad [WPV1 and WPV3], Niger [WPV1], and Sudan [WPV1]) or Indiat (Angola and DRC [both with WPV1]) has persisted for >12 months. Vaccine-Derived PoliovirusesVaccine-derived polioviruses (VDPVs) were detected from AFP cases in 2008 in seven countries.†† Of these, type 2 circulating VDPVs (cVDPVs) were identified in northern Nigeria, where transmission has continued since 2006 (148 cases to date) (5,6), and in DRC and Ethiopia, where new type 2 cVDPV outbreaks in 2008 were detected (two separate outbreaks of two and 11 cases in DRC and an outbreak of two cases in Ethiopia, to date). Routine VaccinationGlobal vaccination coverage of infants with 3 routine doses of trivalent OPV (OPV3) by age 12 months was estimated at 82% in 2007, the most recent year for which data are available.§§ OPV3 coverage estimates for 2007 in the World Health Organization (WHO) regions were 70% in the South-East Asian Region, 73% in the African Region, 87% in the Eastern Mediterranean Region, and >92% in the American, European, and Western Pacific regions. National OPV3 coverage for 2007¶¶ was 83% in both Afghanistan and Pakistan, 62% in India, and 61% in Nigeria. However, routine OPV3 coverage <40% continues to be reported from northern Nigerian states, the Indian states of Bihar and Uttar Pradesh, and parts of Afghanistan and Pakistan. Supplementary Immunization ActivitiesIn 2008, a total of 241*** SIAs were conducted in 36 countries (57 national immunization days, 118 subnational immunization days, and 53 mop-up rounds), using a total of 2.46 billion doses of OPV, which were delivered to approximately 340 million children††† aged <5 years. Use of mOPV1 increased from 26% of all OPV doses administered during SIAs in 2005 to 49% in 2008. A total of 102 (42%) of the 241 SIAs were conducted in the four polio-endemic countries: 42 in India, 26 in Pakistan, 18 in Afghanistan, and 16 in Nigeria. Of the remaining 139 SIAs, 100 (41% of all SIAs) were conducted in 15 countries where WPV was reintroduced in 2008 or earlier,§§§ and 39 (16% of all SIAs) were conducted in 18 countries without confirmed WPV in 2008. Acute Flaccid Paralysis SurveillanceAcute flaccid paralysis (AFP) surveillance is fundamental to monitoring progress toward polio eradication. The AFP surveillance system tracks any case of AFP in a child aged <15 years or any case of paralytic illness in a person of any age when polio is suspected. The quality of surveillance for acute flaccid paralysis (AFP) is monitored by performance indicators.¶¶¶ In 2008, each WHO region maintained the overall sensitivity of AFP surveillance at certification-standard levels (Table). Since 2005, an operational target for all countries reporting WPV and for neighboring countries (considered at high risk for WPV importation) has been to achieve a nonpolio AFP rate of at least two cases per 100,000 children aged <15 years. In 2008, all four polio-endemic countries and the 15 previously polio-free countries with WPV importation reached this target rate nationally, although subnational surveillance quality varied substantially. Reported by: Polio Eradication Dept, World Health Organization, Geneva, Switzerland. Div of Viral Diseases and Global Immunization Div, National Center for Immunization and Respiratory Diseases, CDC. Editorial Note:The Global Polio Eradication Initiative faced a number of challenges and impediments to progress in 2008, both in the four polio-endemic countries and in previously polio-free countries that had transmission resulting from WPV importations. At the end of 2008, two independent advisory bodies to WHO**** reviewed the progress of the eradication initiative and concluded that the remaining technical and operational challenges could be overcome in each of the polio-endemic countries (7,8). The advisory bodies concluded that the ultimate success of global polio eradication depended on 1) ensuring the political commitment of all polio-affected countries to attain the highest possible coverage during SIAs and 2) enhancing routine vaccination and surveillance. Despite these challenges, specific signs of progress during 2008 were noted by the advisory committee. These included the success in interrupting indigenous transmission of WPV1 in western Uttar Pradesh, India, for 12 months and innovative management approaches to SIA implementation in parts of Nigeria, Pakistan, and Afghanistan, which demonstrated that operational challenges can be overcome with sufficient commitment by national and subnational authorities. In India, low OPV effectiveness in the highest-risk communities (believed to be caused by a combination of high incidence of diarrheal diseases, malnutrition, and the high force of WPV infection attributed to crowding) has been identified as the key challenge to interrupting WPV transmission (9,10). Responses being explored include the use of inactivated poliovirus vaccine as a supplement to mOPV, development of a bivalent OPV containing both type 1 and type 3, other novel uses of OPV, and zinc supplementation. In all other countries with ongoing WPV transmission, serious limitations in accessing and vaccinating children remain the major impediments to polio eradication. The type 2 cVDPV outbreaks in Nigeria, DRC, and Ethiopia reveal striking lapses in routine and SIA vaccination in parts of those countries because cVDPVs are biologically similar to WPVs in terms of infectivity and pathogenicity. In Nigeria, the key to success will be to scale-up throughout the country the communication, social mobilization, and operational improvements that were achieved in some areas of northern Nigeria. In Pakistan, SIA coverage gaps must be better addressed, not only in security-compromised areas but in secure areas experiencing ongoing operational challenges. In Afghanistan, the challenge is making progress in the insecure areas. Prolonged transmission after WPV importation into affected countries will require continuing efforts to overcome the long-standing operational impediments limiting routine and SIA vaccination of children. These impediments will require improved engagement of health and political authorities in those countries, the exploration and implementation of other technical and operational innovations, and the continued coordinated effort of partners. References
† As of March 3, 2009, a total of 57 WPV1, 33 WPV3, and one WPV1/WPV3 coinfection cases with onset of paralysis during 2009 have been reported. § Mass campaigns conducted for a brief period (days to weeks) in which 1 dose of OPV is administered to all children aged <5 years, regardless of vaccination history. Campaigns can be conducted nationally or in portions of the country. Mop-up rounds are intensive house-to-house SIAs conducted in a limited area with evidence of recent transmission. ¶ Benin, Burkina Faso, Cote d'Ivoire, Ghana, Mali, and Togo. ** WPV1 from northern Nigeria spread to Benin, Burkina Faso, Chad, Cote d'Ivoire, Ghana, Mali, Niger, and Togo. WPV3 from northern Nigeria spread to Benin and Niger. WPV3 from India was imported into Nepal and into Angola, with subsequent spread to DRC and Central African Republic. WPV3 of Nigerian origin circulating in Chad was imported into Sudan. †† Angola (two), Ethiopia (two), Nigeria (59), Malawi (one), DRC (13), the Russian Federation (one), and Somalia (one). All isolated VDPVs were serotype 2, except in Malawi, where serotype 3 was found. §§ World Health Organization/UNICEF estimates. OPV3 coverage data available at http://www.who.int/vaccines/globalsummary/immunization/countryprofileselect.cfm. ¶¶ Available at http://www.who.int/immunization_monitoring/en/globalsummary/wucoveragecountrylist.cfm. *** Includes 38 single rounds using both mOPV1 and mOPV3, which are counted as two rounds. ††† Most children received OPV doses during more than one SIA round. §§§ WPV cases in Angola, Benin, Burkina Faso, Central African Republic, Chad, Cote d'Ivoire, DRC, Ethiopia, Ghana, Mali, Nepal, Niger, Sudan, and Togo; in Egypt, response SIAs were conducted after isolation of WPV from sewage. WPV1 was detected on two occasions in sewage in Egypt in 2008, representing two separate importation events, genetically linked to poliovirus originating in Sudan and India, respectively. ¶¶¶ Performance indicators are 1) the rate of AFP cases not caused by WPV (the nonpolio AFP rate), with a target for polio-free certification of at least one case per 100,000 children aged <15 years, and 2) the proportion of AFP cases with adequate stool specimens, with a target for certification of >80%. Adequate specimens are two stool specimens, collected at least 24 hours apart, within 14 days of onset of paralysis, and shipped on ice or frozen ice packs to a WHO-accredited laboratory, arriving at the laboratory in good condition. **** The Advisory Committee on Poliomyelitis Eradication and the Strategic Advisory Group of Experts on Immunization. Table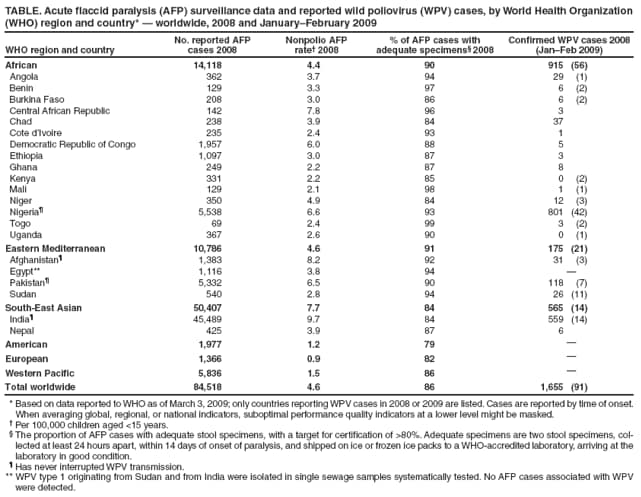 Return to top. Figure 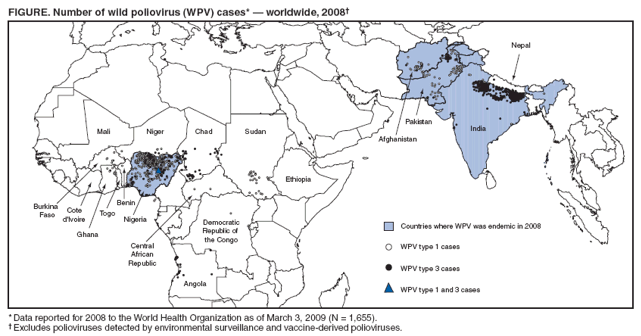 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 4/2/2009 |
|||||||||
|